Abstract
In the present study, proline dithiocarbamate (PRODTC) ligand was radiolabeled with the [99mTc≡N]2+ core successfully to obtain the 99mTcN-PRODTC complex with high radiochemical purity. No decomposition of the complex at room temperature was observed over a period of 6 h. Its partition coefficient indicated that it was a hydrophilic complex. The electrophoresis results showed that the complex was negative. The biodistribution of 99mTcN-PRODTC in mice bearing S 180 tumor showed that the complex accumulated in the tumor with a certain uptake. The tumor/blood and tumor/muscle ratios reached 2.19 and 4.54 at 2 h post-injection, suggesting it would be a promising candidate for tumor imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
[18F]fluorodeoxyglucose [18F]FDG has been the most widely used radiotracer for tumor imaging. However, [18F]FDG can not distinguish tumor from inflammatory tissue or normal brain tissues. Amino acids play an important role in the growth of tumor cells, providing a good opportunity for their use in tumor imaging. The tumor imaging by using radiolabeled amino acids is less influenced by inflammatory processes, suggesting they can overcome the limitation displayed by [18F]FDG [1]. Currently, many amino acids derivatives radiolabeled with positron emitters such as [11C]methionine ([11C]MET) [2, 3], L-3-[18F]fluoro-α-methyl-l-tyrosine ([18F]FMT) [4, 5], O-(2-[18F]-fluoroethyl)-l-tyrosine ([18F]FET) [6–8] and Cis-4-[18F]fluoro-l-proline(Cis-[18F]FPro) [9] have been developed for positron emission tomography (PET). However, a cyclotron is essential to produce these radionuclides, thus restricting their wide use in clinical practice. By comparison, 99mTc has been the isotope of choice for development of novel radiopharmaceuticals owing to its short half-life, optimal γ-energy, inexpensive cost and diverse coordination chemistry. Therefore, achieving 99mTc labeled amino acids derivatives as single photon emission computed tomography (SPECT) probes is considered to be of great interest. Recently, several 99mTc labeled amino acids derivatives as tumor imaging agents have been reported [10–12]. Among them, 99mTc-diethylenetriaminepentaacetic acid-bis(methionine) (99mTc-DTPA-bis(Met)) shows the most promising character to be a tumor imaging agent. However, its chemical structure is poorly defined due to its complicated coordination structure. The preparation of novel 99mTc labeled amino acids derivatives is still considered to be necessary and highly challenging.
The [99mTcN]2+ core has been found to complex well with ligands containing sulfur atoms, as in dithiocarbamates. Recently, some novel 99mTcN labeled dithiocarbamate complexes for infection and tumor imaging have been reported by our group [13–15]. l-proline is a natural amino acid and its molecular structure has an active amine group, thus making it possible to react with carbon disulfide in NaOH solutions to produce the corresponding dithiocarbamate. This background encouraged us to prepare the 99mTcN-PRODTC complex by ligand-exchange reaction with 99mTcN intermediate to find a potential good tumor imaging agent. In this study, the synthesis and biological evaluation of the 99mTcN-PRODTC complex as a potential agent to target tumor are reported for the first time.
Experimental
Materials and methods
l-proline was purchased from J&KCHEMICA, China. Succinic dihydrazide (SDH) kit was obtained from Beijing Shihong Pharmaceutical Center, Beijing Normal University, China. All other chemicals were of reagent grade and were used without further purification. 99Mo/99mTc generator was obtained from the China Institute of Atomic Energy (CIAE). IR spectrum was obtained with an AVATAR 360 FT-IR spectrometer using KBr pellets. NMR spectrum was recorded on a 500 MHz Bruker Avance spectrophotometer. ESI–MS spectrum was recorded on a LC–MS Shimadzu 2010 series. HPLC analysis was carried out with a reversed-phase column (Kromasil 100-5C, 250 4.6 mm), Shimadzu SCL-10AVP series.
Synthesis of proline dithiocarbamate (PRODTC)
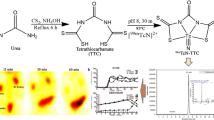
The synthesis of PRODTC was carried out according to the literature [16]. Carbon disulfide (0.5 mL, 8.70 mmol) was dissolved in 5 mL anhydrous diethyl ether and cooled to 0 °C in an ice bath. l-Proline (1,000 mg, 8.70 mmol) and NaOH (692 mg, 17.3 mmol) were dissolved in 10 mL anhydrous methanol and added dropwise to the carbon disulfide solution. The mixture was stirred for 3 h in an ice bath. The solvent was removed and the resulting residue was triturated with diethyl ether. The white oil was dried in vacuo to give PRODTC as a pale yellow solid (1.12 g, 54.6 %).
Radiolabeling of 99mTcN-PRODTC and quality control techniques
The preparation procedure for 99mTcN-PRODTC and thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) analysis conditions are as follows. 1 mL of saline containing [99mTcO4]− (370 MBq) was added to a kit containing 0.05 mg of stannous chloride dihydrate, 5.0 mg of succinic dihydrazide (SDH), 5.0 mg of propylenediamine tetraacetic acid (PDTA). The mixture was kept at room temperature for 15 min. Then, 1.0 mg of PRODTC dissolved in 1.0 mL water was added and the reaction was allowed to stand for 20 min at room temperature. The TLC was performed on a polyamide strip and eluted with saline and acetonitrile respectively. HPLC analysis was carried out with a reversed-phase column (Kromasil 100-5C, 250 × 4.6 mm), Shimadzu SCL-10AVP series, working at a flow rate of 1.0 mL/min. Water (A) and acetonitrile (B) mixtures were used as the mobile phase and the following gradient elution technique was adopted for the preparation (0 min 5 % B, 10 min 50 % B, 20 min 50 % B, 25 min 5 % B, 40 min 5 % B).
In vitro stability study
The stability of the complex was assayed by TLC analysis by measuring the RCP in the reaction mixture for 6 h at room temperature (25 °C). To evaluate the serum stability of 99mTcN-PRODTC, 0.5 mL of 99mTcN-PRODTC (18.5 MBq) was added to 1 mL human serum albumin (1 mg/mL) and incubated at 37 °C. The RCP of the complex was measured by TLC up to 6 h.
Human serum albumin binding assay
10 μL of 99mTcN-PRODTC (370 KBq) was added in 100 μL human serum albumin (100 mg/mL) in the centrifuge tube. After the mixture was incubated at 37 °C for 2 h, the serum protein was precipitated by adding 1 mL trichloroacetic acid (250 mg/mL) to the mixture. The supernatant and precipitate were separated by centrifugation at 2,000g for 5 min. The radioactivities of both phases were measured separately. The above experimental procedure was repeated four times and the percentage of human serum protein binding was determined by the following equation: serum protein binding % = (cpm in precipitate)/(cpm in precipitate + cpm in supernatant) × 100 %.
Determination of the partition coefficient
The partition coefficient was determined by mixing the complex with an equal volume of 1-octanol and phosphate buffer (0.025 mol/L, pH 7.4) in a centrifuge tube. The mixture was vortexed at room temperature for 1 min and then centrifuged at 5,000g for 5 min. From each phase 0.1 mL of the aliquot was pipetted and counted in a well γ-counter. Each measurement was repeated three times. Care was taken to avoid cross contamination between the phases. The partition coefficient, P, was calculated using the following equation:
Usually the final partition coefficient value was expressed as log P.
Paper electrophoresis
1 μL sample was spotted on a piece of Whatman 1 chromatography paper, saturated with 0.05 mol/L pH 7.4 phosphate buffer, in an electrophoresis bath. Across 15 cm of the strip, 150 V was applied for 1.5 h. The strips were dried, and the distribution of radioactivity on the strip was determined.
In vitro cell uptake
Cell uptake studies were carried out by using murine sarcoma S180 cell lines. 99mTcN-PRODTC was incubated at 37 °C for 0.5–4.0 h with 1 × 106 S180 cells. After incubation, the cells were centrifuged at 2,000g for 5 min for pellet formation. The cell pellets were then washed three times. The radioactivity related to cell uptake was expressed in the mean percentages of radioactivity bound to tumor cell compared with the total radioactivity added after correcting for non-specific uptake (i.e. incubations without tumor cell added).
Biodistribution study
Biodistribution study was performed in Kunming male mice (weighing 18-20 g) bearing S180 tumor, which grew to a leg diameter of 10-15 mm. 0.1 mL of 99mTcN-PRODTC (7.4 × 105 Bq) was injected via a tail vein and the injected radioactivity was measured with a well-type NaI(Tl) detector. The mice were sacrificed at 0.5, 2 and 4 h post-injection. The tumor, other organs of interest and blood were collected, weighed and measured for radioactivity. The results were expressed as the percent uptake of injected dose per gram of tissue (%ID/g). All biodistribution studies were carried out in compliance with the national laws related to the conduct of animal experimentation.
Results and discussion
Synthesis and radiolabeling
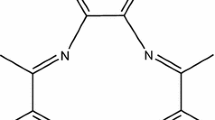
PRODTC was prepared by reacting l-proline with an equivalent amount of carbon disulfide in NaOH solutions. The reaction is schematically shown in Scheme 1. It was characterized by IR, 1H NMR, 13C NMR and ESI–MS spectroscopy. IR (KBr)/cm−1:2,964 (CH2), 1,586 (C=O), 1,005 (C=S). 1H-NMR (D2O) δ: 4.70-4.72 (m, 1H), 3.78-3.83 (m, 1H), 3.71-3.76 (m, 1H), 2.19-2.24 (m, 1H), 1.89-1.92 (m, 3H). 13C-NMR (D2O) δ: 205.2, 179.8, 69.1, 55.3, 31.1, 24.3. ESI–MS (m/s): 190 [M-2Na + H]−.
The preparation of 99mTcN-PRODTC was carried out using the following procedure in Scheme 2.
The method is based on the reaction of [99mTcO4]− with succinic dihydrazide (SDH) in the presence of stannous chloride as reducing agent to form a technetium-99m nitrido intermediate. The [99mTcN]2+ core is a suitable substrate for the substitution reaction with PRODTC to produce the 99mTcN-PRODTC complex at room temperature.
The radiochemical purity of the complex was routinely checked by TLC and HPLC. By TLC, in saline, [99mTcO4]– and 99mTcN-PRODTC remained at the origin while [99mTc≡N] 2+int migrated with the front. In acetonitrile, [99mTcO4]– migrated at Rf = 0.3–0.5, while 99mTcN-PRODTC and [99mTc≡N] 2+int remained at the origin. The HPLC pattern of the complex is shown in Fig. 1. It was observed that the retention time of [99mTcO4]– was 2.8 min and [99mTc≡N] 2+int was 2.5 min, while that of 99mTcN-PRODTC was found to be 10.5 min. The mean radiochemical purity of the product was over 90 % immediately after the preparation. The specific radioactivity of the product was calculated to be 86.95 MBq/μmol.
Based on the previous characterization of 99mTc-nitrido dithiocarbamate complex [17], it seems reasonable to presume that the structure of 99mTcN-PRODTC should have a square pyramidal geometry with an apical Tc≡N bond and two dithiocarbamate ligands spanning the four positions in the basal plane through the four sulfur atoms. Clearly, further studies should be performed, using macroscopic levels of the long-lived 99Tc, to determine and characterize the structure of 99mTcN-PRODTC.
Stability study
In vitro experiments showed that the complex did not decompose over 6 h at room temperature, suggesting it possesses a great stability in the reaction mixture at room temperature. In serum at 37 °C, the RCP of the complex was over 90 % at 6 h after synthesis, suggesting it was stable over 6 h in human serum conditions.
Human serum albumin binding assay
The percentage of serum protein binding of 99mTcN-PRODTC was relatively high (87.42 ± 0.01 %). The similar results are also found in [99mTc(Tyrosine)(H2O)(CO)3] and [99mTc(Lysine)(H2O)(CO)3] complexes [18].
Partition coefficient (log P)
The partition coefficient (log P) value of 99mTcN-PRODTC was calculated as −2.80 ± 0.11, indicating it was hydrophilic.
Paper electrophoresis
The electrophoresis results showed that the complex moved to the anode (percentage of radioactivity >98 %), suggesting it was negative charged.
In vitro cell study
The result of cell uptake of 99mTcN-PRODTC is given in Fig. 2. From Fig. 2, 99mTcN-PRODTC had optimal uptake between 2 h and 3 h post-incubation and reached a high cell uptake. However, at 4 h post-incubation, a decreased uptake of 99mTcN-PRODTC in murine sarcoma S180 cells was observed.
Biodistribution study
The results of biodistribution of 99mTcN-PRODTC in mice bearing S180 tumor are shown in Table 1. Results of biodistribution of recently reported 99mTc complexes as tumor imaging agents are shown in Table 2 for comparison.
As described in Table 1, 99mTcN-PRODTC has a certain tumor uptake (1.10 % ID/g at 0.5 h post-injection). The initial blood uptake is high, but its clearance is faster than that of the tumor so that the tumor/blood ratio is higher. The muscle uptake is low so the T/N ratio is better. The tumor uptake decreases at 4 h post-injection. The results are in accordance with the cell uptake results. Therefore, the optimal imaging time may be conducted between 2 and 3 h post-injection. The initial uptakes of the liver and kidneys are high, suggesting the main routes of excretion are via the urinary tract and through the hepatobiliary. The clearance of the liver and kidneys is rapid, thus making it possible to acquire good imaging results. Further imaging studies in more extensive animal models are in due course.
As seen from Table 2, among the three complexes, 99mTcN-PRODTC exhibits the highest T/N ratio and T/B ratio. As for tumor uptake, a decrease in the order is observed: [99mTc(CO)3(IDA–PEG3–CB)]− > 99mTcN-PRODTC > [99mTc(CO)3(PA-TZ-CHC)]+.
Conclusion
In summary, PRODTC ligand was successfully synthesized and its 99mTc nitrido complex was achieved in high yield. The moderate tumor localization, high tumor/blood and tumor/muscle ratios of the complex in mice exhibited favorable properties, suggesting the possibility of the complex as a novel tumor imaging agent.
References
Jager PL, Vaalburg W, Prium J, De Vries EGE, Langen KJ, Piers DA (2001) J Nucl Med 42:432
Leskinene-Kallio S, Ruotsalainen U, Någren K, Teräs M, Joensuu H (1991) J Nucl Med 32:1211
Nettelbladt OS, Sundin AE, Valind SO, Gustafsson GR, Lamberg K, Långstrom B, Björnsson EH (1998) J Nucl Med 39:640
Inoue T, Tomiyoshi K, Higuichi T, Ahmed K, Sarwar M, Aoyagi K, Amano S, Alyafei S, Zhang H, Endo K (1998) J Nucl Med 39:663
Inoue T, Shibasaki T, Oriuchi N, Aoyagi K, Tomiyoshi K, Amano S, Mikuni M, Ida I, Aoki J, Endo K (1999) J Nucl Med 40:399
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, Stöcklin G (1999) J Nucl Med 40:205
Wang MW, Yin DZ, Cheng DF, Li GC, Wang YX (2006) J Radioanal Nucl Chem 270:439
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H, Tellmann L, Jansen P, Reifenberger G, Hamacher K, Coenen HH, Langen KJ (2009) Nucl Med Biol 36:779
Langen KJ, Jarosch M, Hamacher K, Muhlensiepen H, Weber F, Floeth F, Pauleit D, Herzog H, Coenen HH (2004) Nucl Med Biol 31:67
Kong FL, Zhang YH, Ali MS, Oh C, Mendez R, Kohanim S, Tsao N, Chanda M, Huang WC, Yang DJ (2010) Nucl Med Commun 31:699
Sinha D, Shukla G, Tiwari AK, Chaturvedi S, Chuttani K, Chandra H, Mishra AK (2009) Chem Biol Drug Des 74:159
Hazari PP, Shukla G, Goel V, Chuttani K, Kumar N, Sharma R, Mishra AK (2010) Bioconjugate Chem 21:229
Zhang JB, Guo HX, Zhang SJ, Lin Y, Wang XB (2008) Bioorg Med Chem Lett 18:5168
Zhang JB, Ren JL, Lin X, Wang XB (2009) Bioorg Med Chem Lett 19:2752
Zhang SJ, Zhang WF, Wang Y, Jin ZH, Wang XB, Zhang JB, Zhang YY (2011) Bioconjugate Chem 22:369
Baird IR, Cameron BR, Skerlj RT (2003) Inorg Chim Acta 353:107
Baldas J, Bonnyman J, Pojer PM, Williams GA, Mackay MF (1981) J Chem Soc Dalton Trans 9:1798
Djokić D, Janković D (2007) J Label Compd Radiopharm 50:155
Wang JJ, Duan XJ, Mao HN, Yang J, Tan CM, Tian Y, Wu WS (2013) J Radioanal Nucl Chem 295:227
Wang JJ, Yang J, Yan ZY, Duan XJ, Tan CM, Shen YL, Wu WS (2011) J Radioanal Nucl Chem 287:465
Acknowledgments
The work was financially supported, in part, by National Natural Science Foundation of China (21171024, 81101069), Beijing Natural Science Foundation (7112035), Fundamental Research Funds for the Central Universities (105566) and Beijing Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Lin, X., Song, X. et al. Synthesis and biodistribution of a novel 99mTc nitrido radiopharmaceutical with proline dithiocarbamate as a potential tumor imaging agent. J Radioanal Nucl Chem 298, 1659–1663 (2013). https://doi.org/10.1007/s10967-013-2592-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2592-x