Abstract
Uranium concentration in groundwater reflect both redox conditions and uranium content in host rock. In the present study an attempt has been made to study the uranium concentration and activity ratios of uranium isotopes to present the geochemical conditions of the groundwater in Malwa region of Punjab state, India and the reason for high uranium levels and variation of activity ratios from secular equilibrium conditions. Uranium concentration in groundwater samples was found to be in the range of 13.9 ± 1.2 to 172.8 ± 12.3 μg/l with an average value of 72.9 μg/l which is higher than the national and international guideline values. On the basis of uranium concentration, the groundwater of the study region may be classified as oxidized aquifer on normal uranium content strata (20 %) or oxidized aquifer on enhanced uranium content strata (80 %). The 238U, 235U and 234U isotopic concentration in groundwater samples was found to be in the range of 89.2–1534.5, 4.4–68.5, and 76.4–1386.2 mBq/l, respectively. Activity ratios of 234U/238U varies from 0.94 to 1.85 with a mean value of 1.11 which is close to unity that shows secular equilibrium condition. High value of 234U isotope than 238U may be due to alpha recoil phenomenon. The plot of AR of 234U/238U against the total uranium content in log scale reveals that the groundwaters of the study region either belongs to stable accumulation or normal oxidized aquifer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a naturally occurring radionuclide which has three isotopes namely 238U, 235U and 234U. The radiological half-lives of these isotopes are 4.5 × 109, 7.1 × 108 and 2.5 × 105 years, respectively. 238U and 235U were parent radionuclides of two natural occurring radioactive series. 238U and 235U finally decays to 206Pb and 207Pb through a series of radionuclides consisting of 14 and 11 radionuclides, respectively. The mass percentage of 238U, 235U and 234U in natural uranium is 99.27, 0.72 and 0.005 %, respectively and the activity percentage is 48, 2 and 48 % respectively. The activity ratios of uranium isotopes remain constant unless there is fractionation of isotopes due to natural or anthropogenic activities. Natural fractionation of uranium isotopes is used as a sensitive tool for the various environmental geochemistry studies. Due to long radioactive half-lives, 238U and 234U isotopes are in secular equilibrium in all minerals and rocks greater than one million years old in a closed system or undisturbed minerals since 234U is a daughter product of 238U. Therefore, activity ratio (AR) of 234U to 238U is unity in the bulk of such materials. However, when such rocks and minerals are interacted with groundwater, the ratio may deviate from unity on either side; disequilibrium is the result depending on the geochemical conditions [1, 2]. Variations in this AR have been used as sensitive chemical indicator for identifying isotopically distinct groundwaters and geochemical processes [3]. Fractionation of uranium isotopes in natural environment is due to mainly due to radiogenic phenomenon. Origin of variation of AR of 234U/238U is from preferential leaching of 234U from the rock matrix which is due to the crystal defects due to alpha particle recoil of the 238U parent nuclide [4] and radiation induced oxidation of 234U [5]. As a result of the aforementioned phenomena, the AR in the groundwater varies from near unity to ten with majority of the groundwater between 1 and 4 [6]. Mainly two parameter influence strongly the AR in groundwater were the contact time between the groundwater and the rock matrix and the extent of leaching by the groundwater. In addition to this, the groundwater chemistry also plays a role determining the AR. The groundwater and host rock minerals are strongly interacted, thus both chemical and physical differentiation processes are enhanced according to rock-water ratio, surface area exposed and residence time [7]. Uranium has two geologically significant oxidation states +6 and +4 under different redox conditions. In the oxidized environment, uranium mostly exists in the +6 state and has a strong tendency to form uranyl complexes with carbonates and bi-carbonates and is readily transportable. Uranium remains in +4 state in reduced condition and almost precipitated quantitatively. This behavior is manifested in sand stones where uranium deposits have formed. Because of the unique properties of uranium isotopes depending on the prevailing geochemical environment, the isotopic activity ratios have been used to characterize and trace groundwater in a variety of environmental studies [8, 9]. Groundwater can be classified according two parameters i.e. uranium concentration and 234U/238U AR [10–12]. Uranium concentration levels allow to classify aquifers in oxidized aquifer on ‘‘normal’’ uranium content strata (values between 1 and 10 μg/l), oxidized aquifer on enhanced uranium content strata (values >10 μg/l) and reduced aquifer on low uranium content strata (values <1 μg/l). On the basis of 234U/238U AR, the groundwater is called ‘‘normal’’ groundwater (values between 1 and 2); possible uranium accumulation (values >2), or possible remobilization of a uranium (values <1). High AR (>2) can be due to a higher than normal ratio of leachable uranium in aquifer or amorphous uraninite, enhancing alpha recoil effect. Activity ratio lower than one implies intense dissolution of the rock matrix may be due to the acidic nature of groundwater. Uranium concentration levels reflect both redox conditions and uranium concentrations in host rocks [10, 13]. Therefore, the study of uranium isotopic ratio in groundwater can provide information about the redox condition and source of uranium.
Globally uranium occurs in groundwater in the range of 1–10 μg/l but there are many reports of high uranium in groundwater including few parts of India are a major concern of the scientific community as uranium is both chemically and radiologically toxic. It was reported that occurrence of high uranium in groundwater in Malwa region of Punjab state [14, 15] and Kollar district of Karnataka state [16]. In the present study, groundwater samples of 15 nos. from the Malwa region of Punjab, India have been analysed for uranium isotopic composition using alpha spectrometer to study the variation of AR, the source and prevailing geochemical conditions.
Study area
Groundwater samples of 15 nos. were collected from the Malwa region of state of Punjab in the northern part of India. The study area, Punjab is located in the northern part of India bordered by the Indian states of Himachal Pradesh and Chandigarh to the east, Haryana to the south and south-east and Rajasthan to the south-west as well as the Pakistani province of Punjab to the west, it is also bounded to the north by Jammu and Kashmir. The state of Punjab is well known for its high agricultural products. The geographical location of the study area is south-west of Punjab between latitude 29°07′–30°57′N and longitude 74°05′–76°55′E at an average elevation of 200 m from the mean sea level. The soil of the study area is loose, sandy, calcareous and alluvial, which is an admixture of gravel, sand, silt and clay in varying proportions. The lands in the study are used for agriculture all over the year but many industries like thermal power plants, fertilizer factories, chemical factories, cement factories are established in the region.
Material and method
Sample processing
Groundwater samples of 1 l each were collected from tube wells and bore wells directly in pre-acid cleaned plastic bottles as per standard protocols [17]. After collection of water samples, filtration was carried out using 0.45 μm filter paper and acidified with nitric acid to pH ~2. The filtered water samples were analyzed for total uranium content using laser induced fluorimeter. The details of sample preparation and analysis was given in our earlier publications [18, 19]. Then the samples were processed for measurement of isotopic composition of uranium by alpha spectrometer and the details are given below.
Water samples of 100 ml were digested with concentrated nitric acid as per ISO/DIS 15587-2 method [20]. After complete digestion, the residues were treated with concentrated hydrochloric acid twice to convert all metals to chloride form. Then 5 ml of 8 M HCl was added to the residues and warmed up, now the solution is ready for ion exchange column separation. Dowex-1 × 8 anion exchange resin, chloride form (100–200 mesh size) is used for separation of uranium from other matrices. The resin is preconditioned with 8 M HCl and the prepared sample solution is subject to the resin for separation. After loading the sample solution, the resin was washed with 8 M HCl to eliminate the residual thorium traces and other impurities. Cations of U+6 were retained on the column as chloro complexes and eluted with 25 ml 1 M HNO3. Purified uranium is electrodeposited from sulphuric acid—ammonium hydroxide solution adjusted to a pH 2.2 [21, 22]. Platinum rod was used as anode whereas cleaned buffed stainless steel disc was used as cathode. Electrodeposition was carried out at constant voltage of 6 V and 0.3 A with a distance of 2 cm between the cathode and anode for 2.5 h. Concentrated ammonia of 1 ml was added to the electrodeposition cell before switching off the voltage to avoid the uranium goes back to the electroplating solution which is acidic in nature. Then, the electroplated samples were subjected to Passivated Implanted Planar Silicon detector (PIPS ULTRA, bias 50 V) in an eight chamber alpha spectrometer (Ortec Octect plus). The PIPS detectors were having the active surface area of 450 mm2 and depletion thickness of 100 μm. The efficiency of alpha spectrometer is 15.2 % at 8 mm source to detector distance and the energy resolution is 35 keV at 4.2 MeV 238U alpha energy. The detector is coupled to an integrated 4 K multi-channel pulse height analyzer. The source and sample deposition diameter was 19 mm on a 25 mm stainless steel planchette. The vacuum of the detector chamber maintained at 75 m Torr throughout the counting process using an high efficient vacuum pump (Edward Vacuum Pump, Model: A65401906). Blank, spiked samples and electroplated water samples were counted for 24 h to estimate uranium isotopes to reduce the counting relative standard deviations <5 %. Uranium standard solutions of (1 mg/l, Aldrich make) were processed similarly with each batch to estimate the recovery and few spiked recovery studies were also carried out.
Quality assurance
The accuracy and reliability of the method is verified by cross method analysis, replicate analysis and spike recovery study. All the water samples were analysed by both laser fluorimetry and alpha spectrometry. The results are in good agreement with each other and the correlation coefficient of 0.82 has been observed. The recovery of the spiked uranium concentration is found to be 70–94 %. The ratio of 234U to 238U is spiked uranium concentration was found to close to one which indicates that the solution is of natural uranium. The ratio of 235U to 238U in the spiked uranium is observed to be 0.046 which clearly establish that the uranium is of natural origin also.
All laboratory glass wares used for sample processing were soaked in 10 % nitric acid for 15 days and then rinsed thoroughly twice with ultra pure water (resistivity 18.1 MΩ, Thermo nanopure diamond TII water purification system) before use. Various precautions were taken in order to avoid cross contamination.
Results and discussion
Uranium concentration in groundwater samples was found to be in the range of 13.9 ± 1.2 to 172.8 ± 12.3 with an average value of 72.9 μg/l. The levels of uranium in these groundwaters were higher than that reported for other part of the country but comparable to the values reported for the same region [14, 15]. Out of the 15 samples, 67 % of samples contains uranium more than the international guideline value of 30 ppb [23, 24] and 47 % of samples more than the national drinking water limit of 60 ppb [25]. On the basis of uranium concentration, the groundwater of the study region may be classified as oxidized aquifer on normal uranium content strata (20 %) or oxidized aquifer on enhanced uranium content strata (80 %). High uranium content in groundwater may be due to local natural geology, industrial activities in the region or use of phosphate fertilizers in the region in huge quantity for agricultural purpose as the region is well known for it or due to any other human activities. The present case may be similar to the study observed in Central Valley, California, USA [26]. The groundwater in this region is found to be rich in biocarbonates, nitrate and chlorides anions [14] and soil is calcareous one. As the region is well known for its agricultural activities, plant root respiration and microbial oxidation of organic matter in soil produce carbon dioxide, resulting in CO2 partial pressure in the soil zone that are greater than the atmospheric pressure. Water percolating through the soil equilibrates with the soil atmosphere by dissolving CO2 (g) to form carbonic acid. The carbonic acid reacts with the calcium carbonates (calcareous soil) to form bicarbonate which is a well-known efficient leaching agent for uranium from soils and sediments. Formation of bicarbonate while water is percolating through soil enhances its leaching efficiency. This may be one of the mechanisms which explain the high uranium in groundwater in the regions but other possibilities can’t be ruled out.
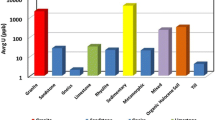
The 238U, 235U and 234U isotopic concentration in groundwater samples was found to be in the range of 89.2–1534.5, 4.4–68.5, and 76.4–1386.2 mBq/l, respectively (Table 1). The distribution of uranium isotopes in groundwater is given in Fig. 1. The AR of 234U/238U and 235U/238U in the groundwater samples were given in Figs. 2 and 3. From Fig. 2, it is evident that the AR of 234U/238U varies from 0.94 to 1.85 with a mean value of 1.11 which is close to unity that shows secular equilibrium condition. In 73 % groundwater samples, the 234U/238U AR is greater than one. High value of 234U isotope than 238U may be due to alpha recoil phenomenon. In case of alpha recoil phenomenon, when 238U isotope in a mineral particle undergoes alpha decay process, the 234Th and alpha particle moves apart on opposite direction to conserve the energy and momentum. 234Th now moves to periphery of the crystal and undergoes two beta particle emission to form 234U which is exposes to pore space which is liable to oxidation and preferential leaching to groundwater. Kronfeld et al. [27] suggested that due to nuclear transformations, the chemical bonds break and cause displacement within crystal structure, create micro-channels that allow for migration of water molecules into mineral grains and auto-oxidation of the daughter nuclide through the loss of two beta particles to the more soluble +6 oxidation state. This process make the 234U isotope more susceptible to groundwater than the parent and allow it to be preferentially leached across rock boundaries. From the AR of 234U/238U, the uranium in the groundwater in Malwa region of Punjab is of natural origin. The fact was also endorsed by the mean AR of 235U/238U (0.045 against the theoretical value of 0.046). From the AR of 234U/238U, it is also confirmed that the groundwater is normal and comparable to worldwide values between 1 and 2. The plot of AR of 234U/238U against the total uranium content in log scale reveals that the groundwaters of the study region either belongs to stable accumulation or normal oxidized aquifer (Fig. 4). The AR of 234U/238U observed in the present study is compared to other reported values in various countries is given in Table 2. The observed values in the present study is comparable to groundwater in South Korea, Syria and Poland but less than that reported for Nevada, USA and Northern Africa. The amount of “excess” 234U above the equilibrium condition with 238U is helpful to identify geochemically similar water. 234U excess is determined by subtracting one (equivalent to secular equilibrium) from AR and multiplied by the 238U concentration in the sample [28]. The plot of uranium mixing diagram for groundwater is given in Fig. 5 which indicates that the uranium in groundwater in the study region is occur in the form carbonate complexes.
Conclusion
Uranium concentration in groundwater samples was found to be in the range of 13.9 ± 1.2 to 172.8 ± 12.3 with an average value of 72.9 μg/l which is higher than the WHO, USEPA and AERB drinking water guideline values. On the basis of uranium concentration, the groundwater of the study region may be classified as oxidized aquifer on normal uranium content strata (20 %) or oxidized aquifer on enhanced uranium content strata (80 %). The 238U, 235U and 234U isotopic concentration in groundwater samples was found to be in the range of 89.2–1534.5, 4.4–68.5, and 76.4–1386.2 mBq/l, respectively. AR of 234U/238U varies from 0.94 to 1.85 with a mean value of 1.11 which is very close to unity that shows secular equilibrium condition. High value of 234U isotope than 238U may be due to alpha recoil phenomenon. The plot of AR of 234U/238U against the total uranium content in log scale reveals that the groundwaters of the study region either belongs to stable accumulation or normal oxidized aquifer.
References
Osmond JK, Cowart JB (1992) Groundwater. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium applications to earth, marine and environmental sciences, 2nd edn. Oxford Science Publications, Oxford
Ivanovich M, Harmon RS (1982) Uranium series disequilibrium: applications to environmental problems. Clarendon Press, Oxford
Osmond JK, Cowart JB (1982) Groundwater. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium: applications to environmental problems, 1st edn. Clarendon Press, Oxford, pp 202–245
Ivanovich M (1994) Uranium series disequilibrium: concepts and applications. Radiochim Acta 64:81
Gascoyne M (1992) Geochemistry of the actinides and their daughters. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium: applications to earth marine and environmental sciences, 2nd edn. Clarendon Press, Oxford, pp 34–61
Osmond JK, Cowart JB (1976) The theory and uses of natural uranium isotopic variations in hydrology. Energy Rev 14:622–679
Chkir N, Guendouz A, Zouari K, Ammar FH, Moulla AS (2009) Uranium isotopes in groundwater from the continental intercalaire aquifer in Algerian Tunisian Sahara (North Africa). J Environ Radioact 100:649–656
Porcelli D, Andersson PS, Baskaran M, Wasserburg GJ (2001) Transport of U- and Th-series nuclides in a Baltic shield watershed and the Baltic Sea. Geochim Cosmochim Acta 65:2439–2459
Reynolds BC, Wasserburg GJ, Baskaran M (2003) The transport of U- and Th-series nuclides in sandy confined aquifers. Geochim Cosmochim Acta 67:1955–1972
Cowart J, Osmon JK (1980) The relationship of uranium isotopes to oxidation/reduction in the Edwards carbonate aquifer of Texas. Earth Planet Sci 48(2):277–283
Osmond JK, Cowart JB (1983) Uranium disequilbrium in ground water as indicator of anomalies. J Appl Radial Isotopes 34:283
Bonotto DM (1999) Applicability of the uranium isotopic model as a prospecting technique in Guarani aquifer, South America. In: Ninth Annual V.M. Goldschmidt Conference, Abstract no 7021, LPI Contribution no 971, Lunar and Planetary Institute, Houston
Bonotto DM, Andrews JN (2000) The transfer of uranium isotopes 238U and 234U to the waters interacting with carbonates from Mendip Hills area (England). Appl Radiat Isot 52:965–983
Kuamr A, Rout S, Narayana U, Mishra MK, Tripathi RM, Singh J, Kumar S, Kushwaha HS (2011) Geochemical modeling of uranium speciation in the subsurface aquatic environment of Punjab state in India. J Geol Min Res 3(5):137–146
Singh J, Singh L, Singh S (1995) High uranium content obserced in some drinking waters of Punjab, India. J Environ Radioact 26:217–222
Babu MNS, Somashekar RK, Kumar SA, Shivanna K, Krishnamurthy V, Eappen KP (2008) Concentration of uranium levels in groundwater. Int J Environ Sci Technol 5(2):263
IAEA (1989) International atomic energy agency, measurement of radionuclides in food and environment, Technical Reports Series no. 295. IAEA, Vienna
Sahoo SK, Mohapatra S, Patra AC, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2009) Distribution of uranium in drinking water and associated age-dependent radiation dose in India. Radiat Prot Dosim 136(2):108–113
Sahoo SK, Mohapatra S, Patra AC, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2010) Determination of uranium at ultra trace level in packaged drinking water by laser fluorimeter and consequent ingestion dose. J Radioprot 45(1):55–66
ISO 17294-2 (2003) Water quality—Application of Inductively Coupled Plasma Mass Spectrometry—Part 2 Determination of 62 elements
Hallastadius L (1984) A method for the electrodeposition of actinides. Nucl Instrum Methods Phys Res 223:266–267
Talvitie NA (1972) Electrodeposition of actinides for alpha spectrometric determination. Anal Chem 44(2):280–283
WHO (2011) World Health Organization guidelines for drinking water quality, 4th edn, pp 430–431
USEPA (2000) United States environmental protection agency, national primary drinking water regulations; radionuclides; final rule, 40 CFR Parts 9, 141, and 142
AERB (2004) Atomic Energy Regulatory Board, limit for uranium in drinking water, India
Jurgens BC, Fram MSF, Belitz K, Burow KR, Landon MK (2009) Effects of groundwater development on uranium: central valley, groundwater, California, USA. doi:10.11111/j.1745-6584.2009.00635.x
Kronfeld J, Godfrey-Smith DI, Johannessen D, Zentilli M (2004) Uranium series isotopes in Avon Valley, Nova Scotia. J Environ Radioact 73:335–352
Cizdziel J, Farmer D, Hodge V, Lindley K, Stetzenbach K (2005) 234U/238U isotope ratios in groundwater from Southern Nevada : a compriosn of alpha counting and magnetic sector ICP-MS. Sci Total Environ 350:248–260
Abdul-Hadi A, Alhassanieh O, Ghafar M (2001) Disequilibrium of uranium isotopes in some Syrian groundwater. Appl Radiat Isotopes 55:109–113
Grabowski P, Bem H (2012) Uranium isotopes as a tracer of groundwater transport studies. J Radioanal Nucl Chem 292:1043–1048
Pietrzak-Flis Z, Kaminska I, Chrzanowski E (2005) Uranium isotopes in public drinking water and dose assessment for man in Poland. Radiat Prot Dosim 113(1):34–39
Lee MH, Choi GS, Cho YH, Lee CW, Shin HS (2001) Concentration and activity ratios of uranium isotopes in the groundwater of the Okchun Belt in Korea. J Environ Radioact 57:105–116
Acknowledgments
Authors gratefully acknowledge the guidance of Dr. A. K. Ghosh, Director and Dr. D. N. Sharma, Associate Director, Health, Safety and Environment Group, BARC. Guidance and help received from Ms. P. D. Sawant, BARC for isotopic analysis of uranium is acknowledged. The suggestions and help from other colleagues during the course of work and preparation of this manuscript are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tripathi, R.M., Sahoo, S.K., Mohapatra, S. et al. Study of uranium isotopic composition in groundwater and deviation from secular equilibrium condition. J Radioanal Nucl Chem 295, 1195–1200 (2013). https://doi.org/10.1007/s10967-012-1992-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1992-7