Abstract
This paper presents the isotope hydrochemical results of groundwaters from southwest Punjab for assessing the uranium contamination and evaluating the factors leading to elevated uranium concentration. A total of 35 samples covering shallow and deep zones were collected for hydrochemistry and isotopes. Uranium concentration ranges between 2.3 and 357 µg L−1 and 66% of the samples are contaminated. Both shallow and deep zones show U contamination but high incidences are noticed in shallow zone. Hydrochemical correlations infer geological sources rather than anthropogenic sources responsible for U contamination. Isotopically there is no clear distinction between high and low U groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater resources are being overexploited in many parts of the country due to rapidly growing population, unfavorable climatic events and recurrent droughts. This scenario is clearly witnessed in human influenced alluvial aquifers of Punjab due to ever increasing demand for irrigation, industrial and domestic needs [1]. In addition to quantity, it was reported that groundwater quality is also deteriorated in many parts of Punjab state by inorganic and organic contaminants [2,3,4,5]. The concern about toxicity related to uranium in groundwater of southwest region of Punjab, India has attracted world attention in recent times, leading to many studies related to identification of source and processes causing high U in groundwater [6,7,8,9]. Occurrence of high concentration of uranium in drinking water and food can lead to potential harmful effects in human beings [10, 11]. Uranium accumulated in human results in chemical and radioactive effects. The principal sites of uranium deposition in the body are the kidneys, the liver and the bones. The toxicity of uranium is a function of the route of exposure (internal or external), particle solubility, contact time, and route of elimination [12]. The concentrations of radiotoxic elements like uranium in drinking water are being monitored by different health organizations. The World Health Organization had recommended a guideline value of 30 μg L−1 U in drinking water [13]. Maximum acceptable level of U in drinking water as per guidelines of India’s Atomic Energy Regulatory Board, Department of Atomic Energy, is 60 μg L−1 [14].
Uranium is generally found in accessory minerals and is mostly immobile in nature; however during weathering it gets released or leached into groundwater [15]. The chemistry of uranium in aqueous systems is mainly controlled by the pH, redox potential and complexing agents such as carbonates, phosphates, vanadates, fluorides, sulfates and silicates [16, 17]. Therefore, it is essential to evaluate the hydrochemical characteristics of the groundwater in U contaminated region. In addition to hydrochemical data, environmental isotopes have been applied in groundwater hydrology to get insight into the occurrence, movement and distribution of water in aquifers [18,19,20,21]. Among environmental stable isotopes, water isotopes (δ2H and δ18O) are commonly employed in many studies related to source and origin of the groundwater [22] interconnection between aquifers [23] and possible sources of contamination [24,25,26].
Most of the studies conducted in Punjab region were limited to profiling in water and soil, while a few attempted to understand the mechanism of U release into groundwater. High U concentration in groundwater was reported by researchers in various parts of Punjab up to 650 µg L−1 by Kumar et al. [6] and up to 1340 µg L−1 by Sharma and Singh [9]. Kochhar et al. [27] suggested high heat producing granites of the adjoining Tusham area can be potential source for U in this region, while Patnaik et al. [28] and Phadke et al. [29] proposed Siwalik sediments as the main cause for increased U in groundwater [27,28,29]. Fertilizer input as the cause for high U in groundwater was suggested by Kumar et al. [6] and Singh et al. [30]. Hydrochemical studies infer that the U occurrence in groundwater is mainly attributed to presence of alkalinity in groundwater along with high TDS and dissolved oxygen [7, 31, 32]. Other hydrochemical studies conducted on water quality of this region have concluded that evaporation is responsible for the increased salinity in groundwater of this region [33]. In the present paper we have attempted to understand the U mobilization process and source of groundwater employing the hydrochemical and environmental stable isotope data.
Materials and methods
Study site description
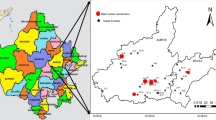
The study area comprises two districts viz., Mansa and Bathinda of southwest Punjab and encompasses an area of about 5538 km2 as shown in Fig. 1. This area falls between 29°32′ to 30°36′ north latitudes and 74°37′ to 75°46′ east longitudes. This region consists of Quaternary alluvial deposits of Recent to Sub-Recent age. These alluvial deposits were formed by the sediments transported from adjoining areas comprising of Siwaliks, granites and other metamorphic rocks [34, 35]. The geological evolution and the source of sediments highlighting Siwalik, granitic and metamorphic source rocks is detailed by Gupta and Thakur, Sharma et al. and Tripathi et al. [36,37,38]. Groundwater exits under both unconfined and confined conditions in these alluvium aquifers. The general slope of the water table is towards SW from North, NE, East and SE. Based on the in situ nature of sediments the alluvial aquifers of this region have been divided in to two aquifers, the shallow aquifer with depth up to 60–70 m bgl (below ground level) and deep aquifer below 70 m bgl [39]. Two aquifer system with similar aquifer thickness was also suggested by other researchers [34, 35, 38]. This area has extensive canal system for irrigation purpose, which are originated from Satluj river. The major canal in the area is Bathinda branch canal, it traverses whole of the area except the southern and extreme north parts. The southern and south-eastern part is traversed by Kotla branch canal and the extreme north part by Abohar Branch canal. 80% of the area is irrigated by canals [34, 35].
The climate of the region can be classified as semi-arid and hot which is mainly dry except in rainy months and characterized by intensely hot summer and cold winter. The average annual rainfall is 400–500 mm, which is unevenly distributed over the study area. The southwest monsoon (June–September) contributes about 82% of annual rainfall. Rest of the rainfall mostly occurs during non-monsoon months of the year. The depth to water level varies from 2.24 to 20.76 m bgl in Bathinda district and 3.89–12.36 m bgl in Mansa district. Rise in water table was observed during post monsoon season and the long term water level fluctuation showed the rising trend, maximum up to 5 m in northeastern part of Mansa [3, 34, 35].
Methodology
Hydrochemistry
A total of 35 samples were collected from bore wells, hand pumps, tube well and canal in the study area during February 2015 (dry period). River Ghaggar flows in the southern tip of the study area and during sampling it is found that electrical conductivity of the samples variation is very high in this part of the study area due to contribution from river in the south direction and water logging in the north direction, hence more number of samples was collected (sample no. 20–27). Depth of the wells was in the range of 0.9–111 m bgl. Before collecting the samples wells were purged by pumping out the stagnant water for 30 min in order to obtain representative sample. Physical parameters like, temperature, pH and electrical conductivity (EC) were measured in situ. Alkalinity was measured by titrating 10 mL of water sample with 0.02 N H2SO4. A mixed indicator (Bromocresol green–Methyl red) was used to mark the end point. 50 mL of water sample was collected from each location and filtered using 0.45 µm filter and stored in acid treated sample bottles for anion measurements. A duplicate set of samples was collected in the same way and acidified to pH 2 using concentrated HNO3. Cation (Na+, K+, Mg2+ and Ca2+) and anion (F−, Cl−, NO3 −, SO4 2− and PO4 3−) analysis was carried out using ion chromatography (Dionex 500). The accuracy of the measurements was better than 5%. The accuracy of the chemical ion data was calculated using charge balance equation given below and the charge balance error (CBE) values of the samples were within the accepted limits of ±5% [40].
The ionic strength and log pCO2 values were calculated by WATEQ4F program [41].
Environmental isotopes
Since the natural variations of environmental stable isotopes (18O and 2H) are usually very small, the only technique that can routinely measure precise isotopic ratios is mass spectrometry (IRMS—isotope ratio mass spectrometer). For stable isotope (2H and 18O) analysis, water samples were collected in 50 mL airtight high density polyethylene bottles and measured by continuous flow IRMS (Isoprime 100). For δ2H analysis, 1 mL of the water sample was equilibrated with H2 in presence of Pt–coated Hokko beads catalyst at 50 °C for 90 min and the gas was introduced into the mass spectrometer [42]. The δ18O of the sample was measured by equilibrating 1 mL of water with CO2 gas at 50 °C for 8 h and the equilibrated gas was introduced into the mass spectrometer [43, 44]. The results are reported in δ-notation and expressed in units of parts per thousand (denoted as ‰). The δ values are calculated using [45]:
where R denotes the ratio of heavy to light isotope (e.g. 2H/1H or 18O/16O) and R x and R s are these ratios in the sample and standard respectively. The precision of measurement for δ2H is ± 0.5‰ and for δ18O is ± 0.1‰ (2σ).
Uranium estimation
Samples for uranium analysis were collected in acid washed high density polyethylene bottles after filtering through 0.45 µm cellulose nitrate filter circles. The Uranium analysis was carried out using laser fluorimeter (UA1, Quantalase), which works on the principle of fluorescence of uranyl complex enhanced by addition of sodium pyrophosphate as fluorescence enhancement reagent and the detection limit was determined by standard addition method [46]. The technical features of laser uranium analyzer are: detection limit: 0.2 μg L−1 of U, range: 0.5–100 μg L−1, excitation source: sealed-off nitrogen laser, wavelength: 337 nm, pulse energy: 20 µ joule, pulse duration: 7 nano second, frequency: 10 Hz and sample size: 3–5 mL. Quantification of unknowns was carried out by standard addition method. Repeat measurements were performed on a few samples for the determination of precision. The accuracy of this technique was validated by comparing the values obtained from inductively coupled plasma mass spectrometry (ICP-MS) technique. The details of the validation are given in Rishi et al. [32]. Uranium concentration was also determined for canal as well as municipal supplies collected in the study area.
Results and discussion
Hydrochemical characteristics
The summary of hydrochemical parameters of groundwater samples is presented in Table 1. pH of the groundwater shows a narrow distribution (mean: 8.2 ± 0.4) and is mostly neutral to alkaline while electrical conductivity of the groundwater samples show wide variation from fresh to saline quality (Table 1). Among major ions high concentrations are noticed for Na+, Cl−, HCO3 − and SO4 2− while the concentrations are low for rest of the ions (Table 1).
Uranium concentration in the study area varies from 2.3 to 357 μg L−1 and 34% of the samples fall within drinking water permissible limit of 30 μg L−1 [13] while 66% of the samples fall in unsafe category (Fig. 2a). The permissible limit for radiological toxicity of U in drinking water is set by Atomic Regulatory Board of India as 60 μg L−1 [14]. As per AERB limits, 63% of the water samples fall in safe category while rest of the samples (37%) fall in unsafe category (Fig. 2b). The municipal supply water as well as canal water showed U concentration <10 μg L−1.
Hydrochemical results indicate a wide variation in EC and HCO3 − concentrations suggesting multiple sources of dissolved ions. The spatial variation of hydrochemical parameters indicates that low ionic concentrations are generally limited to locations near the canals and other surface water bodies while high values are mostly from locations adjacent to agricultural fields. In order to determine the contribution of fertilizers K+, NO3 − and PO4 3− were also measured and their concentrations were found to range from 0.8 to 101 mg L−1, 0.3 to 150 mg L−1and BDL to 200 µg L−1 respectively (Table 1). Results indicate that samples with high U concentration (>100 μg L−1) do not necessarily show high NO3 − or K+ or PO4 3− concentrations in groundwater. High NO3 − in groundwater is observed in both agricultural and non-agricultural areas, mostly near domestic outlets [47, 48]. Moreover high NO3 − is confined mostly to shallow zones while deeper zone groundwater is free from NO3 − contamination. These observations infer that high NO3 − in groundwater may not be only due to agricultural practices but also due to sewage leakage into the ground as the sewer lines may not be well maintained in the study area as also reported in previous study [2, 4].
A vertical distribution of U and associated HCO3 − concentration is depicted in Fig. 3. High variability in U and HCO3 − concentrations are generally noticed in the shallow zone while in the deeper zones the variation is less. Highest concentration of U was found to be 357 μg L−1 in shallow zone of Phul village tapping a shallow zone of 33 m bgl. Elevated levels of U are observed at locations Bhai Rupa, Boha, Bhucho khurd, Jalal and Jatana Kalan where the U concentrations are 222, 212, 176, 145 μg L−1 respectively. High U concentration was found both in shallow as well as deep wells. Low U concentrations are observed prominently in the canal command area, near the Ghaggar river in Mansa.
In order to determine the probable source of U in groundwater, U concentration was plotted against the other ions (Fig. 4a–f). A positive correlation is observed between U and HCO3 − (Fig. 4a) with a correlation coefficient of 0.48 (except three samples encircled), which indicates that high HCO3 − content facilitates U leaching into groundwater. Presence of high alkalinity (mean HCO3 −: 482 mg L−1) and alkaline pH conditions (mean pH: 8.2) favor the formation of stable and soluble species of U such as UO2CO3(aq) and UO2(CO3) 2−2 . Similar trends between U and HCO3 − were reported by other researchers [2, 7, 32, 49]. In the case of ionic strength, it can be observed that the data points fall in two groups. There is only a marginal increase in U concentration with increase in ionic strength of the groundwater (shown by group (i)). This reflects that uranium is not directly associated with ionic strength. Other set of data fall in group (ii) where groundwater shows increasing trend in U content and don’t show a proportional increase in ionic strength. This observation is in contradiction to earlier observations wherein it was concluded that high ionic strength, TDS and electrical conductivity of the groundwater lead to enhanced dissolution of the U from the minerals [25, 50].
Application of fertilizers also can cause U contamination in groundwaters [51]. In order to evaluate the fertilizer input towards U contamination, NO3 −, K+ and PO4 3− concentrations of the groundwater was plotted against corresponding U concentration (Fig. 4c–e). From the plot it can be observed that increase in NO3 − in groundwater has not increased the U concentration (Fig. 4c group (i)) similarly increase in U concentration is not associated with increased NO3 − (Fig. 4c, group (ii)). High U samples (>100 μg L−1) showed low NO3 − concentration (<40 mg L−1). Few samples with moderate U concentration (50–200 μg L−1) showed NO3 − concentration in the range of 80–150 mg L−1. This behavior can be attributed to mixing of groundwater between shallow and deep zones at those specific locations. Shallow and deep zone interconnections were noticed in some parts of the southwest Punjab by earlier researchers [32]. There is a negative correlation of U with K+ concentration as shown by eye-guide line in Fig. 4d. High U samples (>100 μg L−1) showed very low K+ concentration (<10 mg L−1). Fertilizers are manufactured from phosphate ores, hence it is expected that fertilizer contribution towards high U in groundwater could also be associated with high phosphate. Figure 4e represents that even in the case of PO4 3− the trends observed are very similar to that of ionic strength (Fig. 4b) and NO3 − (Fig. 4c). Overall, the trends observed in the plots (Fig. 4 b–d) indicate that contribution of fertilizers towards high U concentration in groundwater is not significant.
Environmental isotope characteristics
Chemical characteristics of groundwater reflect only the dissolved salt component and this signal can be modified by various processes whereas environmental isotopes 2H and 18O are conservative tracers of groundwater as they are part and parcel of water molecule. Deuterium and oxygen-18 have been used in this study to understand the source and origin of groundwater and to relate this with the U contamination.
The stable isotopes vary from −11.24 to −5.03‰ for δ18O and −80 to −41.66‰ for δ2H (Table 1). A wide variation in isotope values is observed in the shallow zone samples while deep zone samples show relatively less variation. The variation in isotopic composition of shallow zone samples is due to presence of multiple sources of recharge, like irrigation return flow, precipitation, canal water, wetlands and other domestic sources. This observation is in agreement with other parameters like EC and uranium. Depleted δ18O values are mostly observed near canals, central and southern parts of the study area where river Ghaggar flows. From the isotope data it can be interpreted that shallow zone groundwater get contribution from both canals and river. In some cases contribution from evaporated water bodies leads to enrichment in isotope values which reaches up to −5.04‰ for δ18O.
A plot of δ2H versus δ18O composition of the groundwaters was presented in Fig. 5. A global meteoric water line (GMWL) with an equation of \(\delta^{ 2} {\text{H}}{\kern 1pt} {\kern 1pt} = {\kern 1pt} {\kern 1pt} 8 \times \delta^{18} {\text{O}}\, +\, 10\) was also shown in the plot [52]. Water samples that are of meteoric origin plot along GMWL but under certain circumstances the isotopic data of groundwater samples deviate from this line, which include evaporation and mixing [18, 21]. Most of the groundwater sample data in this region fall on the GMWL indicating precipitation as the source of recharge to these waters. Best fit line for the isotopic data of the groundwater samples shows a slope of 5.7, indicating slight evaporation (Fig. 5). Groundwater samples falling on the evaporation line indicate contribution from irrigation return flow or from other surface water bodies. It is observed that stable isotopic variations are similar in both low and high U groundwater and mostly fall on the GMWL. This indicates that the source and origin is common for both high and low U groundwaters and it is mainly precipitation. However, shallow zone also receives additional recharge from canal and irrigation return flow and hence show a wide variation in isotopic composition.
A plot of U versus δ18O composition of the groundwaters was presented in Fig. 6. It can be observed from the figure that groundwaters fall in two groups. Group (a) samples show an increase in U concentration (10 to 350 μg L−1) with enrichment in isotopic content (δ18O: −11.2 to −5.0‰).Group (b), on the other hand, doesn’t show any systematic increase in U concentration with almost similar range of increase in δ18O values (δ18O: −9.2 to −5.0‰). It can be inferred that group (a) samples derive U from interaction of sediments with evaporated water most likely in shallow zone while group (b) samples derive U from sediment–water interaction in deep zone. The deep groundwater is recharged by lateral flows from NE part of the study area [28, 34, 35, 38]. This observation suggests that the source of U in groundwater is not different for low or high U samples. In shallow zone, the groundwater has a significant input from irrigation return flow which is also high in bicarbonate. This mobilizes U from sediments in shallow zone. A positive correlation between HCO3 − and U and no or negative correlation of U with fertilizer related ions indicate that fertilizers as such might not be the source for U but the HCO3 − contributed by root zone activity is mainly responsible for leaching U from the sediments, especially in shallow zone. In the case of deep zone, it is the long residence time of groundwater with the sediments containing carbonate minerals (like kankar) that allows accumulation of U in the groundwater. This process also increases ionic strength moderately. The geology of this region reports presence of kankar in the formation [34, 35].
From hydrochemistry and isotope data, it can be inferred that the significant factor aiding in the release of U from the sediments could be elevated alkalinity in the shallow zone. Shallow groundwaters receive inputs from irrigation return flow which is high in HCO3 − concentration due to evaporation and also root respiration. This facilitates easy mobilization of U from minerals present in sediments of shallow zone. The log pCO2 values in the groundwater range from −4.0 to −1.9. Shallow zone (<60 m bgl) show slightly lower mean log pCO2 value (−2.5) compared to deep zone (−2.8). There is no correlation found between log pCO2 and U (Fig. 4f). Since there is a no or negative correlation between U and NO3 −, K+ and PO4 3−, concentrations in groundwater, fertilizer or other anthropogenic factors do not seem to contribute high U in groundwaters of this region.
Summary
An isotope and hydrochemical study was carried out in two districts of Punjab viz., Bathinda and Mansa in order to determine the source of uranium contamination in groundwater and also to characterize groundwaters. U concentration in groundwater ranges from 2.4 to 357 μg L−1. About 66% samples exceed the prescribed limit by WHO (30 µg L−1) while 37% samples exceed AERB limit (60 μg L−1). Depth profile of the U concentration indicates that high U was more prominent in the shallow zones while in deep zone it is less. A positive correlation was observed between HCO3 − and U indicating U occurrence is mainly due to leaching from sediments that are derived from granitic rocks. Correlation between ionic strength and U indicates that high U in groundwater is not necessarily governed by the salinity of the groundwater. Ions related to agricultural activities such as K+, NO3 − and PO4 3− showed no or negative correlation with U, indicating that the role of anthropogenic factors is negligible towards U contamination. Isotopically there is no clear distinction between high and low U groundwaters suggesting that the source of water is same. Impact of evaporation in groundwater samples is clearly seen from enriched isotopic composition.
References
Bhattacharya P, Ramanathan AL, Mukherjee AB, Bundschuh J, Chandrasekharam D, Keshari AK (2008) Groundwater for sustainable development: problems, perspectives and challenges. CRC Press, Boca Raton
Sharma DA, Rishi MS, Keesari T (2016) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in Southwest Punjab, India using hydrochemical Approach. Appl Water Sci. doi:10.1007/s13201-016-0456-6
Sharma DA, Rishi MS, Keesari T (2016) Understanding the factors responsible for groundwater contamination in parts of southwest Punjab, India. J Energy Environ Carbon Credits 6:7–23
Sharma DA, Rishi MS, Keesari T, Sinha UK (2016) Assessment of groundwater quality of Bathinda district, Punjab with reference to nitrate contamination. J Appl Geochem 18(4):480–489
Thakur T, Rishi MS, Naik PK, Sharma P (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ Earth Sci 75:467–471
Kumar A, Usha N, Sawant PD, Tripathi RM, Raj SS, Mishra M, Rout S, Supreeta P, Singh J, Kumar S, Kushwaha HS (2011) Risk assessment for natural uranium in subsurface water of Punjab state, India. Hum Ecol Risk Assess 17:381–393
Keesari T, Chawla D, Sinha UK, Kumar A, Rishi M (2015) In: Kannan S, Kannan S, Mukerjee SK, Ramakumar KL (eds) Occurrence of uranium in parts of southwest Punjab its interrelation with physicochemical parameters. Board of Research in Nuclear Sciences, India
Singh J, Singh L, Singh G (1995) High U-contents observed in some drinking waters of Punjab, India. J Environ Radioact 26:217–222
Sharma N, Singh J (2016) Radiological and chemical risk assessment due to high uranium contents observed in the ground waters of Mansa District (Malwa region) of Punjab state, India: an area of high cancer incidence. Expo Health. doi:10.1007/s12403-016-0215-9
ATSDR (2011) Toxicological profile for uranium: agency for toxic substances and disease registry. U.S. Department of Health and Human Services. Public Health Service, Atlanta
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Vaisanen SB, Penttila IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110(4):337–342
ATSDR (2013) Toxicological profile for uranium. U.S. Department of Health and Human Services. Public Health Service. Agency for toxic substances and disease registry. Atlanta https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf
WHO (2012) Uranium in drinking-water. Background document for preparation of WHO guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/118/Rev/1)
AERB, DAE (2004) Drinking water specifications in India. Atomic Energy Regulatory Board, Mumbai
Guthrie VA, Kleeman JD (1986) Changing uranium distributions during weathering of granite. Chem Geol 54:113–126
Hess CT, Michel J, Horton TR, Prichard HM, Conigilio WA (1985) The occurrence of radioactivity in public water supplies in the United States. Health Phys 48:553–586
Langmuir D (1997) Aqueous environmental geochemistry, vol 7th ed. Prentice Hall, New York
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Fontes CH (1981) Environmental isotopes in groundwater hydrology, In: Fritz P and Fontes JCH (eds.) Handbook of environmental isotopes geochemistry, The terrestrial environment A, Vol 1. Elsevier Scientific Publishing Co, Amsterdam, 1980, pp 75–140
Herczeg A, Lamontagne S, Pritchard J, Leaney F, Dighton J (1992) Groundwater-surface water interactions: testing conceptual models with environmental tracers. 8th Murray Darling Basin groundwater workshop, Victor Harbor, South Australia.6B.3
Gat JR (1981) Stable isotope hydrology: deuterium and oxygen-18 in the water cycle In: Gat JR and Gonfiantini R (eds.), IAEA technical reports series, No. 210. IAEA (International Atomic Energy Agency), Vienna
Shivanna K, Tirumalesh K, Noble J, Joseph TB, Singh G, Joshi AP, Khati VS (2008) Isotope techniques to identify recharge areas of springs for rainwater harvesting in the mountainous region of Gaucher area, Chamoli District, Uttarakhand. Curr Sci 94(8):1003–1011
Rao SM, Kulkarni KM (1997) Isotope hydrology studies on water resources in western Rajasthan. Curr Sci 72:55–61
Keesari T, Shivanna K, Jalihal AA (2007) Isotope hydrochemical approach to understand Fluoride release into groundwaters of Ilkal Area, Bagalkot District, Karnataka, India. Hydrogeol J 15:589–598
Keesari T, Mohokar HV, Sahoo BK, Mallesh G (2014) Assessment of environmental radioactive elements in groundwater in parts of Nalgonda district, Andhra Pradesh, South India using scintillation detection methods. J Radioanal Nucl Chem 302:1391–1398
Keesari T, Shivanna K, Noble J, Narayan KK, Xavier KT (2007) Nuclear techniques to investigate source and origin of groundwater pollutants and their flow path at Indian Rare Earths Ltd, Cochin, Kerala. J Radioanal Nucl Chem 274:307–313
Kochhar N, Dadwal V, Rishi M, Sharma NK, Balaram V (2012) Evaluation of chemical quality of groundwater in parts of Sirsa (Harayana), Mansa, Bhatinda and Muktsar Districts, SW Punjab with emphasis on uranium in relation to human health. In: Proceedings of 5th IGWC-2012 on the assessment and management of groundwater resources in hard rock systems with special reference to basaltic terrain, 611–626
Patnaik R, Lahiri S, Chahar V, Naskar N, Sharma PK, Avhad DK, Bassan MKT, Knolle F, Schnug E, Srivastava A (2016) Study of uranium mobilization from Himalayan Siwaliks to the Malwa region of Punjab state in India. J Radioanal Nucl Chem 308(3):913–918
Phadke AV, Mahadevan TM, Narayandas GR, Saraswat AC (1985) Uranium mineralisation in some phanerozoic sandstones of India, In: Geological environments of sandstone-type uranium deposits. IAEA-TECDOC-328,121–134
Singh L, Kumar R, Kumar S, Bajwa BS, Singh S (2013) Health risk assessments due to uranium contamination of drinking water in Bathinda region, Punjab state,India. Radioprotection 48(02):191–202
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2015) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10(1):13–19
Rishi MS, Keesari T, Sharma DA, Pant D, Uday Sinha K (2017) Spatial trends in uranium distribution in groundwaters of Southwest Punjab, India-a hydrochemical perspective. J Radioanal Nucl Chem 311(3):1937–1945
Krishan G, Rao MS, Kumar CP, Semwal P (2013) Identifying salinization using isotopes and ion chemistry in semi-arid region of Punjab, India. J Geol Geosci 2:4. doi:10.4172/jgg.1000129
CGWB (2013) Groundwater information booklet. Bathinda District, Punjab A report. http://cgwb.gov.in/District_Profile/Punjab/Bathinda.pdf
CGWB (2013) Groundwater information booklet. Mansa District, Punjab A report. http://www.cgwb.gov.in/District_Profile/Punjab/Mansa.pdf
Gupta S, Thakur RS (1989) Report on hydrogeology of District Bathinda, Punjab State (F.S.P. 1989–93). Central Ground Water Board, North Western Region, Chandigarh
Sharma M, Sharma YC, Basu B, Chhabra J, Gupta RK, Singh J (2000) Uranium mineralization in the sandstones of Dharamsala, Tileli area, Mandi district, Himachal Pradesh, India. CurrSci 78:897–899
Tripathi A, Mishra AK, Verma G (2016) Impact of preservation of subsoil water act on groundwater depletion: the case of Punjab, India. Environ Manag 58(1):48–59
Dhiman SC (2012) Aquifer systems of India. Central Ground Water Board, Ministry of Water Resources, Government of India, Guwahati, p 111
Hounslow AW (1995) Water quality data—analysis and interpretation. Lewis Publishers, CRC Press, Florida
Plummer LN, Jones BF, Truesdell AH (1976) WATEQF—A Fortran IV Version Of WATEQ, A computer program for calculating chemical equilibrium of natural waters. US Geol Surv Water Resources Investigations Report, 76–13
Ohsumi T, Fujino H (1986) Isotopic exchange technique for preparation of hydrogen gas in mass spectrometric D/H analysis of natural waters. Anal Sci 2:489–490
Epstein S, Mayeda T (1953) Variation of 18O contents of water from natural sources. Geochim Cosmochim Acta 4:213–224
Horita J, Ueda A, Mizukami K, Takatori I (1989) Automatic δD and δ18O analyses of multi-water samples using H2- and CO2-water equilibration methods with a common equilibration set-up. Int J Radiat Appl Instrum 40:801–805
Coplen TB (1996) New guidelines for reporting stable hydrogen, carbon and oxygen isotope-ratio data. Geochim Cosmochim Acta 60:3359–3360
Sahoo SK, Mohapatra S, Chakrabarty A, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2010) Determination of uranium at ultra trace level in packaged drinking water by laser fluorimeter and consequent ingestion dose. Radioprotection 45:55–66. doi:10.1051/radiopro/2009030
Kass A, Gavrieli I, Yechieli Y, Vengosh A, Starinsky A (2005) The impact of freshwater and wastewater irrigation on the chemistry of shallow groundwater: a case study from the Israeli Coastal Aquifer. J Hydrol 300(1):314–331
Nolan BT, Hitt KJ (2006) Vulnerability of shallow groundwater and drinking-water wells to nitrate in the United States. Environ SciTechnol 40(24):7834–7840
Thivya C, Chidambaram S, Keesari T, Prasanna MV, Thilagavathi R, Adithya VS, Singaraja C (2016) Lithological and hydrochemical controls on distribution and speciation of uranium in groundwaters of hard-rock granitic aquifers of Madurai District, Tamil Nadu (India). Environ Geochem Health 38(2):497–509
Thivya C, Chidambaram S, Tirumalesh K, Prasanna MV, Thilagavathi R, Nepolian M (2014) Occurrence of the radionuclides in groundwater of crystalline hard rock regions of central Tamil Nadu, India. J Radioanal Nucl Chem 302:1349–1355. doi:10.1007/s10967-014-3630-z
Nolan J, Weber KA (2015) Natural uranium contamination in major US aquifers linked to nitrate. Environ Sci Technol Lett 2(8):215–220
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Acknowledgements
Authors sincerely acknowledge the constant support and encouragement by Shri K. S. S. Sarma, Head, Isotope and Radiation Application Division and Dr. B. S. Tomar, Director, Radiochemistry and Isotope Group, Bhabha Atomic Research Centre, Mumbai. The authors would like to acknowledge the BRNS, DAE for providing the financial support (letter no. 35/14/11/2014-BRNS-193).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, D.A., Rishi, M.S., Keesari, T. et al. Distribution of uranium in groundwaters of Bathinda and Mansa districts of Punjab, India: inferences from an isotope hydrochemical study. J Radioanal Nucl Chem 313, 625–633 (2017). https://doi.org/10.1007/s10967-017-5288-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5288-9