Abstract
Groundwater contributes to the highest exposure level of naturally occurring uranium (U) to biosphere, and hence, the source and concentration of uranium in groundwater needs to be monitored periodically. In the present study, groundwaters from different lithologic locations were collected and measured for uranium concentration and major ions in order to establish any possible link with the lithology on the uranium distribution in central parts of Tamil Nadu, South India. About 11% of the samples contain U in excess of the permissible limit of WHO (Guidelines for drinking-water quality, WHO, Geneva, 2011), and the contamination was limited to mostly hard rock terrain, which is granitic in nature. The correlations among U, major ions, and environmental isotopes were studied to understand the mechanism governing uranium dissolution and transport in groundwater of this region. Observations lead us to infer that the older water with near-neutral pH and oxidizing condition contains higher dissolved U compared with relatively young groundwater. The results also reflect the possible health risk to the local population through long-term consumption of uranium-containing groundwater without any pretreatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium (U) is a naturally occurring radioactive element that is commonly present in groundwater. Studies show that contribution of ingested U through food stuff accounts to 15%, whereas drinking water contributes 85% of ingested U (Tanner 1980). Hence, the health risk due to consumption of uranium-containing groundwater poses a greater risk compared to other causes. Higher U concentration was reported in groundwater in comparison to surface waters due to accumulation of U through rock–water interactions and also due to the presence of complexing ions in groundwater, which can leach U from the rock and form stable and soluble U complexes in aqueous media (Langmuir 1978). Carbonate, phosphate, fluoride, and sulfate commonly complex with U in water depending on the dissolved concentrations of the individual ions and pH ranges (Závodská et al. 2008). Uranium mobility is governed by various factors, among which pH and redox potential (Eh) plays a crucial role (Závodská et al. 2008). The concentration of U in groundwater also depends upon regional geology and structure. Generally siliceous igneous rocks contain higher U which increases further with increase in the silica content as seen in pegmatites as compared to basalts (Langmuir 1997). Hard rocks especially granites report highest U concentration in groundwater (Brindha and Elango 2013). In India, excess U in groundwater was first reported from Punjab state from Amritsar and Bhatinda districts (Singh et al. 1995). The subsequent studies on U occurrence in SW Punjab region indicated that not only hard rock terrains, even alluvial formations are prone to U contamination (Rishi et al. 2017; Sharma et al. 2017; Pant et al. 2017).
Uranium in drinking water is known for its radiological and toxicological impacts to living organisms and many studies have been carried out to assess the health hazards of this radioactive element (Singh et al. 2014; Leggett 1989; McDonald-Taylor et al. 1997; Kim et al. 2004; Kurttio et al. 2002). There were many studies on the identification of the sources and processes related to high U concentration groundwater (Garg et al. 2014; Singh et al. 2009a, b; Ansoborlo et al. 2015; Bajwa et al. 2015; Alrakabi et al. 2012; White et al. 1984). U concentrations ranging from 500 to 4700 ppb is noted in naturally occurring igneous rocks and sedimentary rocks whereas carbonate rocks report an average level of 2000 ppb. U content of unconsolidated beach sands is about 3000 ppb. For the soil U content, often the bedrock is controlling factor. Uranium concentration in the range of 0.2 to 0.6 ppb is noted in river water (Bleise et al. 2003; Irwin 1997; Kim 1986; Meinrath et al. 2003; Závodská et al. 2008). Uranium concentrations (Gascoyne 1989) in various lithologies throughout the world are shown in Fig. 1. The occurrence of higher U in groundwater is reported to be more in sedimentary formation followed by Granites. Very high U concentrations of 14,870 ppb and 80,000 ppb were reported in granitic and sedimentary formations, respectively (Gascoyne 1989). Gneissic terrains report comparatively lower U concentration. The occurrence of various uranium deposits across the world is listed by IAEA (1996). 33% of the total uranium resources are present in unconformities followed by sandstone. Breccia and QPC types in terms of occurrence are the next highest U deposits found worldwide. There are other types of U deposits which also contribute to the total uranium resource in significant quantities (Fig. 2).
Occurrence of radioactivity levels in groundwater with respect to lithology (Modified after Gascoyne 1989)
A simplified list of uranium deposits/resources with respect to their types of occurrence (after IAEA 1996)
A study by Dahlkamp (1993) correlated the types of U deposits with their corresponding geological age and the number of U deposits in each type (Fig. 3). It is seen that the occurrence of sandstone-type Uranium deposits during the Mesozoic to Cenozoic period dominates over the other types, while quartz pebble conglomerate (QPC) is the major dominant type of U deposit to occur during the Archean period. A few Uranium deposits were also reported in unconformities during the Proterozoic period.
Occurrence of uranium deposits with respect to different geological ages (after Dahlkamp 1993)
Countries like Norway, Jordan, United States, Egypt, Spain, Finland, Bangladesh and India report uranium ranges in groundwater far exceeding the recommended limit of WHO (30 ppb). The highest value is found in Helsinki, Finland in the range of 9400–19,600 ppb (Piirainen 1968). An alarmingly high concentration of above 500 ppb is reported in India (Bajwa et al. 2015; Rishi et al. 2017). An exposure to about 0.1 mg/kg of body weight of soluble natural Uranium results in severe chemical damage to kidneys (Health Physics Society 2002). Thus, kidney and lungs are found to be the major target organs of U toxicity, and the intake of drinking water contaminated with Uranium, in very high acute doses, can lead to necrosis and tubular degeneration (WHO 2004). Uranium is also known for its radiological toxicity as a carcinogenic element along with possible respiratory diseases (e.g., fibrosis, emphysema), potential neurological damage, and reproductive disorders (WHO 2004).
In the present study, uranium concentrations in groundwaters from different geological units were measured along with other hydrochemical parameters in order to identify the geological controls on U distribution in groundwater.
Study Area
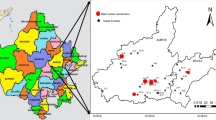
The study area falls in the central part of Tamilnadu comprising parts of Madurai, Dindigul, Trichy, Pudukottai, and Sivaganga districts with a total area of 4311 km2. The geographical area spans across East 09°53′24″–10°20′60″ latitudes and North 78°1′48″–78°48′36″ longitudes (Fig. 4). A major seasonal river Vaigai originates in Western Ghats and flows from NW to SE along the central Tamil Nadu. Pambar and Manimuttar are the other major tributaries of Vaigai River. The Geological succession established by GSI (2006) reports that the crystallines (Dharwars) are composed of meta-sediments intercalated with Charnockite series. Pelitic and semipelitic members associated with massive Quartzites, Banded Calcareous, and psammitic members that constitute the meta-sediments. Conglomerate beds occur in the contact areas between Sedimentary formations (in the East) and Hard rock terrain (in the West). South-eastern (SE) region is dominated by Sandstone and Shales followed by sand, silt, and clay partings along with conglomerate. Oldest among the lithological units are the Archean outcrops of Garnet Sillimanite Gneiss that are exposed as a small patch in the North part. Fissile hornblende biotite gneiss (FHBG) is the major rock type occurring in central, Northern, Western, and Southern parts of study area, along with Charnockites in the western side, followed by Hornblende biotite gneiss in the NE part. Granite occurs as intrusions in the central and NE regions with Quartzite in the North.
In the study area, groundwater exists in the hard rocks under water table conditions at shallow levels and under semiconfined-to-confined conditions in the fractured, jointed, and sheared zones. One-third area of the region is underlain by sedimentary formations. In the sedimentaries, groundwater occurs under water table and confined conditions. Two main aquifer systems are encountered in the region such as Porous formations, viz., alluvium, sandstone etc., as well as weathered and fractured crystalline formations consisting of Charnockite and Granite.
An average rainfall of 950 mm per annum is reported in the region (CGWB 2008). Major water-bearing formations in the western side are fractured crystallines, Granite, Charnockite, and Gneisses, while the eastern side possesses alluvium and Sand stone formations. Water level trends in 10 years (1998–2007) indicate a fluctuation of 0.004 to 1.523 m/year (CGWB 2008). The study area chosen for the present study is a complex geological terrain consisting of hard rocks, sedimentary formations of Cenozoic age with conglomerate beds, and Granitic intrusions.
Methodology
Groundwater sampling was carried out during July 2014 (South West Monsoon), and a total of 54 samples were collected from hand pumps and bore wells based on spatial variance and lithological coverage. Physical parameters such as pH, temperature, total dissolved solids (TDS), and electrical conductivity (EC) of the samples were measured in situ using a portable water-analysis kit (Thermo Orion 5 star). Sampling and analysis were carried out using standard analytical methodology. Major ions such as Ca2+, Mg2+, Na+, K+, F−, Cl−, HCO3−, NO3−, H4SiO4, PO43−, and SO42− were analyzed in laboratory. Ca+, Mg+ were measured by EDTA Titrimetric method; Cl− by argentometric method; and CO3− and HCO3− by H2SO4 Titration method. Na+ and K+ were measured by flame photometer (ELICO CL 378), SO42− by Turbidimetric method, PO4− by Ascorbic acid Method, NO3− by UV Spectrophotometric Screening Method, and H4SiO4 by Molybdo-silicate Method. A UV Spectrophotometer (HACH, DR 6000) was used for the measurements. Accuracy of analysis was checked by error percentage calculation using the equation.
where TA and TC are the total number of anions and cations in milli equivalents, respectively. The percentage of error is found to vary within ± 10%.
Total U concentration in groundwater samples was analyzed by Laser fluorimetry using LED Fluorimeter (Model LF -2a, Quantalase). This method can measure concentration of uranium in trace and ultra-trace levels in aqueous medium. It works on the principle of measurement of fluorescence of uranyl complex (uranyl phosphate), which is formed by addition of a fluorescence-enhancement reagent like Fluran (sodium pyrophosphate) to an aqueous solution containing uranium. The excitation is done under 337.1 nm laser pulse.
Groundwater samples were analyzed for oxygen-18 (δ18O), and deuterium (δD) isotopes. Polyethylene bottles of 60 ml capacity were used for collecting groundwater samples after the stagnant water was pumped out, in order to get representative samples. Proper care was taken to preserve the sample bottles in airtight condition after filling them completely in order to avoid any evaporation. Stable isotopes such as δ18O and δD were measured using mass spectrometer (Finnigan Deltaplus Xp, Thermo Electron Corporation, Bermen, Germany) based on gas equilibration method with precisions of 0.5 and 0.1‰ (2σ criterion), respectively. The results of stable isotopes were compared using VSMOW (Vienna Standard Mean Ocean water) in units δ (‰) where
where R = D/H or 18O/16O.
A few selected groundwater samples were collected for Tritium analysis. The environmental tritium was analyzed by liquid scintillation counting method using Quantulus liquid scintillation counter, available at Isotope Hydrology Section, Bhabha Atomic Research Centre, Mumbai.
Results and Discussion
Table 1 shows that Uranium concentration in groundwater ranges from 0.79 to 71.93 ppb in the study area. It is reported that the solubility and mobility of U are highly dependent on redox conditions and are influenced by pH, complexation, and sorption reactions (Sharp 2014). On an average, U concentration of 1800 ppb is reported in soils (Závodská et al. 2008). The formation of a highly mobile carbonated species of uranium is favored by neutral to alkaline pH of natural aqueous systems with a relative atmospheric pCO2 of 0.03%. These conditions of any given water type lead to transport of U from soils to rivers, lakes, and groundwater. The concentration of U in continental surface waters is normally found to vary from 0.1 to 500 ppb (Závodská et al. 2008). Seawater is a major deposit of U with a content of about 3000 ppb. Table 2 shows distribution of uranium in various states in India. The highest reported U concentration is in Kolar district of Karnataka (1443 ppb), and the lowest is in Maharashtra (0.03 ppb). The range of concentrations observed in study area matches with that of Hyderabad, Telangana, and the highest value is noted in Allampati village (71.93 ppb).
Uranium Contour
Uranium concentration of groundwater samples was compared to the WHO standard, and it was found that six samples contain Uranium concentrations above the safe limit of 30 ppb (WHO 2011). The spatial distribution of Uranium (Fig. 5) shows the highest concentrations associated with Granite bodies in two patches; one in the central part of study area, and the other along the north-eastern side. Apart from this, samples taken from the Charnockite rocks exposed along NW–NE sides also shows higher concentrations of Uranium. In general, water samples belonging to the Hard rock terrains located on the NW side show higher values of Uranium, whereas those from Sedimentary rocks on the SE side show negligible concentrations.
The groundwater flow through fractures and fissures in crystalline rocks like Charnockites and FHBG; therefore, the extent of contact between water and the rock matrix varies depending on the availability and interconnectivity of the fracture. The interaction of water and rock is greater in rocks with primary porosity (sedimentary rocks) compared to crystalline rocks. Further, the silicate minerals in crystalline rocks tend to weather slowly leading to limited interconnected pores; therefore, the residence time of groundwater in metamorphic and plutonic igneous rocks is more. The low rainfall and excessive evaporation conditions in arid and semiarid regions favor the formation of groundwaters with higher ionic concentrations (Hem 1985). The process of illumination enriches U in groundwater by leaching and precipitation process, thereby adding dissolved loads of natural U, through natural weathering of rocks such as granite (Brindha and Elango 2013). As a result, the highest U concentration is noted in granitic terrains followed by FHBG, and then Charnockites. It is observed that the concentrations are lower in the Sedimentary terrain or along the contact area between the hard rock and the sedimentary aquifers.
pH
pH governs the dissolution of minerals in water and also governs the distribution of uranium in groundwater. Figure 6 shows that higher uranium concentration is distributed in the near-acidic-to-neutral pH conditions irrespective of the formation (6.72–7.24). Most of the samples of FHBG are above pH 7.24 and have lower U concentration. The study on the variation of uranium species with respect to pH (Fig. 7) showed that UO2(CO3)2−2 and UO2(CO3)4−3 are more predominant in acidic-to-near-neutral pH conditions. Under closed conditions with constant amount of dissolved carbonate and low acidic-to-neutral pH, the formation of carbonate–uranium complexes is reported which is mainly due to transformation of U(VI) species with the increasing pH (Krestou and Panias 2004). Near-acidic condition favors the formation of uranyl carbonate, and with further removal of more and more CO2 from the water by complexing with the uranyl ion makes the system more acidic. Thus, the system is made more aggressive toward the dissolution of U in these regions with more complexation of UO2(CO3)4−3.
Uranyl phosphate complexes can also form at near-neutral pH, and the aqueous species UO2(HPO4)2−2 could be important in neutral-pH waters (Drever 1997). However, the present study shows that there is no considerable correlation of phosphate complexes of U with pH. The other U complexes expected to form are sulfide and fluoride species, which can occur under acidic conditions (Langmuir 1978; Drever 1997). It is also inferred that the chloride and sulfate act as important ligands in saline waters (Porcelli and Swarzenski 2003). Stable complexes of U(VI) also form in association with dissolved organic carbon (Cothern and Lappenbusch 1983; Higgo et al. 1993; Arey et al. 1999). Thus, organic-rich waters can significantly enhance the transport of U.
Redox
Oxidation and reduction reactions play an important role in releasing U from the rock/soil matrix. Reduction of U(VI) to insoluble U(IV) is favored in low Eh thus immobilizing U from water. The oxygen content in groundwater determines the dissolution capacity of the U from the minerals present in the host rocks (Fig. 8). Under oxidizing conditions, uranyl ion, UO22+, is the dominant dissolved species of U(VI). In general, the ORP values in the groundwater of the study area are positive (68–223 mV). The spatial representation of these values (Fig. 9) shows that the values increase from the central part represented near the granitic patch and the highest values are observed in the north-western part of the study area covered by FHBG. Since the groundwater is under oxic condition, leaching of U from the source rock to the groundwater is favored.
Conceptual diagram depicting variation in U concentration along groundwater flow path (after Ivanovich et al. 1991)
pCO2
Groundwater CO2 is the theoretical pCO2 that exists in chemical equilibrium with the solution if a gas phase were present. The relationship of pCO2with HCO3− and pH is given as
where Ct = log10(K1,T) − log10(Kh,T); pH is in standard pH units (− log10 of the activity of H +); HCO3− has units of chemical activity; K1,T is the temperature dependent first association constant of H2CO03*, H2CO03* is the sum of H2CO03 and CO02, and Kh, T is the temperature dependent Henry’s Law constant expressing the relation between H2CO03* and pCO2. The volume-percent of CO2 in dry air is 0.032%, and it corresponds to a partial pressure of 3 × 10−4 (10−3.5) atm.
Higher log PCO2 value is indicative of closed system (Chidambaram et al. 2011). Values in the range from -3 to -4 of log pCO2 indicate open system, as -3.75 is atmospheric pCO2. In open system, water is directly connected to atmosphere, but in closed system, it is not connected to atmosphere and the pressure of CO2 increases. High pCO2 increases H+ion in solution, through the dissociation of H2CO3, thereby triggering chemical weathering of felsic and mafic silicates in weathered and hard rock terrains of the study area.
Uranyl–carbonates are the most important aqueous species of U. The higher partial pressure of CO2 (about 10−2 atm) in the deep subsurface enhances the concentration of carbonates that are naturally abundant. The concentration of CO2 further increases up to 10−2 M in groundwaters by reactions with carbonate-containing mineral phases (e.g., dolomite (CaMg(CO3)2) and calcite (CaCO3)) (Langmuir 1997; Clark et al. 1995; Runde 2000). Transport of U in natural waters is intensified by Uranyl–carbonate complexes by increasing the solubility of U minerals, simulating U(IV) oxidation, and hindering adsorption of U in oxidized waters (Langmuir 1997; Závodská et al. 2008; Wazne et al. 2003).
The graph (Fig. 10) of log pCO2 versus Uranium shows that all samples except two fall within the log pCO2 range from − 2 to − 1. It is observed that for the same pCO2 value, there are samples with low U concentration as well as higher U value concentration. Groundwater samples from Hornblende Biotite Gneiss show a wide range of pCO2 values indicating the presence of both open and closed systems. However, higher U values are noted only in the higher pCO2 region indicating role of higher HCO3 concentration in mobilizing U from the rock. These observations reveal that a closed system predominant in hard rock regions leads to higher uranium concentrations in the study area, while in sedimentary formations, an open system exists with low U concentrations in groundwater.
HCO3
In acidic environment, the release of U into the solution is favored with the increase in HCO3−; this subsequently slowly turns the groundwater to near-neutral condition and changes H2CO3 to HCO3−. Higher uranium values show a linear trend with the increasing bicarbonate indicating a positive correlation between U and HCO3 in FHBG (Fig. 11), but all higher HCO3 values do not necessarily show a linear trend with higher U concentrations. Similarly, it is interesting to note that the trend is well maintained in the case of FHBG than in other formations. This is partly attributed to the intensity of weathering in these rock types, FHBG being the most easily weathered unit (Appelo and Postma 2005). High pH favors the formation of the carbonate complex and desorption of U(VI) from aquifer sediment containing metal oxides/oxyhydroxides and clay minerals, releasing U into groundwater.
Oxygenated groundwater is best suited for U transport, whereas reducing environment aids in formation of stable U minerals. In oxic environments, Uranium exists in groundwater with a valency of +6 [U(VI)], and uranyl ion (UO+22) forms in the absence of complexing species, such as dissolved inorganic carbon (DIC) (Langmuir 1997). Very high concentrations of U (greater than 1000 ppb) are a major requisite for formation of stable U minerals in oxic environments. Uranyl adsorption to iron oxy-hydroxide coatings on sediment grains, clay mineral edges, or to organic matter reduces the aqueous concentration of U(VI) (His and Langmuir 1985; Waite et al. 1994; Davis and Curtis 2004; Catalano and Brown 2005). However, the presence of complexing ions, particularly DIC species, can increase the solubility and mobility of U (VI) (Langmuir 1997; His and Langmuir 1985; Waite et al. 1994; Davis and Curtis 2004). This gives an idea that since hard rock terrains have less connectivity to the surface due to lack of interconnected pores or primary porosity, increased residence time will enable leaching of Uranium from the rocks and hence gives higher uranium concentrations in those regions.
Ionic Strength
In order to evaluate the relation between dissolved U and total salt load, ion strength was estimated in the water samples. Fresh waters usually have ionic strength less than 0.02 and that of oceanic water is about 0.7 mol kg. Typical fresh water has ionic strength of less than 0.005 (Domenico and Schwartz 1990).
Ionic strength (IS) of the groundwater samples ranges from 0.0023 to 0.0183 in the study area. Figure 12 depicts increasing trends in ionic strength for an increase of U concentrations in varying proportions in hard rocks. Most of the higher uranium samples have higher ionic strength, and it shows a particular trend as noticed in FHBG and Charnockites. This also reflects higher residence time of groundwater in the system and the presence of uraniferous minerals in the aquifer. On the contrary, groundwater from granitic terrain shows higher concentration of uranium with lesser ionic strength, which could be due to flow of groundwater through the granitic intrusions with U-rich minerals. Groundwater reacts with U-rich minerals present in granites, resulting in uranium dissolution from rock matrix even though residence time is less and ionic strength is low.
δ 18O
Environmental isotopes indicate two kinds of groundwater. Samples with depleted isotope values are typically recharged by rain water, while samples with enriched stable isotope composition are recharged by evaporated water bodies (local tanks, reservoirs, etc.) (Fig. 13). In general, groundwater samples of the region show both enriched and depleted isotope values, but groundwater from charnockite, quartzite, sandstone formations, and half of the FHBG formation are represented by the depleted isotopes. Those samples with enriched stable isotope values show lesser uranium concentrations.
Groundwater which is mainly recharged by the precipitation indicates depleted isotopic composition compared to groundwater recharged by evaporated water. Fluctuation of water level was noted in the Charnockite and FHBG aquifers, by the earlier researchers. The increase of groundwater level also enhances the dissolved uranium concentration (Brindha et al. 2010). Samples were collected in the month of July during the onset of monsoon which might also enhance the dissolution of U in groundwater from the minerals near the surface (shallow depths). This happens when the infiltrating rainfall flushes uranium from the unsaturated zone and simultaneously results in the increased uranium concentration in solution as well as rise in the water table as noted in the Charnockite and FHBG. This observation is supported by the depleted stable isotope concentration of groundwater. Enriched isotopic values are noted in groundwater of granitic terrains with higher U concentration, which reflect evaporated surface water as the main recharge source.
The classification of Tritium levels in water (Clark and Fritz 1997) shows that the 3H values < 0.8 TU represent sub-modern water recharged prior to 1952; 0.8 to ~ 4 TU ranges signify the mixture of sub-modern and recent recharge; The range of values from 5 to 15 TU shows modern recharge with recharge times of 5–10 years. Figure 14 depicts a plot between Uranium and Tritium for selected samples. Groundwater can be classified into three types in the study area, viz i) sub-modern, ii) Mixture of sub-modern and recent recharge and iii) modern. All samples except two belonged to the mixture of types between sub-modern and recent-recharged types. In general, the highest uranium concentration is observed in this mixed water type belonging to rock types such as Fissile Hornblende Biotite Gneiss, Charnockite, and Granite. This inference is found to match with the observations obtained from ionic strength patterns. Higher U groundwaters associated with hard rocks have longer residence time which in turn led to higher uranium content. It is found that modern waters contain lower uranium due to dilution by infiltrating rain water.
Hazard Zonation
Exposure to the radionuclide uranium has numerous long-term health risks. The mode of exposure, contact time, solubility of uranium, and preventive measures are the factors determining the severity of damage. The presence of uranyl compounds in edible parts of plants is reported by many researchers (Saric et al. 1995; Sheppard et al. 1989). Vegetable food stuff such as cauliflower, carrots, cucumber, and red peppers were reported to contain relatively high amounts of Uranium compared to other organic products (Sheppard et al. 1989). After being consumed through drinking and cooking water, most of the uranium and its radioactive daughter products are eliminated from the human body. However, due to its non-biodegradable nature, small amounts of this heavy metal finds its way to enter the blood stream. In blood plasma, uranyl ion combines with bicarbonate, citrate, and forms complexes like UO3(CO2)3 (Durbin 1984). The UO22+ ion binds with the red blood cells (Sethy et al. 2011). During the kidney functioning and purification of blood, Uranium gets filtered and deposited in the kidneys and damages the proximal tubules (WHO 2011). This affects the urinary albumin (Mao et al. 1995), glucose, and calcium (Arzuaga et al. 2010). Role of uranium as a cytotoxicity biomarker is studied by Zamora et al. (1998). Continuous exposure to U leads to high cancer risk and also liver damage (ATSDR 1999). Brain dysfunction, diminished bone growth, DNA damage, and developmental defects are reported in infants due to critical exposure during their growth (Brugge et al. 2005; Brugge and Buchner 2011).
Ingestion of uranium in human body is mainly through drinking water and due to its high ionization power, it tends to be hazardous. Consumption of uranium through drinking water by population residing around the uranium-bearing rock formations has been considered in the present study. Spatial distribution of Uranium hazard has been arrived by assuming an average per capita consumption of 4 L of water per day. Population details for each sample points were collected using Census 2001 for Tamilnadu and multiplying it with the average daily per capita water consumption (4 L) and with corresponding total uranium concentrations for each sample of that village (Fig. 15).
Four liters × total population of the village × U concentration in groundwater of the village = average U-contaminated groundwater consumed per day by village (Lpd).
Higher hazard values are detected along the eastern side of the study area where the population is higher with an average higher U concentration in groundwater, which covers an area of 83.49 km2 in the central–eastern segment of the study area.
Conclusion
Sampling was carried out to understand the source, distribution, occurrence, and potential risk zones of Uranium hazard in the study area. A comparison is made with worldwide U occurrences over various lithologies, and it is observed that groundwater in granite formation reports the highest uranium content followed by sedimentary formations. This is found to match with the highest observed value of 71. 93 ppb reported from central granitic intrusions of the study area. Fissile Hornblende Biotite gneiss reports the next higher uranium content followed by Charnockites located along the NW part. In general, samples from hard rock terrain contain higher uranium concentrations than those of the sedimentary formations. It’s observed that the highest dissolution of uranium is taking place in the near-neutral to acidic pH conditions of groundwater. In the highest uranium samples, uranium is found to be in complex form predominantly as UO2(CO3)2−2 and UO2(CO3)4−3 supporting the near-neutral and acidic pH environment. Uranyl species such as UO2(HPO4)2−2 is also present supporting an acidic environment. Redox potential values observed in the region clearly indicate the oxidizing condition, which is favorable for uranium transport and dissolution. The role of pCO2 in assisting chemical weathering of felsic and mafic silicates in weathered and hard rock terrains of the study area is observed. Log PCO2 values reveals a closed system of hard rocks having higher uranium concentrations and an open system of sedimentary formations with low U concentrations. The positive trend observed between U and bicarbonate in FHBG reveals that the presence of complexing ions such as HCO3 increases uranium solubility and mobility. Isotopic composition is found to be enriched in groundwater of the granitic terrain with higher U concentration, probably due to the recharge from an evaporated surface water source. It is also inferred that relatively older waters characterized by low tritium values have higher uranium concentrations. Since hard rock terrains have less connectivity to the surface due to lack of interconnected pores or primary porosity, the residence time of groundwater in the system is more, and it allows for enhanced leaching of Uranium from the rocks giving rise to higher dissolved U concentrations. Higher ionic strengths observed in FHBG and Charnockites are another manifestation of longer residence times of groundwater, which again promotes higher release of U into groundwater.
References
Alrakabi M, Singh G, Bhalla A, Kumar S, Kumar S, Srivastava A, Rai B, Singh N, Shahi JS, Mehta D (2012) Study of uranium contamination of ground water in Punjab state, in India using X-ray fluorescence technique. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-011-1585-x
Ansoborlo E, Jacobs LL, Prat O (2015) Uranium in drinking-water: a unique case of guideline value increases and discrepancies between chemical and radiochemical guidelines. Environ Int 77:1–4
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. CRC Press, Boca Raton, ISBN 9780415364218 - CAT# SW1652
Arey JS, Seaman JC, Bertsch PM (1999) Immobilization of uranium in contaminated sediments by hydroxyapatite addition. Environ Sci Technol 33:337–342
Arzuaga X, Rieth SH, Bathija A, Cooper GS (2010) Renal effects of exposure to natural and depleted uranium: a review of the epidemiologic and experimental data. J Toxicol Environ Health B 13:527–545
ATSDR (Agency for Toxic Substances and Disease Registry) (1999) Toxicological profile for uranium. Atlanta. www.atsdr.cdc.gov/toxprofiles/tp150.html
Babu MNS, Somashekar RK, Kumar SA, Shivanna K, Krishnamurthy V, Eappen KP (2008) Concentration of uranium levels in ground water. Int J Environ Sci Technol 5(2):263–266
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2015) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10(1):13–19
Balbudhe Y, Srivastava SK, Vishwaprasad K, Srivastava GK, Tripathi RM, Puranik VD (2011) Assessment of age dependent uranium intake due to drinking water in Hyderabad, India. Radiat Prot Dosim 148(4):502–506
Bansal V, Azam A, Srivastava DS (1985) Measurement of uranium content of water using plastic track detectors. In: Sharma KK (ed) Application to earth sciences. IV National Seminar-Cum-Workshop on SSNTDs, vol 14. Shiva Offset Press, Dehradun, pp 89–98
Bansal V, Tyagi RK, Prasad R (1988) Determination of uranium concentration in drinking water samples by fission track method. J Radiol Nucl Chem 125:439–443
Bhangare RC, Tiwari M, Ajmal PY, Sahu SK, Pandit GG (2013) Laser flourimetric analysis of uranium in water from Vishakhapatnam and estimation of health risk. Radiat Prot Environ 36(3):128–132
Bleise A, Danesi PR, Burkart W (2003) Properties, use and health effects of depleted uranium (DU): a general overview. J Environ Radioact 64:93–112
Brindha K, Elango L (2013) Occurrence of uranium in groundwater of a shallow granitic aquifer and its suitability for domestic use in southern India. J Radioanal Nucl Chem 295:357–367. https://doi.org/10.1007/s10967-012-2090-6
Brindha K, Rajesh R, Murugan R, Elango L, Nair RN (2010) Spatial and seasonal variation in groundwater level and uranium concentration in Peddagattu and Seripalli area of Nalgonda District, Andhra Pradesh, India. In Proceedings of the Seventh National Symposium on Environment, pp 256–260
Brugge D, Buchner V (2011) Health effects of uranium: new research findings. Rev Environ Health 26:231–249
Brugge D, de Lemos JL, Oldmixon B (2005) Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: a review. Rev Environ Health 20:177–193
Catalano JG, Brown GE (2005) Uranyl adsorption onto montmorillonite: evaluation of binding sites and carbonate complexation. Geochem Cosmochim Acta 69(12):2995–3005
CGWB (2008) Central Ground Water Board, Annual report 2008–2009
Chidambaram S, Prasanna MV, Karmegam U, Singaraja C, Pethaperumal S, Manivannan R, Anandhan P, Tirumalesh K (2011) Significance of pCO2 values in determining carbonate chemistry in groundwater of Pondicherry region, India. Front Earth Sci 5(2):197–206. https://doi.org/10.1007/s11707-011-0170-5
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York, p 328
Clark DL, Hobart DE, Neu MP (1995) Actinide carbonate complexes and their importance in actinide environmental chemistry. Chem Rev 95:25–48
Cothern RC, Lappenbusch WL (1983) Occurrence of Uranium in Drinking Water in the US. Health Phys 45(1):89–99
Dahlkamp FJ (1993) Uranium ore deposits. Springer, Berlin, p 460
Davis JA, Curtis GP (2004) Application of surface complexation modeling to describe uranium (VI) adsorption and retardation at the Uranium Mill Tailings Site at Naturita, Colo. U.S. Nuclear Regulatory Commission, Rockville
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, N.Y.
Drever JL (1997) The geochemistry of natural waters—surface and groundwater, 3rd edn. Prentice Hall, New Jersey
Durbin PW (1984) Metabolic model for uranium. In: Moore RH (ed) Biokinetics and analysis in man 1984. United States Uranium Registry, National Technical Information Service, Springfield
Garg VK, Yadav A, Singh K, Singh M, Bishnoi M, Pulhani V (2014) Uranium Concentration in Groundwater in Hisar City, India. Int J Occup Environ Med\ 5:112–114
Gascoyne M (1989) High levels of uranium and radium in ground waters at Canada’s Underground Research Laboratory, Lac du Bonnet, Manitoba, Canada. Appl Geochem 4:577–591
GSI (2006) Geology and mineral resources of the states of India. Part VI—Tamil Nadu and Pondicherry
Health Physics Society (2002) Ask the expert. http://www.hps.org/publicinformation/ate/q1906.html
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. Department of the Interior, US Geological Survey, Reston USGS water supply paper
Higgo JJW, Kinniburgh DG, Smith B, Tipping E (1993) Complexation of Co+2 , Ni+2 , UO22+ and Ca2+ by humic substances in groundwaters. Radiochim Acta 61:91–103
His CKD, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides: application of the surface complexation site-binding model. Geochim Cosmochim Acta 49:1931–1941
International Atomic Energy Agency (IAEA) (1996) World distribution of uranium deposits (map rolled). IAE, Vienna
Irwin RJ (1997) Environmental contaminants encyclopedia, Uranium entry. National Park Service, Fort Collins
Ivanovich M, Fröhlich K, Hendry MJ (1991) Uranium-series radionuclides in fluids and solids, Milk River aquifer, Alberta, Canada. Appl Geochem 6:405–418
Kansal S, Mehra R, Singh NP (2011) Uranium concentration inground water samples belonging to some areas of Western Haryana, India, using fission track registration technique. J Public Health Epidemiol 3:352–357
Keesari T, Mohokar HV, Sahoo BK, Mallesh G (2014) Assessment of environmental radioactive elements in groundwater in parts of Nalgonda district, Andhra Pradesh, South India using scintillation detection methods. J Radioanal Nucl Chem 302(3):1391–1398
Kim JI (1986) Chemical behaviour of trans uranic elements in natural aquatic systems. In: Keller FAJ (ed) Handbook on the physics and chemistry of the actinides. Elsevier Science Publisher, Amsterdam, pp 413–455
Kim YS, Park HS, Kim JY, Park SK, Cho BW, Sung IH, Shin DC (2004) Health risk assessment for uranium in Korean groundwater. J Environ Radioact 77(1):77–85
Kochhar N, Gill GS, Tuli N, Dadwal V, Balaram V (2003) Chemical quality of groundwater in relation to incidence of cancer in parts of SW Punjab, India. Asian J Water Environ Pollut 4:107–112
Krestou A, Panias D (2004) Uranium (VI) speciation diagrams in the UO22+/CO32−/H2O system at 25°C. Eur J Miner Process Environ Prot 4(2):113–129 1303–0868
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Mäkeläinen I, Väisänen SB, Penttilä IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110(4):337–342
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim Cosmochim Acta 42(6):547–569
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall Inc, Upper Saddle River, p 600
Leggett RW (1989) The behaviour and chemical toxicity of U in the kidney: a reassessment. Health Phys 57(3):365–383
Mao Y, Desmeules M, Schaubel D, Berube D, Dyck R, Brule D, Thomas B (1995) Inorganic components of drinking water and microalbuminuria. Environ Res 71:135–140
McDonald-Taylor CK, Singh A, Gilman A (1997) Uranyl nitrate-induced proximal tubule alterations in rabbits: a quantitative analysis. J Toxicol Pathol 25(4):381–389
Meher PK, Sharma P, Khare A, Mishra KP (2015) Uranium in ground water of Eastern Uttar Pradesh, India: a preliminary study. Int Res J Environ Sci 4(6):70–74
Mehra R, Singh S, Singh K (2007) Uranium studies in water samples belong to malwa region in Punjab by track etching technique. Radiat Meas 42(3):441–445
Meinrath A, Schneider P, Meinrath G (2003) Uranium ores and depleted uranium in the environment, with a reference to uranium in the biosphere from the Erzgebirge/Sachsen, Germany. J Environ Radioact 64:175–193
Pant D, Keesari T, Sharma D, Rishi M, Singh G, Jaryal A, Tripathi RM (2017) Study on uranium contamination in groundwater of Faridkot and Muktsar districts of Punjab using stable isotopes of water. J Radioanal Nucl Chem 313(3):635–639
Piirainen T (1968) Die petrologie un die Uranlägerstatten desKoli-Kaltimogebiets in Finnischen Nordkarelien. Bulletindes Commentaires Geológiques Finlande 237:1–99
Porcelli D, Swarzenski PW (2003) The behavior of U- and Th-series nuclides in groundwater. Rev Mineral Geochem 52:317–361 (Uranium-Series Geochemistry)
Ramola RC, Singh S, Virk HS (1988) Uranium and radon estimation in some water samples from Himalayas Part D. Nucl Tracks Radiat Meas 15(1–4):791–793
Rani A, Singh S (2006) Analysis of uranium in drinking water samples using laser induced fluorimetry. Health Phys 91:101–107
Rani A, Mehra R, Duggal V, Balaram V (2013) Analysis of uranium concentration in drinking water samples using icpms. Health Phys 104(3):251–255
Rao SR, Shah SM (1976) Elemental contents in environmental samples. BARC, Mumbai
Rishi MS, Keesari T, Sharma DA, Pant D, Sinha UK (2017) Spatial trends in uranium distribution in groundwaters of Southwest Punjab, India-A hydrochemical perspective. J Radioanal Nucl Chem 311(3):1937–1945
Runde W (2000) The chemical interactions of actinides in the environment. Los Alamos Sci 26:392–411
Sahu SK, Maity S, Bhangare RC, Pandit GG, Sharma DN (2014) Determination of uranium in ground water using different analytical techniques Government of India Atomic Energy Commission, Bhabha Atomic Research Centre, Mumbai, BARC/2014/E/011, pp 30
Saric MR, Stojanovic M, Babic M (1995) Uranium in plant species grown on natural barren soil. J Plant Nutr 18:1509–15018
Sethy NK, Tripathi RM, Jha VN, Sahoo SK, Shukla AK, Puranik VD (2011) Assessment of natural uranium in the ground water around Jaduguda Uranium Mining Complex, India. J Environ Prot 2:1002–1007
Sharma DA, Rishi MS, Singh R, Pant D, Keesari T, Sinha UK (2017) Groundwater chemistry and uranium distribution in Southwest Punjab-findings from isotope hydrochemical study. In Proceedings of the thirteenth DAE-BRNS nuclear and radiochemistry symposium
Sharp JM (2014) Uranium distribution in ground water from fractured crystalline aquifers in Norway, Fractured rock hydrogeology. CRC Press, Boca Raton, p 271
Sheppard SC, Evenden WG, Pollock RJ (1989) Uptake of natural radionuclides by field and garden crops. Can J Soil Sci 69:751–767
Singh J, Singh L, Singh S (1995) High U-contents observed in some drinking waters of Punjab, India. J Environ Radioact 26(3):217–222
Singh P, Rana NPS, Azam A, Naqvi AH, Srivastava DS (1996) Levels of uranium in waters from some Indian cities determined by fission track analysis. Radiat Meas 26(5):683–687
Singh B, Singh G, Sandhu AS, Singh S (1999) Uranium estimation in water samples collected from some areas of Himachal Pradesh, India. Radiat Meas 31:683–685
Singh S, Malhotra R, Kumar J, Singh B, Singh L (2001) Uranium analysis of geological samples, water and plants from Kulu Area, Himachal Pradesh, India. Radiat Meas 34:427–431
Singh S, Rani A, Mahajan RK, Singh Walia TP (2003) Analysis of uranium and its correlation with some physicochemical properties of drinking water samples from Amritsar, Punjab. J Environ Monit 5:917–921
Singh H, Singh J, Singh S, Bajwa BS (2009a) Uranium concentration in drinking water samples using the SSNTDs. Indian J Phys 83(7):1039–1044
Singh J, Singh H, Singh S, Bajwa BS (2009b) Estimation of uranium and radon concentration in some drinking water samples of Upper Siwaliks, India. Environ Monit Assess 154:15–22. https://doi.org/10.1007/s10661-008-0373-8
Singh B, Kishore N, Garg VK, Pulhani V, Yadav P (2014) Uranium in groundwater from Western Haryana. J Radioanal Nucl Chem, India. https://doi.org/10.1007/s10967-014-3133-y
Talukdar BC, Chaudhary PK, Pathak KM (1983) On uranium concentration in water. J Pure Appl Phys 21:381–382
Tanner AB (1980) Radon migration in the ground: a supplementary review. In: Gesell TF, Lowder WM (eds) The natural radiation environment III, National Technical Information Services, Springfield. CONF-780422.1, pp 5–56
Thivya C, Chidambaram S, Thilagavathi R, Nepolian M, AdithyaV S (2014) Evaluation of drinking water quality index (DWQI) and its seasonal variations in hard rock aquifers of Madurai district, Tamilnadu. Int J Adv Geosci 2(2):48–52
Waite TD, Davis JA, Payne TE, Waychunas GA, Xu N (1994) Uranium(VI) adsorption to ferrihydrite: application of a surface complexation model. Geochim Cosmochim Acta 58(24):5465–5478
Wazne M, Korfiatis GP, Meng X (2003) Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide. Environ Sci Technol 37:3619–3624
White AF, Delany JM, Narasimhan TN, Smith A (1984) Groundwater contamination from an inactive uranium mill tailings pile: 1. Application of a chemical mixing model. Water Resour Res 20(11):1743–1752. https://doi.org/10.1029/WR020i011p01743
WHO (2004) Guidelines for drinking-water quality, recommendations, 3rd edn. World Health Organization, Geneva
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
WHO (World Health Organization) (2006) Guidelines for drinking water quality recommendations, vol 1, 3rd edn. WHO, Geneva, p 515
Zamora ML, Tracy BL, Zielinski JM, Meyerhof DP, Moss MA (1998) Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol Sci 43:68–77
Závodská L, Kosorínová E, Ščerbáková L, Lesný J (2008) Environmental chemistry of uranium. HEJ, ENV-081221-A, pp 1–19
Acknowledgements
The authors express their sincere thanks to the Board of Research in Nuclear Sciences (BRNS), India, for providing the necessary financial support to this Research Project vide Ref. No. 2012/35/12/BRNS/1918 dated 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adithya, V.S., Chidambaram, S., Keesari, T. et al. Occurrence of Uranium in Groundwater Along the Lithological Contacts in Central Tamilnadu, India: An Isotope Hydrogeochemical Perspective. Expo Health 11, 277–290 (2019). https://doi.org/10.1007/s12403-017-0269-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-017-0269-3