Abstract
Tumors such as prostate, small cell lung cancer, breast, gastric and colon cancer are known to overexpress receptors to bombesin (BBN). In this study, a new bombesin analogue was labeled with 99mTc via HYNIC and tricine/EDDA as coligands and investigated further. HYNIC-GABA-Bombesin (7–14) NH2 was synthesized using a standard Fmoc strategy. Labeling with 99mTc was performed at 100 °C for 10 min and radiochemical analysis involved ITLC and HPLC methods. The stability of radiopeptide was checked in the presence of humane serum at 37 °C up to 24 h. The receptor bound internalization and externalization rates were studied in GRP receptor expressing PC-3 cells. Biodistribution of radiopeptide was studied in nude mice bearing PC-3 tumor. Labeling yield of >98% was obtained corresponding to a specific activity of ~2.6 MBq/nmol. Peptide conjugate showed good stability in the presence of human serum. The radioligand showed high and specific internalization into PC-3 cells (14.63 ± 0.41% at 4 h). In biodistribution studies, a receptor-specific uptake was observed in GRP-receptor-positive organs so that after 4 h the uptakes in mouse tumor and pancreas were 1.31 ± 0.18 and 1.2 ± 0.13% ID/g, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A lot of malignant human cancer cells overexpress different receptors on their cell surfaces. These receptors have become important and useful as targets for molecular imaging and therapy of tumors [1, 2]. Several receptors such as somatostatin, neurotensin and bombesin receptors have attracted considerable interest in recent years [3]. Bombesin is a 14-aminoacid peptide isolated from frog skin [4]. The mammalian counterparts of the frog peptide are neuromedin B (NMB) and gastrin-releasing peptide (GRP). Over-expression of receptors for both NMB and GRP have been reported to be found on the cell surfaces of several malignant tissues, particularly in the cancers like prostate [5, 6], breast [7, 8], colon [9], and small cell lung cancer [10]. Therefore the presence of GRP receptor may form a molecular basis for diagnosis and treatment of relevant tumors by GRP receptor targeted imaging.
Since this time, several exciting new approaches toward design of specific diagnostic or therapeutic radiopharmaceuticals that target the GRP receptor have occurred. Bombesin analogs have been radiolabeled with different radionuclides including 111In [11–13], 68Ga [14, 15], 64Cu [16–19], 18F [14, 15, 20], 125I [21–23], 177Lu [12, 24–26], 90Y [12] and 188Re [27–29] and 99mTc [30, 31]. 99mTc based conjugates of bombesin and other peptide derivatives continue to be attractive due to the availability of low cost 99Mo/99mTc generator, favorable physical characteristics of 99mTc (t 1/2 of 6 h, γ 140 keV 89% abundance) and high specific activity of radionuclide. Recently, several new BBN conjugates radiolabeled with 99mTc have been reported [32]. Radiolabeling has been performed either directly or indirectly via a ligand including N3S [33], N2S2 [34–36], N4 [37, 38], Hydrazino nicotinic acid (HYNIC) and carbonyl [39–43]. Lin et al. [36] reported a conjugate contains of the DTPA as a pharmacokinetic modifier on the N-terminus of the BBN conjugate and a diaminodithiol ligand for metal coordination. This new 99mTc-based bombesin conjugate demonstrated high affinity for GRP receptor on PC-3 cells and was excreted primarily via the renal/urinary pathway. Also Alves et al. [43] reported a hydrophilic 99mTc-BBN conjugate that utilizes a tridentate pyrazolyl ligand to stabilize the 99mTc metal center after labeling with [99mTc(CO)3(H2O)3]+ by heating. This new conjugate cleared efficiently from the bloodstream via the renal and was specify for GRP receptors. Recently Kunstler et al. [44] reported 99mTc-BBN (7–14) conjugates labeled using the novel ‘4 + 1’ mixed ligand system, which Tc is coordinated by a monodentate isocyanide linker bearing the peptide and the tetradentate chelator NS3. Biodistribution studies showed rapid hepatobiliary excretion of the conjugates and the low uptake in target specific pancreatic tissue. We should consider various limitations are associated with some of the above labeling procedure, such as low radiochemical yield, extended reaction time, high temperature, high pH values, and high lipophilicity of the radiometal chelate.
In recent years one of the bifunctional chelating ligand that has received considerable interest is HYNIC. The use of the 99mTc-HYNIC core was first reported for the labeling of IgG and since then, has been conjugated to various biomolecules including antibodies and peptides [45]. Since HYNIC could only occupy one or two coordination position on the radionuclide, coligands are necessary to complete the coordination sphere of the technetium core [45]. Usually a co-ligand, such as tricine or ethylenediamine diacetic acid (EDDA), is included in the 99mTc labeling of HYNIC conjugates. The co-ligand can sometimes play an important role in the stability, lipophilicity, clearance property and protein binding potency of the radiolabels. For example, while providing higher labeling efficiency, the tricine coligand also provides higher non-specific protein binding potential over the EDDA complexes. In labeling process coligands tricine and EDDA can be used separately or together via exchange labeling method. Yurt Lambrecht et al. [46] recently described optimization of radiolabeling condition for a bombesin like peptide (Q-Litorin) based on HYNIC using EDDA or tricine as a separate coligand. They demonstrated that it is possible to produce Litorin based radiolabeling agents 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin at high yields. Faintuch and co-workers [45] have investigated the effect of coligand and spacer length on design and development of new 99mTc-HYNIC-BBN derivatives. The conjugates prepared by tricine/EDDA exchange labeling showed relatively high specific activity and demonstrated excellent radiochemical stability even out to 24 h post incubation. As we know in development of new BBN analogs 99mTc-chelator complex could coupled directly or via a spacer group to the N-terminus of the peptide. It has been shown the best results achieved by conjugates which 99mTc complex placed at a certain distance from the N-terminus of peptide [45].
We also recently reported the evaluation of a new somatostatin analogues labeled via bifunctional chelating agents HYNIC and EDDA/tricine as coligands [47]. In continuation of our efforts to create a new 99mTc-labelled peptide for tumor targeting, lately we prepared a bombesin peptide with HYNIC conjugation and labeled it to a 99mTc solution with a high specific activity using tricine as coligand [48, 49]. The radiolabeled bombesin internalized rapidly into GRP receptor positive PC3-tumor cells. To extend our previous study and to increase the stability and hydrophilicity, and in order to improve the tumor uptake of the labeled peptide in tumor bearing mice we prepared a EDDA/tricine complex of 99mTc-HYNIC-Bombesin (7–14) peptide which gamma amino butyric acid (GABA) as a three carbon chain spacer was between HYNIC and N-terminus of the peptide.
Here we present data on the synthesis of HYNIC-GABA-Bombesin (7–14) NH2 and describe optimum condition for radiolabeling of conjugate with 99mTc using tricine/EDDA as coligands. In addition we studied stability in human serum, receptor bound internalization, efflux in PC-3 cells and in vivo tumor uptake and tissue biodistribution of radiolabeled compound.
Experimental
Materials and methods
Rink amide MBHA (4-methylbenzhydrylamine) resin and all of the Fmoc-protected amino acids were commercially available from NovaBiochem (Laufelfingen, Switzerland). The prochelator HYNIC-Boc was synthesized according to Abrams et al. [50]. Other reagents were purchased from Fluka, and used without further purification.
The reactive side chains of the amino acids were masked with one of the following groups: Trp, t-butoxycarbonyl (Boc); His and Gln, Triphenylmethyl (Trt). The cell culture medium was Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), amino acids, vitamins and penicillin/streptomycin from Gibco. Sodium pertechnetate (Na99mTcO4) obtained from commercial 99Mo/99mTc generator (Radioisotope Division, Atomic Energy Organization of Iran). Analytical reverse phase high performance liquid chromatography (RP-HPLC) was performed on a JASCO 880-PU intelligent pump HPLC system equipped with a multiwavelength detector and a flow-through Raytest-Gabi γ-detector. CC 250/4.6 Nucleosil 120-5 C18 column from Teknokroma was used for analytical HPLC, and a VP 250/10 Nucleosil 100-5 C18 column was used for semipreparative HPLC. The gradient systems consisted of 0.1% trifluoroacetic acid/water (Solvent A) and acetonitrile (Solvent B). For analytical HPLC, Gradient I was used: 0 min 95% A (5% B), 5 min 95% A (5% B), 25 min 0% A (100% B), 27 min 0% A (100% B), 30 min 95% A (5% B), flow = 1 mL/min, γ = 280 nm; for semipreparative HPLC Gradient II: 0 min 80% A (20% B), 2 min 80% A (20% B), 17 min 50% A (50% B), 19 min 0% A (100% B), 21 min 0% A (100% B), 25 min 80% A (20% B), flow = 2 mL/min, γ = 280 nm. Mass spectrum was recorded on an Agilent 1100/Bruker Daltonic (Ion trap) VL instrument (LC/MS). Quantitative gamma counting was performed on an EG&G/ORTEC (Ametek; Advanced Measurement Technology Division) Model 4001M Mini Bin & Power Supply counter.
Synthesis
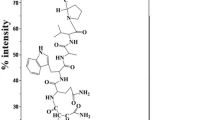
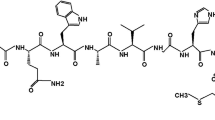
The peptide was synthesized by standard Fmoc solid phase synthesis on Rink Amide MBHA resin with substitution, 0.69 mmol/g. Coupling of each amino acid was performed in the presence of 3 mol excess of Fmoc-amino acid, 3 mol excess of N-hydroxybenzotriazole (HOBt), 3 mol excess of diisopropylcarbodiimide (DIC) and 5 mol excess of diisopropylamine (DIPEA) in dimethylformamide (DMF). Completeness of coupling reactions was monitored by the Kaiser test and the Fmoc groups were removed by adding 20% piperidine in DMF. Coupling of HYNIC to peptide was performed in the presence of 1.2 mol excess of HYNIC-Boc 2.5 mol excess of (2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) (HATU), 5 mol excess of diisopropyletylamine (DIPEA) in dimethylformamide (DMF). The peptide HYNIC conjugate was removed from the resin and amino acid side chains were also deprotected by treatment with a cocktail of trifluoroaceticacid (TFA), triisopropylsilane and water (95:2.5:2.5). After removing the organic solvents in vacuum, the crude product was precipitated with cold petroleum ether and diisopropyl ether (50:50). The crude peptide HYNIC conjugate was dissolved in water/methanol and purified by semi-preparative RP-HPLC; then the purified product was characterized by LC/MS and analytical HPLC.
Labeling of HYNIC-GABA-Bombesin (7–14) NH2 with 99mTc
A stock solution of HYNIC-GABA-Bombesin (7–14) NH2 (concentration 1 mmol/L) was prepared by dissolving the peptide in distilled water. Radiolabeling of peptide was performed by adding 17.24 μL of the stock solution (20 μg of peptide), 15 mg of tricine and 5 mg of EDDA co-ligands in 0.5 mL of water. To this solution was added 40 μg SnCl2 (20 μL of 2 mg/mL SnCl2, 2H2O in nitrogen-purged 0.1 M HCl). Finally, 370–1,480 MBq of 99mTcO4 − in 0.5 mL saline was added to the solution and incubated for 10 min at 100 °C. After cooling down to room temperature the preparation was checked for bound and free 99mTc.
Radiochemical analysis of 99mTc-labeled HYNIC-GABA-Bombesin (7–14) NH2
99mTc-labeled HYNIC-GABA-Bombesin (7–14) NH2 was characterized by analytical RP-HPLC (gradient I) and ITLC on silica gel 60 (Merck) using different mobile phases: 2-butanone for free 99mTcO4− (Rf = 1), 0.1 M sodium citrate (pH = 5) to determine the non-peptide bound 99mTc coligand and 99mTcO4− (Rf = 1) and methanol/1 M ammonium acetate 1/1 for 99mTc colloid (Rf = 0). The radioactivity was quantified by cutting the strip (1.5 × 10 cm2) into 1 cm pieces and counting in a well type gamma counter.
Serum stability
To 1 mL of freshly prepared human serum, we added 86 MBq 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 and mixture was incubated in 37 °C environment. At different time points, 100 μL aliquots was removed and treated with 100 μL of alcohol. Sample was centrifuged for 15 min at 3,000 rpm to precipitate serum proteins. Supernatant was removed and activity in the supernatant compared with the activity in sediment to give the percentage of radiopeptide or radiometal bound or transferred to the serum proteins. Supernatant was analyzed with HPLC Gradient I to determine the stability of labeled compound.
Cell culture
The PC-3 cells were cultured in DMEM supplemented with 10% FBS, 2 mM glutamine and penicillin–streptomycin. Cells were maintained in a humidified 5% CO2/air atmosphere at 37 °C. For all cell experiments, the cells were seeded at a density of 1 million cells per well in six-well plates and incubated over night with internalization medium (DMEM with 1% FBS).
Internalization and nonspecific membrane binding
Medium was removed from the six-well plates contain PC-3 cells with density of 1 million cells per well and cells were washed once with 2 mL of internalization medium (DMEM with 1% FBS). Furthermore, 1.5 mL internalization medium was added to each well, and the plates were incubated at 37 °C for about 1 h. Afterwards, about 150 kBq (2.5 pmol total peptide mass per well) was added to the medium, and the cells were incubated at 37 °C for various time periods. To determine nonspecific membrane binding and internalization, we incubated cells with the radioligand in the presence of 150 μL, 1 μmol/L bombesin. The cellular uptake was stopped at appropriate time periods (30 min, 1, 2 and 4 h) by removing medium from the cells and washing twice with 1 mL of ice-cold phosphate-buffered saline (PBS). An acid wash for 10 min with a glycine buffer (pH = 2.8) on ice was also performed twice. This step was to distinguish between membrane-bound (acid releasable) and internalized (acid resistant) radioligand. Finally, the cells were treated with 1 N NaOH. The culture medium and the receptor-bound and internalized fractions for both with and without cold peptide were measured radiometrically in a gamma counter.
Externalization
For externalization studies, the PC-3 cells (106/well) were incubated with radioligand. After 2 h internalization at 37 °C and 5% CO2, the medium was removed and the cells were washed twice with 1 mL ice cold PBS. Acid wash for a period of 5 min twice with a glycine buffer of pH 2.8 was done to remove the receptor bound ligand. Cells were then incubated again at 37 °C with fresh internalization medium. After different time points (15, 30 min, 1, 2 and 4 h), the external mediums were removed for quantification of radioactivity in a gamma counter. The cells were solubilized in 1 N NaOH and removed, and the internalized radioactivity was quantified in a gamma counter. The externalized fraction was expressed as percentage of the total internalized amount per 1 million cells.
Biodistribution
Animal experiments were performed in compliance with the regulations of our institution and with generally accepted guidelines governing such work. A suspension of human PC-3 cells (1 × 107) in PBS buffer was subcutaneously injected in the right flank of each nude mouse. Seven to 10 days after inoculation, the tumors were inducted and then, an activity of 20 MBq (0.35 nmol) of 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 was injected via the femoral vein. In order to determine the non-specific uptake of the radiopeptides, in receptor-positive organs, a group of three animals were injected with 100 μg cold peptide in 50 μL saline as a co-injection with the radiopeptides (blocked animals). After 1, 4 and 24 h, the mice in groups of three animals were killed, organs of interest were collected, weighed and radioactivity was measured in a gamma-counter. The percentage of the injected dose per gram (% ID/g) was calculated for each tissue.
Statistical methods
The calculations of means and standard deviations for internalization and biodistribution were performed on Microsoft Excel. Student’s t test was used to determine statistical significance. Differences at the 95% confidence level (p < 0.05) were considered significant [48].
Results
Synthesis
HYNIC-GABA-Bombesin (7–14) NH2 was synthesized by Fmoc strategy supplying an overall yield of 45% based on the removal of the first Fmoc group after cleavage, purification and lyophilization (Fig. 1). The composition and structural identity of purified HYNIC-peptide was verified by analytical HPLC and LC–MS (Table 1). The purity was 97.3% as confirmed by HPLC method.
Radiolabeling
Radiochemical purity of 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 conjugate was evaluated by RP-HPLC using the gradient systems consisted of 0.1% trifluoroacetic acid/water (Solvent A) and acetonitrile (Solvent B). The labeling yield of 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 was >98%, acquired via HPLC and also ITLC at a specific activity of 80 GBq/μmol. The HPLC elution times (Gradient I) were 4.27 min for 99mTcO4 − and 15.05 min for 99mTc-peptide (Fig. 2).
In vitro internalization and stability
Figure 3 shows the result in respect of the time-dependent and specific internalization of the radioligand into PC-3 cells. During 60 min, the radioligand showed 4.99 ± 0.95% specific cell uptake, which increased to 14.63 ± 0.41% up to 4 h. In all experiments, the internalization was strongly reduced in the presence of excess cold. In fact, nonspecific internalization was 0.87 ± 0.22% after 4 h, and the surface-bound peptide (acid removable) was 1.3 ± 0.43% of the added activity after 4 h.
In stability study the protein fraction obtained in sedimentation form contained 8.9 ± 1.4% of radioactivity. Up to 24 h incubation in human serum the radiochemical purity remained >90%.
Externalization
The externalization in PC-3 cells was evaluated after 2 h of internalization of the radioligand (Fig. 4). After 15 min, 15.67 ± 1.32% of radioactivity was externalized, which increased to 50.31 ± 1.65% at 4 h. With more time, the percentage of externalization reaches a plateau.
Animal biodistribution
Results from biodistribution studies using the 99mTc-labeled peptide are presented in Table 2 as the percentage of injected dose per gram of tissue (% ID/g). 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 displayed rapid blood clearance with 0.11 ± 0.02% ID/g at 4 h. Fast clearance from the GRP receptor-negative tissues except the kidneys was found as well. Labeled peptide shows high uptake values in the PC-3 tumor and in the GRP receptor-positive organs. By blocking the receptor through prior injection of cold peptide, the uptake in tumor and pancreas is diminished and this confirms the specificity of radioconjugate. Reduction uptake were 77% (1.31 vs. 0.29% ID/g at 4 h) and 89% (1.2 vs. 0.13% ID/g at 4 h), respectively (Fig. 5). On the other hand, the uptake reduction in non-targeted tissues due to blocking dose was not significant.
Discussion
GRP receptors were overexpressed on a variety of human tumors. Based on this fact, and the experience with other peptides like somatostatin [47] we conclude that targeting the GRP receptor with optimized analogue of bombesin is very important for imaging of prostate and breast tumors. As we previously reported Bombesin analog [99mTc-HYNIC0, D-Tyr6, D-Trp8] Bombesin (6–14) NH2 is capable of visualizing GRP receptor positive tumors in vivo [48, 49]. In the present study, we investigated a new HYNIC coupled bombesin analog with sequences bombesin (7–14) without any replacement. In order to improve binding affinity, we attached HYNIC chelator via a GABA spacer to the N-terminus of bombesin (7–14) peptide.
A variety of chelators have been used as bifunctional chelating agent (BFCA) in labeling proteins, peptides and other biologically active molecule with 99mTc. These include N3S triamidethiols [51], N2S2 diamidedithiols [52], N2S2 diaminedithiols [53], PnAo propyleneamine oxime [54], tetramines [55] BATOs [56] and HYNIC [57]. Among these, HYNIC is the best candidate because we can achieve labeling in high specific activity followed by using various coligands, which permit control of the hydrophilicity and pharmacokinetics of the labeled peptide [12, 24, 50, 58–65]. In the group of different coligands, tricine gives the best radiolabeling efficiency. However, it have been reported that with tricine as a coligand 99mTc-complex was not stable, particularly in dilute solutions, due to different bonding modalities of the hydrazine moiety of the HYNIC and the tricine coligand [48–50, 59]. The coligand EDDA is also of particular interest because it is a potentially tetradentate ligand and is expected to form a more symmetrical and stable complex with technetium when compared to tricine [48–50, 59]. It has been shown that using both coligands together to produce 99mTc-HYNIC-peptide via a trans-metallation type of reaction, produces very good results [61]. In this study we used 20 μg of HYNIC-peptide with tricine and EDDA together as a coligand in amounts of 15 and 5 mg in final volume of labeled solution, respectively. We obtained high radiochemical yield >98% with very low amount of 99mTc-pertechnetate (<0.2) 99mTc-radiocolloid (<0.5) and 99mTc-coligands (<1.0). In RP-HPLC analysis we observed a single major peak without any impurities due to isomeric forms of the new 99mTc-HYNIC conjugate. In comparison to those report regarding 99mTc/tricine-HYNIC complex instability [64], our new labeled peptide conjugate was stable up to a 24 h post labeling period in the room temperature. These high labeling yield and stability may be due to optimization of formulation in amount of materials and also in our exchange labeling method.
High rate of internalization was observed in this conjugate (14.63 ± 0.41% up to 4 h) which was not unexpected since Bombesin (7–14) NH2 sequence offers agonistic property to compound. Besides efflux curve of 99mTc-HYNIC-GABA-Bombesin (7–14) NH2 in PC-3 cells after 2 h of internalization showed an acceptable intercellular trapping. Pervious studies of 111In-DOTA-8-Aoc-BBN (7–14) NH2 [39] and [111In]-DTPA or [111In]-DOTA-GABA-[D, Tyr6, β-Ala11, Thi13, Nle14] BN (6–14) [BZH1 and BZH2] [12] also demonstrate internalization and receptor mediated trapping of labeled compounds. Compare with our pervious compound [99mTc/tricine/HYNIC0, D-Tyr6, D-Trp8] BN (6–14) NH2 [48, 49] this new analog showed higher rate of internalization after 4 h in PC-3 cells (14.63 ± 0.41 vs. 10.7 ± 1.2%). It could be due to replacement of a three carbon chain spacer (GABA) instead of D-Tyr6. It also could be explained that by placing a bifunctional chelating agent farther from the receptor binding region of peptide, the negative effect of chelator on the receptor binding is reduced. It has been shown that positive charge in sequence of peptide tends to interact faster and in an effective way with proteins, besides that is not targeted to a specific receptor [64]. Considering a fast and receptor specific internalization which was demonstrated with uptake results in GRPr blocked cell experiments is an indication of balance of charges for the complex.
Our BBN analog showed metabolic stability in human serum up to 24 h after labeling and incubation. Results from Hanwen et al. [12] show relatively low metabolic stability for [111In]-BZH1 and [111In]-BZH2. They found two degradation sites in their peptides sequences, one between β-Ala11 and His12 and another between Gln7 and Trp8. Also the study by M. de Visser et al. [65] shows that changes in the bombesin amino acid sequence can have a marked effect on the peptides stability. They also found that the substitution of native amino acids in bombesin sequences can enhance receptor affinity but not the serum stability. The stability of our compound could be attributed to the use of intact sequence of bombesin (7–14) NH2 and directly connected GABA spacer to Gln7 in the sequences.
In biodistribution studies, clearance from the blood circulation was fast with <0.14% ID/g remaining in the blood at 4 h and the whole body clearance proceeded via the urinary system. Clearance from GRP receptor negative tissues was also rapid except from the kidneys. Accumulation of radiopeptide in bombesin receptor positive tissues like the pancreas, the stomach, the intestines and the xenografted tumor was observed. Compared with our pervious study [48, 49], the uptake of radioactivity in pancreas increased as the lipophilicity of the conjugate increased with the replacement of a three carbon chain spacer instead of D-Tyr6 (1.51 ± 0.15 vs. 1.04 ± 0.11% ID/g at 1 h). The uptake in tumor, pancreas and intestine was specific and receptor mediated, as shown by the co-injection of cold peptide, indicating that these organs are also GRP receptor positive.
The tumor and pancreas accumulation of this radioconjugate and its good pharmacokinetic behavior such as low tendency to accumulate in liver and intestine and its high kidney excretion due to moderate lipophilicity are the major advantages of this compound.
Conclusion
In this study, we have shown synthesis and radiolabeling of HYNIC-GABA-Bombesin (7–14) NH2. The labeling was completed within a very short time in high specific activity (~80.9 GBq/μmol). Furthermore, this conjugates prepared by labeling of peptide, GABA, HYNIC and tricine/EDDA as a coligands demonstrated an excellent radiochemical stability even up to 24 h post labeling. Our new labeled conjugate had a specific cell binding and internalization followed by a good stability in human serum at 37 °C for at least 24 h and no significant impurities were detected by HPLC. The prepared conjugate showed high accumulation in tumor and pancreas as a positive GRP receptors targeted tissues followed by excretion via the kidney. These promising characteristics make our new designed labeled peptide conjugate as a very suitable candidate for diagnostic of malignant tumors.
References
Okarvi SM (2004) Med Res Rev 24(3):357
Hoffman TJ, Quinn TP, Volkert WA (2001) Nucl Med Biol 28(5):527
Reubi JC (2003) Endocr Rev 24:389
Anastasi A, Erspamer V, Bucci M (1971) Experientia 27(2):166
Markwalder R, Reubi JC (1999) Cancer Res 59:1152
Sun B, Halmos G, Schally AV, Wang X, Martinez M (2000) Prostate 42:295
Gugger M, Reubi JC (1999) Am J Pathol 155:2067
Halmos G, Wittliff JL, Schally AV (1995) Cancer Res 55:280
Preston SR, Woodhouse LF, Jones-Blackett Miller GV, Primrose JN (1995) Br J Cancer 71:1097
Toi-Scott M, Jones CL, Kane MA (1996) Lung Cancer 15:341
Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, Volkert WA (2003) J Nucl Med 44:823
Zhang H, Chen J, Waldherr C, Hinni K, Waser B, Reubi JC, Maecke HR (2004) Cancer Res 64:6707
Maina T, Nock BA, Zhang H, Nikolopoulou A, Waser B, Reubi JC, Maecke HR (2005) J Nucl Med 46:823
Meyer GJ, Maecke HR, Schuhmacher J, Knapp WH, Hofmann M (2004) Eur J Nucl Med 31:1097
Schuhmacher J, Zhang H, Doll J, Maecke HR, Matys R, Hauser H, Henze M, Haberkorn U, Eisenhut M (2005) J Nucl Med 46:691
Rogers BE, Bigott HM, McCarthy DW, Manna DD, Kim J, Sharp TL, Welch MJ (2003) Bioconjug Chem 14:756
Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, Conti PS (2004) J Nucl Med 45:1390
Parry JJ, Andrews R, Rogers BE (2007) Breast Cancer Res Treat 101:175
Yang Y, Zhang X, Xiong Z, Chen X (2006) Nucl Med Biol 33:371
Zhang X, Cai W, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen X (2006) J Nucl Med 47:492
Rogers BE, Rosenfeld ME, Khazaeli MB, Mikheeva G, Stackhouse MA, Liu T, Curiel DT, Buchsbaum DJ (1997) J Nucl Med 38:1221
Rogers BE, Curiel DT, Mayo MS, Laffoon KK, Bright SJ, Buchsbaum DJ (1997) Cancer 89:2419
Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M (2002) Clin Cancer Res 8:1139
Smith CJ, Gali H, Sieckman GL, Hayes DL, Owen NK, Mazuru DG, Volkert WA, Hoffman TJ (2003) Nucl Med Biol 30:101
Waser B, Eltschinger V, Linder K, Nunn A, Reubi JC (2007) Eur J Nucl Med 34:95
Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, Thomas R, Eaton SM, Bogdan NJ, Arunachalam T, Reubi JC, Raju N, Metcalfe EC, Luttuada L, Linder KE, Swenson RE, Tweedle MF, Nunn AD (2006) J Nucl Med 47:1144
Safavy A, Khazaeli MB, Qin H, Buchsbaum DJ (1997) Cancer 89(12 Suppl):2354
Smith CJ, Sieckman GL, Owen NK, Hayes D, Mazuru DG, Volkert WA, Hoffman TJ (2003) Anticancer Res 23:63
Moustapha ME, Ehrhardt GJ, Smith CJ, Szajek LP, Eckelman WC, Jurisson SS (2006) Nucl Med Biol 33:81
Karra SR, Schibli R, Gali H, Katti KV, Hoffman TJ, Higginbotham C, Sieckman GL, Volkert WA (1999) Bioconjug Chem 10:254
Van de Wiele C, Dumont F, Dierckx RA, Peers SH, Thornback JR, Slegers G, Thierens H (2001) J Nucl Med 42:1722
Scopinaro F, Vincentis GD, Varvarigou AD (2005) J Clin Oncol 23(13):3170
Block D, Feitsma HIJ, Kooy YMC, Welling MM, Ossendorp F, Vermeij P, Drijfhout JW (2004) Nucl Med Biol 31:815
Baidoo KE, Lin K, Zhan Y, Finley P, Scheffel U, Wagner HN (1998) Bioconjug Chem 9:218
Lin K, Luu A, Baidoo KE, Hashemzadeh-Gargari H, Chen MK, Pili R, Pomper M, Carducci M, Wagner HN (2004) Bioconjug Chem 15:1416
Lin K, Luu A, Baidoo KE, Hashemzadeh-Gargari H, Chen MK, Brenneman K, Pili R, Pomper M, Carducci MA, Wagner HN (2005) Bioconjug Chem 16:43
Nock B, Nikolopoulou A, Chiotellis E, Loudos G, Maintas D, Reubi JC, Maina T (2003) Eur J Nucl Med 30:247
Nock B, Nikolopoulou A, Galanis A, Cordopatis P, Waser B, Reubi JC, Maina T (2005) J Med Chem 48:100
La Bella R, Garcia-Garayoa E, Bahler M, Blauenstein P, Schibli R, Conrath P, Tourwe D, Schubiger PA (2002) Bioconjug Chem 13:599
La Bella R, Garcia-Garayoa E, Langer M, Blauenstein P, Beck-Sickinger AG, Schubiger PA (2002) Nucl Med Biol 29:553
Smith CJ, Sieckman GL, Owen NK, Hayes DL, Mazuru DG, Kannan R, Volkert WA, Hoffman TJ (2003) Cancer Res 63:4082
Blauenstein P, Garcia-Garayoa EG, Ruegg D, Blanc A, Tourwe D, Beck-Sickinger A, Schubiger PA (2004) Cancer Biother Radiopharm 19:181
Alves S, Paulo A, Correia JDG, Gano L, Smith CJ, Hoffman TJ, Santos I (2005) Bioconjug Chem 16:438
Kunstler JU, Veerendra B, Figueroa SD, Sieckman GL, Rold TL, Hoffman TJ, Smith CJ, Pietzsch HJ (2007) Bioconjug Chem 18(5):1651
Faintuch BL, Santos RLSR, Souza ALFM, Hoffman TJ, Greeley M, Smith CJ (2005) Chemistry 35:43
Lambrecht FY, Durkan K, Bayrak E (2010) J Radioanal Nucl Chem 284:539
Gandomkar M, Najafi R, Shafiei M, Mazidi M, Ebrahimi SES (2007) Nucl Med Biol 34:651
Sadeghzadeh N, Gandomkar M, Najafi R, Shafiei M, Sadat Ebrahimi SE, Shafiee A, Larijani B (2010) J Radioanal Nucl Chem 283:181
Sadeghzadeh N, Gandomkar M, Shafiee M, Mazidi M, Goudarzi M, Mirfallah SM, Sadat Ebrahimi SE (2009) Iran J Nucl Med 17(1):18
Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss W, Fischman AJ (1990) J Nucl Med 31:2022
Liu S, Edwards DS, Looby RJ, Poirier MJ, Rajopadhye M, Bourque JP, Carroll TR (1996) Bioconjug Chem 7(2):196
Fritzberg AR, Abrams PG, Beaumier PL, Kasina S, Morgan AC, Rao TN, Reno JM, Sanderson JA, Srinivasan A, Wilbur DS, Vanderheyden JL (1988) Proc Natl Acad Sci USA 85:4025
Kasina S, Rao T, Srinivasan A, Sanderson JA, Fitzner JN, Reno JM, Beaumier PL, Fritzberg AR (1991) J Nucl Med 32:1445
Maina T, Stolz B, Albert R, Bruns C, Koch P, Maecke HR (1994) Eur J Nucl Med 21(5):437
Maina T, Stolz B, Albert R, Bruns C, Koch P, Maecke H R (1995) In: Nicolini M, Badoli G, Mazzi U (eds) Technetium and rhenium in chemistry and nuclear medicine. SGE Editoriali, Padova, p 395
Linder KE, Wen MD, Nowotnik DP, Malley MF, Gougoutas JZ, Nunn AD, Eckelman WC (1991) Bioconjug Chem 2(3):160
Babich JW, Solomon H, Pike MC, Kroon D, Graham W, Abrams MJ, Tompkins RG (1993) J Nucl Med 34:1964
Scopinaro F, De Vincentis G, Varvarigou AD, Laurenti C, Iori F, Remediani S (2003) Eur J Nucl Med Mol Imaging 30:1378
Liu S, Edwards DS, Looby RJ, Harris AR, Poirier MJ, Barrett JA, Heminway SJ, Carrol TR (1996) Bioconjug Chem 7:63
Liu S, Edwards DS, Barrett JA (1997) Bioconjug Chem 8:621
Gabriel M, Froehlich F, Decristoforo C, Ensinger C, Donnemiller E, von Guggenberg E, Heute D, Moncayo R (2004) Eur J Nucl Med Mol Imaging 31:330
Plachcinska A, Mikolajczak R, Maecke HR, Miodkowska E, Kunert-Radek J, Michalski A, Rzeszutek K, Kozak J, Kusmierek J (2003) Eur J Nucl Med 30:1402
von Guggenberg E, Behe M, Behr T, Saurer M, Seppi T, Decristoforo C (2004) Bioconjug Chem 15:864
Babich JW, Fischman AJ (1995) Nucl Med Biol 22(1):25
de Visser M, Bernard HF, Erion JL, Schmidt MA, Srinivasan A, Waser B, Reubi JC, Krenning EP, de Jong M (2007) Eur J Nucl Med Mol Imaging 34(8):1228
Acknowledgments
The authors wish to thank Mr. Mirfallah and Mr. Talebi of the radioisotope department (AEOI) for providing sodium pertechnetate and assistance in quality control tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirmardi, S.P., Gandomkar, M., Mazidi, M. et al. Synthesis and evaluation of a new bombesin analog labeled with 99mTc as a GRP receptor imaging agent. J Radioanal Nucl Chem 288, 327–335 (2011). https://doi.org/10.1007/s10967-011-0985-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-0985-2