Abstract
Bombesin (BNN)-like peptides have very high binding affinity for the gastrin-releasing peptide (GRP) receptor. The goal of the current study was to optimize the labeling conditions of a new 99mTc-radiolabeled BNN-like peptide based on the bifunctional chelating ligand HYNIC using different co-ligands (EDDA and tricine). The radiolabeling conditions (pH, amount of co-ligand, amount of stannous chloride, temperature and reaction time) for newly-formed 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin were optimized and evaluated by RHPLC and RTLC. Radiochemical yields for 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin were 98.0 ± 1.7 and 97.5 ± 2.5%, respectively. When EDDA was used as co-ligand, the labeling of 99mTc-EDDA-HYNIC-Q-Litorin was optimal in the following reaction mixture: HYNIC-peptide: EDDA: 10 μg/5 mg, pH 3, SnCl2 concentration: 12 μg/0.1 mL, reaction temperature: 100 °C, reaction time: 15 min. Besides, the optimum conditions were HYNIC-peptide:tricine: 10 μg/50 mg, pH 5, SnCl2 concentration: 12 μg/0.1 mL, reaction temperature: 100 °C, reaction time: 15 min for preparing 99mTc-tricine-HYNIC-Q-Litorin. The manufactured 99mTc-HYNIC-Q-Litorin conjugates may offer new possibilities for imaging cancer cells expressing bombesin receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small radiolabeled peptides have become an important class of radiopharmaceuticals for diagnostic tumor imaging and other diseases in nuclear medicine. Technetium-99m (99mTc) is one of these highly used isotopes with sufficiently long half life and hence wider commercial availability [1–8]. To effectively deliver 99mTc to the targeted cells, the isotope is attached to a peptide specific to a particular receptor expressed in the cells. For example, gastrin releasing peptide receptor (GRPr) is expressed in several human tissues such as breast, prostate, lung and pancreatic cancer. As a promising class of ligands to GRPr, bombesin (BNN) or BNN-like peptide such as litorin function as growth stimulant and therefore plays an important role in carcinogenesis [1, 9–11]. In our preliminary study, we have shown that the compound obtained by simply labeling litorin with 99mTc (99mTc-litorin) has been highly uptaken by the pancreas in normal rats [11]. This preliminary study demonstrated potential of litorin in radiopharmacetical studies and justified further work.
Another way of labeling a BNN-analogue peptide with 99mTc is to use a bifunctional chelating agent (BFCA) conjugated to a peptide. This configuration provides a high specific activity at the binding site for Technetium. A variety of BFCAs have been developed and currently available [6–8, 12–21]. One of the attractive BFCAs is 6-hydrazino nicotinatic acid (HYNIC). HYNIC is attached to the amino group at the N-terminus of the BNN-like peptide via solid phase peptide synthesis method. HYNIC makes high efficiency labeling with 99mTc possible, and the final product very often requires no purification and exhibits high specific activity even with very low concentrations of the peptide [19, 22]. Chelating HYNIC with 99mTc requires additional co-ligands such as tricine or ethylenediamine-N,N′-diacetic acid (EDDA) [6, 21]. The HYNIC chelated BNN-like peptides were shown to exhibit significant uptake in prostate cancer cell line (PC-3) and pancreas in mouse models [14, 23], colon cancer [2] and breast with malignant tumors [9]. But, HYNIC chelated peptide litorin has yet to be studied and the role of HYNIC conjugation in tumor detection has yet to be explored. Therefore, we extended our previous study with 99mTc-litorin to investigate the potential of litorin conjugated to HYNIC as a radiopharmaceutical agent to image GRPr expressing tumors. HYNIC-Q-Litorin was synthesized by conjugating HYNIC to the N-terminal glutamine of Q-Litorin [1]. As a first step, our investigation focused on optimizing the radiolabeling conditions for the production of this new end radiopharmaceutical product. We prepared conjugates with two different co-ligands (tricine/EDDA) and amounts, and vary reaction conditions such as pH, reaction temperature, reaction time, and stannous chloride in the manufacturing process. The co-ligands were provided to complete the coordination sphere so that 99mTc could bind to HYNIC by allowing easy modification of the hydrophilicity and pharmacokinetics of 99mTc-radiolabeling peptide conjugates [1, 19]. In this paper, we give the details of these optimization procedures and the final parameter values that led to maximum yield in the production.
Materials and methods
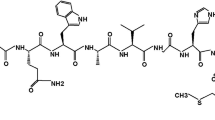
Conjugating HYNIC to litorin required adding amino acid Gln to the peptide sequence. We outsourced the synthesis of the desired conjugate HYNIC-Q-Litorin [Q-litorin: Gln-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 (Fig. 1), MW 1238.8 g/mol] to a company (PiChem Inc., Graz, Austria). The synthesized agent had purity higher than 98% as analyzed by the reverse phase High Performance Liquid Chromatography (HPLC) and mass spectroscopy instrumentation. Other reagents were purchased from another company (Sigma-Aldrich Chemical Co., Germany). Na99mTcO4 was supplied by Department of Nuclear Medicine in Ege University, as 99Mo/99mTc generator eluent (Monrol, Inc., Istanbul, Turkey).
Tricine as co-ligand
A mixture of 10 μg of HYNIC-Q-Litorin and 50 mg/150 μL water of tricine co-ligand was first obtained in a sealed reaction vial. After adding SnCl2 in concentration of 12 μg/100 μL H2O (fresh 1 mg/ml in water), the mixture was purged with argon for 5 min. Next, 111 MBq Na99mTcO4 was added to the mixture. This process resulted in 5 for the pH of the reaction. The solution was incubated at 100 °C for 15 min [2, 21]. After being cooled down to room temperature, a sample of the resulting solution was analyzed by using radio High Performance Liquid Chromatography (RHPLC) and Radio Thin Layer Chromatography (RTLC).
EDDA as co-ligand
Ten micrograms of HYNIC-Q-Litorin was incubated with 5 mg of EDDA co-ligand in 500 μL phosphate tampon. After the addition of 12 μg/100 μL SnCl2·H2O (fresh 1 mg/ml in water), the mixture was purged with argon for 5 min. The sample was further mixed with 111 MBq Na99mTcO4 and the solution was heated at 100 °C for 15 min. The pH of reaction solution was measured as 3 [1, 16]. After being cooled down to the room temperature, a sample of the resulting solution was analyzed with RHLC and RTLC. The specific activity for these conjugates was 13,875 MBq/μmol.
Yield curve of the final compound obtained with each co-ligand (tricine or EDDA) has been produced for different amounts of the co-ligand and SnCI2·H2O, and by varying the pH, reaction temperature and time.
Preparations of 99mTc-tricine and 99mTc-EDDA
To test whether 99mTc was properly attached to tricine-HYNIC-Q-Litorin or EDDA-HYNIC-Q-Litorin during the procedures described above or bonded to tricine or EDDA alone, we separately prepared 99mTc-tricine and 99mTc-EDDA batches for quality control purposes. For preparing 99mTc-tricine, fifty mg of tricine was dissolved in water (150 μL) in a tube and 50 μg/100 μL stannous chloride solution along with 111 MBq Na99mTcO4 were added to the solution. The pH of the reaction was 4.6. The tube was kept at at room temperature for 30 min. For preparing 99mTc-EDDA, 50 mg of EDDA was dissolved in pH 7 tampon (500 μL) in a tube. 50 μg/100 μL stannous chloride solution and about 111 MBq Na99mTcO4 were added to the tube. The tube was stored at room temperature for 30 min. The quality control of the resulting samples was carried out using RHPLC and RTLC.
Evaluation of radiochemical purity
The radiological purity of the specimens obtained by using the above procedures was evaluated with the help of TLC on silica gel sheets (TLC-SG, Merck, Germany) and different solvent systems [(A = 1% NaCl/acetone/acetonitrile (2/1/1), citrate-dextrose buffer solution (ACD), serum phiologic (SF) and 50% acetonitrile (ACN)]. The thin sheets were scanned with BioScan TLC-scanner (Bioscan AR-2000, Washington, DC). Relative front (R f) values and labeling efficiencies were measured from the chromatograms.
RHPLC analysis
The product 99mTc-HYNIC-tricine/EDDA-Q-Litorin was also characterized by low pressure gradient HPLC system. HPLC analysis was performed on LC-10 ATvp quaternary pump, UV detector (Shimadzu SPD-10ATvp, Macherey-Nagel, EC 250/4.6 Nucleodur 100-5 C18 column) and 20 μL loop and settled with a Cd(Te) detector equipped with a RAD-501 single channel analyzer. HPLC solvents consisted of 0.1% TFA in H2O (solvent A) and 0.1% TFA in CH3CN (solvent B) at a flow rate of 1 mL/min. The HPLC gradient system begins with a solvent composition of 100% A:0% B from 0 to 3 min, 50% A:50% B from 3 to 23 min, 30% A:70% B from 23 to 26 min and 100% A:0% B from 26 to 30 min. The UV detector was settled at 215 nm.
Results and discussion
Results of RTLC and RHPLC analysis
Table 1 lists the R f values measured from RTLC analysis performed on radiolabeled compounds produced in this study using four different solvent systems. According to the results in Table 1, 99mTcO4 −, 99mTc-tricine and 99mTc-EDDA have shown strong reaction to all four solvents, as indicated by the high R f values. Increase in R f value indicated migration of the samples from the origin to the front of the TLC sheet. The compound reduced 99mTc had reaction in only ACD solution. Both 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin had lower readings for two solvent systems ACD and SF, indicating that their samples remained at the origin of the TLC sheet. When other solvents (A and 50% acetonitrile) were used, these samples migrated from origin to the front of the sheet and attained higher R f values. Close analysis of the results in Table 1 also suggested that only ACD can differentiate successfully 99mTc labeling in both 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin, as indicated by the lower R f values therein.
The RHPLCs of 99mTc-tricine and 99mTc-tricine-HYNIC-Q-Litorin obtained in this study were over plotted in Fig. 2. The chromatograms of 99mTc-tricine and 99mTc-tricine-HYNIC-Q-Litorin depicted single peak for each compound with retention times (R t) of 3.84 and 13.6 min, respectively. Similarly, when the co-ligand EDDA was used, one peak was again observed in chromatogram of each compound (Fig. 3). The corresponding R ts were 3.83 and 13.7 min, respectively. Combining the RTLC results in Table 1 with the RHPLC results in Figs. 2 and 3 together indicates a fairly high labeling yield for both 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin.
In order to optimize the radiochemical yield, different amounts of co-ligands and stannous ion concentrations and pH values were employed, and the temperature and reaction time were manipulated during the radiolabeling procedures. The effect of pH variation on the yield of the final product 99mTc-HYNIC-Q-Litorin was demonstrated in Fig. 4, when tricine or EDDA was used as co-ligands. The labeling yields for both showed dependence on the reaction pH when three pH values 3, 5 and 7 were employed. In the presence of tricine, the mean radiolabeling yield had parabolic behavior, meaning it measured at 84.1 ± 5.0% when pH was low at 3, then reached to a peak value of 92.0 ± 2.7% at pH of 5 and dropped back to 76.0 ± 2.0% level when pH was 7. The labeling with the EDDA conjugate, on the other hand, exhibited a monotonically declining trend with the increased pH levels. The yield was maximum at 70.1 ± 5.2% at pH = 3, but dropped to 41.0 ± 5.0% at pH = 5 and remained at 40.0 ± 7.0% when pH = 7. The literature relevant to the different construct of the 99mTc-HYNIC-peptides has reported variations in the pH value measured form the reported reaction products. In few studies carried out with HYNIC-bombesin (7,14)NH2 was labeled with 99mTc using co-ligand tricine, pH value was reported as 7 [1]. In other studies, the pH of reaction solution was indicated as 5 [2, 15, 21]. Because of these differences in the reported pH values in the literature, we opted to examine the influence of pH on the labeling yield in the current study, and performed experiments at three different pH values 3, 5 and 7, as described above. From this extensive analysis, we determined the optimum pH as 5 for the new peptide conjugate 99mTc-tricine-HYNIC-Q-Litorin when tricine was used as the co-ligand. When EDDA was used, we found out that 3 was the optimum pH value. However, few publications reported pH value of 7 when the experiments were performed with EDDA [1, 16, 21]. From Fig. 4, the yield was 70.1 ± 5.2% at pH = 3 but the yields at pH = 5 or 7 were only about 40%. This clearly demonstrates that the previously published procedures are not consistent with our results and produces substantially less yield. However, our peptide is a new design and structurally different from the ones used in the previous publications. Therefore, this point must carefully be taken into considerations when interpreting our findings and comparing our new results with those published by others.
Effect of pH on radiolabeling yield of 99mTc-HYNIC-Q-Litorin conjugates. Error bars denote standard deviation. Yields for tricine were obtained with tricine = 50 mg, reaction temperature = 100 °C, reaction time = 15 min, and stannous chloride = 100 μg. Yields for EDDA were similarly obtained with EDDA = 5 mg, reaction temperature = 100 °C, reaction time = 15 min, and stannous chloride = 50 μg
As we stated before, we used tricine and EDDA as co-ligands in this study. Tricine is one of the most used co-ligands in producing 99mTc-HYNIC-peptide conjugates. But the selection of the amount of tricine has been a crucial matter, affecting the radiochemical purity of the 99mTc-HYNIC-peptide. The different concentrations of tricine were used in previous publications [1, 2, 12, 13, 18, 19, 21]. The existing literature has also reported that the absence of excess co-ligand causes instability of the 99mTc-peptide conjugate and lower tricine concentration (<10 mg/mL) induces formation of 99mTc-colloid (99mTcO2)[1]. In radiolabeling HYNIC-BN conjugate, optimum tricine amount was determined as 20 mg and the yield was about 98% [23]. To determine if we can reach the same level of yield with lesser quantity of tricine, we investigated the radiolabeling yield by using 50 mg and also at reduced amounts of 25 and 12 mg of tricine in our experiments. The results obtained with these tricine amounts are presented in Fig. 5. From the curve in the figure, the yield can be seen as increasing monotonically with the amount of tricine and reads 82.2 ± 2.2, 92.1 ± 2.7 and 98.1 ± 2.7% for the amounts tested. We attained maximum yield at 50 mg of tricine, which is consistent with the previous report by King et al. [18], also produced for our conjugate as well.
In the previous studies, the maximum radiolabeling yield was obtained with 5 mg of EDDA [1, 16, 18]. To test if this amount is also optimal for producing our conjugate, we have selected two more data points around this optimal value and used 3, 5 and 7 mg EDDA. Figure 6 depicts the percentage yield values associated with these amounts. The mean yield values obtained with 3 and 5 mg were close to each other at 68.7 ± 1.5 and 70.0 ± 5.2% (maximum yield), respectively, but it was 41.4 ± 5.2% for 7 mg of EDDA. The standard deviation for the measurement with 5 mg was lower than that of 3 mg. When this difference is considered, the results at 3 and 5 mg may not be different at a statistically significant level. The consequence of this is that the optimal EDDA amount may lie between 3 and 5 mg for our conjugates.
The effect of the amount of stannous chloride on the radiolabeling yield is summarized in Fig. 7. Initially, we used 12 μg of stannous chloride, same as the amount reported by others [1]. At this amount, we observed both tricine and EDDA produced nearly maximum yield of about 98% for both conjugates. Because of attaining nearly perfect yield at 12 μg and our determination to define the behavior of the yield with larger amounts of stannous chloride, we tested the yield efficiency at two more data points at 25 and 50 μg of stannous chloride. The data from these experiments showed that the tricine did not have significant influence on the yield of its conjugate, but the yield for the EDDA containing conjugate reduced with increase in stannous chloride amount in the medium. The reduction was monotonic and noticable at 86.1 ± 7.2% and 70.0 ± 5.2% for 25 and 50 μg of stannous chloride, respectively.
Effect of stannous chloride on the radiolabeling yield of 99mTc-HYNIC-Q-Litorin conjugates. Error bars denote standard deviation. Yields for tricine were obtained with tricine = 50 mg, reaction temperature = 100 °C, reaction time = 15 min, and pH = 5. Yields for EDDA were similarly obtained with EDDA = 5 mg, reaction temperature = 100 °C, reaction time = 15 min, and pH = 3
The labeling yields for both tricine and EDDA conjugates were demonstrated for three different reaction temperatures of 25, 50 and 100 °C in Fig. 8. The corresponding yields were very close at 98.0 ± 1.7 and 97.5 ± 2.5% and maximized when the temperature was 100 °C, respectively. The effects of reaction time on the yields are shown in Fig. 9. The highest labeling yields were obtained when the time was 15 min for both conjugatees. Our optimal temperature and reaction time findings are in agreement with the results from other studies carried out with BNN-like other peptides conjugated to 99mTc with co-ligand tricine or EDDA [1, 2, 15, 16, 18]. It is important to note that the optimal temperature and reaction time depends on underlying peptide and in some cases, maximum yield can be obtained not necessarily at 100 °C, but at room temperature [16].
Effect of temperature on the radiolabeling yield of 99mTc-HYNIC-Q-Litorin conjugates. Error bars denote standard deviation. Yields for tricine were obtained with tricine = 50 mg, stannous chloride = 12 μg, reaction time = 15 min, and pH = 5. Yields for EDDA were similarly obtained with EDDA = 5 mg, stannous chloride = 12 μg, reaction time = 15 min, and pH = 3
Effect of reaction time on the radiolabeling yield of 99mTc-HYNIC-Q-Litorin conjugates. Error bars denote standard deviation. Yields for tricine were obtained with tricine = 50 mg, stannous chloride = 12 μg, reaction temperature = 100 °C, and pH = 5. Yields for EDDA were similarly obtained with EDDA = 5 mg, stannous chloride = 12 μg, reaction temperature = 100 °C, and pH = 3
In above sections, we showed that the radiolabeling efficiencies of 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin were 98.0 ± 1.7 and 97.5 ± 2.5%, respectively. Liu et al. suggested that EDDA is a potentially tetradentate ligand and therefore is expected to be more stable 99mTc-conjugate than that of tricine [21]. The higher symmetry associated with EDDA conjugate is suggested as the reason for stability because it results in fewer coordination isomers than those obtained with tricine. However, our findings indicated no remarkable differences in the radiolabeling yields. This is not a surprising finding because similar observations were made with different peptide conjugatees including tricine and EDDA in the past [1].
Conclusion
In this study, we demonstrated that it is possible to produce litorin based radiolabeling agents 99mTc-tricine-HYNIC-Q-Litorin and 99mTc-EDDA-HYNIC-Q-Litorin at high yields. The optimal conditions for 99mTc-tricine-HYNIC-Q-Litorin are: HYNIC-peptide:tricine: 10 μg/50 mg, pH 5, SnCl2 concentration: 12 μg/0.1 mL, reaction temperature: 100 °C, reaction time: 15 min. The corresponding values for 99mTc-EDDA-HYNIC-Q-Litorin are: HYNIC-peptide:EDDA: 10 μg/5 mg, pH 3, SnCl2 concentration: 12 μg/0.1 mL, reaction temperature: 100 °C, reaction time: 15 min. It remains to the future work to investigate the biological activity profiles of these conjugates for imaging GRPr expressing tumor cells.
References
Faintuch BL, Santos RLSR, Souza ALFM, Hoffman TJ, Greeley M, Smith CJ (2005) 99mTc-HYNIC-Bombesin (7–14)NH2: radiochemical evaluation with co-ligands EDDA (EDDA = Ethylenediamine-N,N′-diacetic acid), Tricine, and Nicotinic acid. Synth React Inorg Met-Org Nano-Met Chem 35:43–51
Shi J, Jia B, Liu Z, Yang Z, Chen K, Chen X, Liu S, Wang F (2008) 99mTc-labeled bombesin (7–14)NH2 with favorable properties for SPECT imaging of colon cancer. Bioconj Chem 19:1170–1178
Miranda-Olvera AD, Ferro Flores G, Pedraza-Lopez M, Arteaga de Murphy C, De Leon Rodriguez LM (2007) Synthesis of oxytocin HYNIC derivatives as potential diagnostic agents for breast cancer. Bioconj Chem 18:1560–1567
Babich JW, Coco WG, Barrow S, Fischman AJ, Femia FJ, Zubieta J (2000) 99mTc-labeled chemotavtic peptides: influence of coligand on distribution of molecular species and infection imaging properties. Synthesis and structural characterization of model complexes with the Re(η2-HNNC5H4 N)(η1-NNC5H4N) core. Inorg Chim Acta 309:123–126
Banerjee SR, Maresca KP, Stephenson KA, Valliant JF, Babich JW, Graham WA, Barzana M, Dong Q, Fischman AJ, Zubieta J (2005) N,N-Bis(2-mercaptoethyl) methylamine: a new coligand for Tc-99m labeling of hydrazinonicotinamide peptides. Bioconj Chem 16:885–902
Decristoforo C (1999) Mather S.J: 99mTc-Technetium-labeled peptide-HYNIC conjugates: effects of lipophilicity and stability on biodistribution. Nucl Med Biol 26:389–396
Gandomkar M, Najafi R, Shafiei M, Mazidi M, Ebrahimi SES (2007) Precilinical evaluation of [99mTc/EDDA/Tricine/HYNIC0, 1-NaI3, Thr8]-octreotide as a new analogue in the detection of somatostatin-receptor-positive tumors. Nucl Med Biol 34:651–657
Decristoforo C, Mather S (1999) Technetium-99m somatostatin analogues: effect of labeling methods and peptide sequence. Eur J Nucl Med 26:869–876
Santos-Cuevas CL, Ferro-Flores G, Murphy CA, Pichardo-Romero PA (2008) Targeted imaging of gastrin-releasing peptide receptors with 99mTc-EDDA/HYNIC-[Lys3]-Bombesin: biokinetics and dosimetry in women. Nucl Med Commun 29(8):741–747
La Bella R, Garcia-Garayoa E, Bahler M, Blauenstein P, Schibli R, Conrath P, Tourwe D, Schubiger PA (2002) A 99mTc(I)-postlabeled high affinity bombesin analogue as a potential tumor imaging agent. Bioconj Chem 13:599–604
Durkan K, Yurt Lambrecht F, Unak P (2007) Investigation of radiopharmaceutical potential and labeling methods of bombesin like peptide: litorin with Tc-99m. Bioconj Chem 18:1516–1520
Melendez-Alafort L, Maria Ramirez F, Ferro-Flores G, Murphy GA, Pedreza-Lopez M, Hnatowich DJ (2003) Lys and Arg in UBI: a specific site for a stable Tc-99m complex. Nucl Med Biol 30:605–615
Ono M, Arano Y, Uehara T, Fujioka Y, Ogawa K, Namba S, Mukai T, Nakayama M, Saji H (1999) Intracellular metabolic fate of radioactivity after injection of technetium-99m-labeled hydrazino nicotinamide derivatized proteins. Bioconj Chem 10:386–394
Ferro-Flores G, Arteaga de Murphy C, Rodriguez-Cortes J, Pedraza-Lopez M, Ramirez-Iglesias MT (2006) Preparation and evaluation of 99mTc-EDDA/HYNIC-[Lys3]-bombesin for imaging gastrin-releasing peptide receptor-positive tumours. Nucl Med Commun 27:371–376
Purohit A, Liu S, Casebier D, Edwards DS (2003) Phosphine-containing HYNIC derivatives as potential bifunctional chelators for 99mTc-labeling of small biomolecules. Bioconj Chem 14:720–727
Decristoforo C, Mather SJ (1999) Preparation, 99mTc-labeling, and in vitro characterization of HYNIC and N3S modified RC-160 and [Tyr3]octreotide. Bioconj Chem 10:431–438
Gandomkar M, Najafi R, Shafiei M, Ebrahimi SES (2007) Confirmation of hydrazone formation in HYNIC-peptide conjugate preparation, and its hydrolysis during labeling with 99mTc. Appl Rad Isot 65:805–808
King RC, Bashir-Uddin Surfraz M, Biagini SCG, Blower PJ, Mather SJ (2007) How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies. Dalton Trans 43:4998–5007
Uddin-Surfraz MB, King R, Mather SJ, Biagini S, Blower PJ (2009) Technetium-binding in labeled HYNIC-peptide conjugates: role of coordinating amino acids. J Inorg Biochem 103:971–977
Rennen HJJM, Boerman OC, Koenders EB, Oyen WJG, Cornstens FHM (2000) Labeling proteins with Tc-99m via hydrazinonicotinamide (HYNIC): optimization of the conjugation reaction. Nucl Med Biol 27:599–604
Liu G, Wescott C, Sato A, Wang Y, Liu N, Zhang YM, Rusckowski M, Hnatowich DJ (2002) Nitriles form mixed-coligand complexes with 99mTc-HYNIC-peptide. Nucl Med Biol 29:107–113
Decristoforo C, Mather SJ (2002) The influence of chelator on the pharmacokinetics of 99mTc-labeled peptides. Q J Nucl Med 46:195–205
Sadehzadeh N, Gandomkar M, Najafi R, Shafiei M, SadatEbrahimi SE, Shafiee A, Larijani B (2010) Preparetion and evaluation of a new 99mTc labeled bombesim derivative for tumor imaging. J Radioanal Nucl Chem 283:181–187
Acknowledgements
The authors gratefully acknowledge the financial support received from the Scientific and Technological Research Council of Turkey, Scientific Project (TUBITAK Number 108S200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yurt Lambrecht, F., Durkan, K. & Bayrak, E. Labeling bombesin-like peptide with 99mTc via hydrazinonicotinamide: description of optimized radiolabeling conditions. J Radioanal Nucl Chem 284, 539–545 (2010). https://doi.org/10.1007/s10967-010-0530-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0530-8