Abstract
Purpose
It has been shown that some primary human tumours and their metastases, including prostate and breast tumours, overexpress gastrin-releasing peptide (GRP) receptors. Bombesin (BN) is a neuropeptide with a high affinity for these GRP receptors. We demonstrated successful scintigraphic visualisation of BN receptor-positive tumours in preclinical studies using the radiolabelled BN analogue [111In-DTPA-Pro1,Tyr4]BN. However, the receptor affinity as well as the serum stability of this analogue leave room for improvement. Therefore new 111In-labelled BN analogues were synthesised and evaluated in vitro and in vivo.
Methods and results
The receptor affinity of the new BN analogues was tested on human GRP receptor-expressing prostate tumour xenografts and rat colon sections. Analogues with high receptor affinity (low nM range) were selected for further evaluation. Incubation in vitro of GRP receptor-expressing rat CA20948 and human PC3 tumour cells with the 111In-labelled analogues resulted in rapid receptor-mediated uptake and internalisation. The BN analogue with the best receptor affinity and in vitro internalisation characteristics, Cmp 3 ([111In-DTPA-ACMpip5,Tha6,βAla11,Tha13,Nle14]BN(5–14)), was tested in vivo in biodistribution studies using rats bearing GRP receptor-expressing CA20948 tumours, and nude mice bearing human PC3 xenografts. Injection of 111In-labelled Cmp 3 in these animals showed high, receptor-mediated uptake in receptor-positive organs and tumours which could be visualised using planar gamma camera and microSPECT/CT imaging.
Conclusion
With their enhanced receptor affinity and their rapid receptor-mediated internalisation in vitro and in vivo, the new BN analogues, and especially Cmp 3, are promising candidates for use in diagnostic molecular imaging and targeted radionuclide therapy of GRP receptor-expressing cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bombesin-like peptides, including gastrin-releasing peptide (GRP) and neuromedin B (NMB), are involved in the regulation of a large number of biological processes in the gut and central nervous system (CNS) [1]. They mediate their action by binding to G protein-coupled receptors [2].
Four subtypes of the bombesin (BN) receptor are known. Three of them, the NMB receptor (BB1), the GRP receptor (BB2) and BN receptor subtype 3 (BRS-3 or BB3), are mammalian receptors, whereas the fourth subtype (BB4) is found only in amphibians [3–6]. Except for the GRP receptor, these receptor subtypes are not characterised very well with regard to their distribution and function in human tissues [7, 8].
BN receptors are expressed in high densities on several primary human tumours and their metastases, including prostate, breast, small cell lung and pancreatic cancers [9–16]. Prostate cancer is the most frequently diagnosed malignancy and the third leading cause of cancer mortality among men in the Western world [17]. Although the commonly used hormonal therapies increase the survival of patients with hormone-dependent prostate tumours, patients with hormone-independent tumours still have a poor prognosis. Therefore new diagnosis and treatment methods for these tumours are still very welcome.
Prostate tumours overexpress GRP receptors [11, 14, 18–21]. In an autoradiographic study, Markwalder and Reubi found the GRP receptor to be expressed in high density on invasive prostate carcinomas and proliferative intraepithelial prostate lesions, mostly prostatic intraepithelial neoplasias, whereas normal prostate tissue and, in most cases, hyperplastic prostate tissue were GRP receptor-negative [14]. These findings suggest that the GRP receptor can be used as a molecular basis for diagnosing and treating prostate tumours with, for example, GRP receptor-targeted scintigraphy, radionuclide therapy and cytotoxic therapy [14, 22]. It has been proposed that GRP receptor-expressing tumours can be visualised and treated using radiolabelled BN analogues in a manner similar to what has been found for somatostatin receptor-expressing tumours, which have been successfully imaged and treated using radiolabelled somatostatin analogues [23–25].
A number of researchers continue to work on the development of radiolabelled BN analogues that specifically target GRP receptor-expressing tumours. For example, 99mTc- and 111In-coupled BN analogues have been developed for diagnostic SPECT imaging and 64Cu- and 68Ga-labelled analogues for PET imaging of GRP receptor-expressing tumours [26–39]. In addition, 90Y- and 177Lu-labelled analogues have been described as promising tools for targeted radiotherapy of these tumours [26, 40].
We previously developed and evaluated the radiolabelled peptide [111In-DTPA-Pro1,Tyr4]BN, a BN analogue which internalised rapidly into GRP receptor-positive tumour cells in vitro and in vivo [37–39]. In addition, GRP receptor-expressing CA20948 and AR42J tumours could be visualised using gamma camera imaging in rats after injection with this radiolabelled BN analogue [37, 38].
Our goal in this study was to extend our previous study by developing an improved BN analogue with an increased uptake in GRP receptor-expressing tumours. Because the BN analogue, Cmp 1 [DTPA-Pro1,Tyr4]BN is an analogue with minimal modifications of the native peptide, we synthesised new BN analogues with shortened amino acid sequences including stable amino acid derivatives to increase the receptor affinity of these peptides. We selected five of these new BN analogues on the basis of their receptor affinity and compared their in vitro characteristics with the characteristics of Cmp 1. We determined the in vivo tumour uptake and tissue biodistribution of the compound found to have the most promising in vitro characteristics.
Materials and methods
Peptide synthesis
The new BN analogues were synthesised by solid phase peptide synthesis using an Applied Biosystems Model 432A “Synergy” Peptide synthesiser (Applied Biosystems, Foster City, CA, USA) employing the Fmoc (9-fluorenylmethoxy-carbonyl) strategy. The instrument protocol required 25 μmol of starting resin and 75 μmol of subsequent Fmoc-protected amino acids activated by a combination of N-hydroxybenzotriazole (HOBt) and (2-(1-H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU). Tri-t-butyl DTPA (75 μmol) was placed at the appropriate location in an amino acid column to prepare DTPA-coupled derivatives [19]. The arginine and lysine derivatives used were purchased from RSP Amino Acid Analogues (Hopkinton, MA, USA). All other Fmoc-protected amino acids were purchased from Novobiochem/EMD Biosciences (Madison, WI).
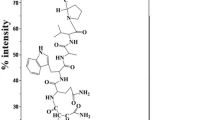
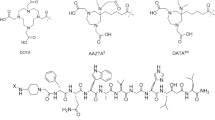
The cleavage and deprotection were accomplished using 85% TFA:5% thioanisole:5% phenol:5% water. We isolated the crude peptide by precipitating with t-butyl methyl ether (Sigma, St. Louis, MO, USA), and purified the peptide by reverse phase high-performance liquid chromatography (HPLC) using an acetonitrile/water gradient containing 0.1% TFA, with final yields ranging from 10% to 25% of the starting resin scale. The molecular weights of compounds 1 through 6 were determined by mass spectrometry operating in the electrospray mode (Varian, Palo Alto, CA, USA); M/z determined for the peptides were: Cmp 1, 1,016.0 (M+2H), consistent for a compound of expected MW of 2,030.0; Cmp 2, 1,515.6 (M+H); Cmp 3, 1,644.7 (M+H); Cmp 4, 1,638.8 (M+H), Cmp 5, 1,504.8 (M+H); Cmp 6, 1,431.8 (M+H).
Peptide radiolabelling
The DTPA-conjugated BN analogues were radiolabelled with 111In (111InCl3, Tyco Healthcare, Petten, the Netherlands, DRN 4901, 370 MBq/ml in HCl, pH 1.5–1.9) to a maximum specific activity of 200 MBq/nmol as described previously [41]. Consecutive quality control by instant thin-layer chromatography and SEP-PAK C18 reverse phase chromatography (Waters) was performed as described previously [42]. The radiolabelling yield and radiochemical purity were always >95%.
Receptor affinity
Receptor affinity of the BN analogues was determined on frozen sections of the GRP receptor-expressing human prostate tumour xenograft PC-295 [43] and rat colon using in vitro autoradiography. The 10-μm sections were incubated for 1 h at room temperature with 0.1 nM 111In-Cmp 1 (111In-DTPA-Pro1,Tyr4-bombesin) (200 MBq/nmol) in 167 mM Tris (pH 7.6), 5 mM MgCl2, 1% bovine serum albumin (BSA), 40 μg/ml bacitracin. To generate competitive inhibition curves, the sections were incubated in the presence of increasing amounts of the selected non-radioactive 115In-labelled BN analogues. After incubation, the sections were washed two times for 5 min each in 167 mM Tris (pH 7.6), 5 mM MgCl2, 0.25% BSA (4°C), for 5 min in 167 mM Tris (pH 7.6), 5 mM MgCl2 (4°C) and finally rinsed in MilliQ water (4°C). The sections were then dried and exposed to phosphor imaging screens for 72 h. The imaging screens were read using the Cyclone Storage Phosphor System and the autoradiograms were quantified using Optiquant Software (Packard, Meriden, USA).
Cell culture
The BN analogues were tested using the GRP receptor-expressing rat pancreatic tumour cell lines CA20948 and AR42J [44] and the human prostate tumour cell line PC3. We have previously used the CA20948 tumour model to characterise BN analogues. Many other groups, however, have used the AR42J tumour model. In this study we used both models to enable comparisons with other studies.
The CA20948 cells were grown in Dulbecco’s modified Eagle’s medium (Gibco, Life Technologies, Breda, the Netherlands) supplemented with 10% foetal bovine serum, Glutamax I (1x), 0.2 mM sodium pyruvate, 0.25 mg/l Fungizone and 200 IU/ml penicillin/streptomycin (Gibco, Life Technologies, Breda, the Netherlands). The AR42J and PC3 cells were grown in RPMI medium (Gibco, Life Technologies, Breda, the Netherlands) supplemented with 10% foetal bovine serum, Glutamax I (1x), 0.2 mM sodium pyruvate, 0.25 mg/l Fungizone and 200 IU/ml penicillin/streptomycin (Gibco, Life Technologies, Breda, the Netherlands).
Internalisation
The internalisation characteristics of the 111In-labelled BN analogues were determined as previously described [45]. Subconfluent cell cultures were transferred to six-well plates 24 h before internalisation experiments. For increasing incubation times, the CA20948 and PC3 cells were incubated in triplicate with 1 ml incubation medium (RPMI supplemented with 20 mM HEPES and 1% bovine serum albumin) containing 80–100 kBq of 111In-labelled peptide (concentration as indicated).
Cellular uptake was stopped by removing medium from the cells and washing with 2 ml of ice-cold PBS. Surface-bound activity was removed by incubation with 1 ml of 20 mM sodium acetate in PBS (pH 5.0) for 10 min. Internalised and surface-bound radioactivity was determined separately by measuring the different fractions in an LKB-1282-Compugamma system (Perkin Elmer, Oosterhout, the Netherlands) and expressed as a percentage of the total dose applied per milligram cellular protein. Protein was determined using a commercially available kit (Protein assay, BioRad, Veenendaal, the Netherlands). To test the receptor specificity of internalisation, an increasing concentration (10−10 M to 10−6 M) of unlabelled [Tyr4]BN was added to the incubation medium to compete with the binding of radiolabelled BN analogues to the GRP receptor.
Biodistribution experiments
The biodistribution experiments were done using male Lewis rats implanted with an average of 1 million CA20948 cells and male NMRI nu/nu mice implanted with an average of 5 million PC3 cells. After 14–21 days (tumour size ca. 2–4 cm2 for CA20948 and 1 cm2 for PC3) the animals were injected intravenously with 2–4 MBq/0.1 μg of 111In-labelled peptide. In this biodistribution study we compared the uptake of the most promising compound (based on the binding affinity and internalisation results), 111In-Cmp 3, with the uptake of compound 111In-Cmp 1 in the selected organs and tumours. The injection volume was 0.50 ml in rats and 0.20 ml in mice. To discriminate between receptor-specific (receptor-mediated) and non-specific binding, some animals were co-injected with an excess of unlabelled [Tyr4]BN (100 μg in rats and 50 μg in mice). We sacrificed the animals 4, 24, 48 and 72 h (mice only 4 h) after injection and collected the organs and tissues of interest for counting radioactivity and calculating the uptake (% injected dose per mg tissue). Statistical analysis was performed using the Mann Whitney and unpaired t test. A probability of less than 0.05 was considered significant.
Serum stability
The serum stability of 111In-Cmp 1 and 3 was evaluated by incubation in human serum [50% serum:50% phosphate-buffered saline (PBS)] of healthy donors at 37°C for 4 h. The percentage of intact peptide after incubation was determined by separating degradation products by reverse phase HPLC using a Vydac C-18 column connected to a radiometric detector, and using a 15 min, 0–70% linear acetonitrile gradient (0.1% TFA/water).
Tumour visualisation
The tumour visualisation experiments were done using a male Lewis rat implanted with an average of 1 million CA20948 cells in the right flank and AR42J cells in the left flank. After 14–21 days, the rat was intravenously injected with 4 MBq/0.1 μg 111In-Cmp 3. Directly after injection of the radiotracer, a dynamic planar scan of the rat was made over 1 h using a one-headed gamma camera (Siemens, Erlangen, Germany). At 4 h after injection, a static image was acquired for 1 min. Immediately after imaging at 4 h post injection, the rat was sacrificed and a biodistribution study was performed.
For microSPECT/CT imaging a female Swiss nu/nu mouse was injected with ca. 1 million CA20948 cells. After 14 days the mouse was intravenously injected with 10 MBq/0.1 μg 111In-Cmp 3. Four hours after injection, SPECT/CT imaging was performed with a four-headed multiplexing multi-pinhole NanoSPECT/CT (Bioscan Inc., Washington D.C.). After the acquisition, the data were reconstructed iteratively with the HiSPECT software (Bioscan Inc., Washington D.C., USA), a dedicated ordered subsets-expectation maximisation (OSEM) software package for multiplexing multi-pinhole reconstruction. The CT and SPECT images were fused using Pmod image fusion software (Mediso Ltd., Budapest, Hungary). Immediately after imaging at 4 h post injection the mouse was sacrificed and a biodistribution study was performed.
Results
Peptides
Figure 1 shows the sequences and the molecular weights of compounds 1–6 used in this study and the structures of the non-natural amino acid derivatives.
Receptor affinity
The receptor affinity of the BN analogues was determined on GRP receptor-expressing human prostate tumour xenografts (PC-295) and rat colon sections. The IC50 values of the BN analogues are presented in Table 1. Cmp 2, Cmp 3 and Cmp 4 show increased receptor affinity for both the human (0.50, 0.36 and 0.41 nM respectively) and rat (0.22, 0.08 and 0.31 nM respectively) GRP receptor compared with analogue Cmp 1 (human 1.40 nM and rat 2.28 nM). However, all analogues show improved receptor affinity for the rat GRP receptor.
Internalisation
All the 111In-labelled BN analogues internalised into CA20948 and PC3 cells. Figure 2 shows the time course of internalisation into the CA20948 (a) and PC3 (b) cells expressed as percent of the dose per milligram cellular protein of the different radiolabelled peptides. All the 111In-labelled BN analogues internalised time dependently, but, compared with 111In-Cmp 1, the new BN analogues were more rapidly internalised into the CA20948 tumour cells. The internalisation characteristics of the analogues in the human PC3 cells were in some cases significantly different from those found in the rat CA20948 cells. In addition, the rank orders of the analogues based on these internalisation data area under the curve (AUC)] are notably different in the rat and human tumour cells (Fig. 2).
Time curve of internalisation of 0.5 nM 111In-Cmp 1 and four new 111In-labelled BN analogues in rat CA20948 tumour cells (a) and human PC3 tumour cells (b). Results are presented as the average percentage of the total dose per milligram cellular protein (% dose/mg protein; mean±SD). AUC: area under the curve
111In-Cmp 3 showed the highest rate of internalisation in the first hours of incubation and had the highest AUC in both the rat and the human tumour model. On the basis of these internalisation data and the data on receptor affinity for the human and the rat GRP receptor, we selected analogue 111In-Cmp 3 for further in vitro and in vivo characterisation.
To prove that the internalisation of 111In-Cmp 3 is receptor specific, we added increasing amounts of unlabelled [Tyr4]BN to the incubation medium, which contained 10−10 M 111In-Cmp 1 or 111In-Cmp 3. The results in Fig. 3 show that addition of 1–10 nM unlabelled [Tyr4]BN effectively caused this peptide to compete with the uptake of either 111In-Cmp 1 or 111In-Cmp 3 for receptor binding and internalisation into CA20948 cells and PC3 cells. Consistent with the findings in the time curve internalisation study (Fig. 2), the superior internalisation characteristics of 111In-Cmp 3 over 111In-Cmp 1 are less impressive in the human PC3 cells than in the rat CA20948 cells.
Internalisation of 0.1 nM 111In-Cmp 1 and 111In-Cmp 3 in the rat CA20948 (a) and human PC3 (b) tumour cells after 1-h incubation with increasing amounts of unlabelled [Tyr4]BN. Results are the average percentage of the total dose per milligram cellular protein (% dose/mg protein); mean±SD of three experiments
Biodistribution
In the in vivo biodistribution studies we compared the uptake of 111In-Cmp 3 with that of 111In-Cmp 1 in rats bearing CA20948 tumours and nude mice bearing PC3 tumours. Compared with 111In-Cmp 1, the uptake of 111In-Cmp 3 was two to three times higher (4 h post injection) in the rat BN receptor-positive pancreas, stomach, caecum, small intestine and colon (p<0.007) (Table 2). The same was true for the uptake in the receptor- positive CA20948 tumour (p<0.015). The radioactivity in the background organs and blood, except for the kidneys, was also two to three times higher for 111In-Cmp 3 than for 111In-Cmp 1, but was still very low, indicating rapid clearance. Co-injection of 100 μg unlabelled [Tyr4]BN resulted in an 89% reduction in 111In-Cmp 3 uptake in the CA20948 tumour and also in a reduction in uptake in other GRP receptor-positive organs, e.g. 94% in the pancreas and 88% in the colon. The uptake reduction found for 111In-Cmp 1 was comparable to that for 111In-Cmp 3: 90% in the tumour, 99% in the pancreas and 88% in the colon. As presented in Table 2, 111In-Cmp 3 showed good retention in receptor-positive organs and tumour up to 72 h post injection. Due to rapid clearance from the body, good tumour-to-background ratios were found (Table 2). The respective tumour-to-blood and tumour-to-muscle ratios were 50 and 126 for 111In-Cmp at 1 and 4 h post injection, and 49 and 146 for 111In-Cmp 3 at 4 h post injection; for 111In-Cmp 3 these ratios respectively reached 133 and 89 at 72 h post injection.
The results found in the PC3 tumour-bearing nude mice at 4 h post injection were partly consistent with the results found in the CA20948 tumour-bearing rats; 111In-Cmp 3 showed a two times higher uptake in the BN receptor-positive organs compared with 111In-Cmp 1 (p<0.005 for pancreas, caecum, small intestine and colon). The uptake of 111In-Cmp 3 in the human PC3 tumour was 1.3-fold higher compared with 111In-Cmp 1 (Table 3). However, the radioactivity accumulation in the mouse kidneys was four times lower for the new compound 111In-Cmp 3 (p<0.0001). The uptake reduction in the blocking experiments in the mice was consistent with the uptake reductions found in the rats: 86% in PC3 tumour for both compounds, 96% for Cmp 3 in mouse pancreas (Cmp 1: 99%) and 95% for Cmp 3 in mouse colon (Cmp 1: 96%). Respective tumour-to-blood and tumour-to-muscle ratios in these PC3 tumour-bearing mice were 28 and 36 for 111In-Cmp 1 and 18 and 49 for 111In-Cmp 3 at 4 h post injection.
Serum stability
The in vitro serum stability of the 111In-Cmp 3 was determined and compared with the stability of 111In-Cmp 1. The percentage of intact peptide after the 4-hour incubation of 111In-Cmp 1 in human serum was 67%. Under the same conditions 111In-Cmp 3 showed a slightly increased stability of 74% intact peptide.
Tumour visualisation
To visualise GRP receptor-expressing tumours in rats using the newly developed BN analogue, we injected a Lewis rat bearing GRP receptor-expressing tumours [CA20948 (right flank) and AR42J, which is commonly used by many other research groups (left flank)] with 4 MBq/0.1 μg 111In-Cmp 3. Gamma camera imaging was performed directly after injection and at 4 h after injection. Figure 4 shows scan images at 1, 10, 30 and 60 min and at 4 h after injection.
Planar gamma camera images of a rat bearing a CA20948 tumour in the right flank and an AR42J tumour at the left flank 1, 10, 30 and 60 min and 4 h after injection of 0.1 μg 111In-Cmp 3 (4 MBq). Biodistribution data obtained from this rat immediately after imaging at 4 h post injection are shown in the adjacent table. The uptake values are presented as %ID/g tissue
The scan images in Fig. 4 demonstrate rapid uptake of 111In-Cmp 3 in GRP receptor-positive AR42J tumour; uptake in this tumour could already be detected at 1 min post injection. At 4 h post injection, the AR42J tumour was still clearly visible. In this experiment the uptake of 111In-Cmp 3 in the CA20948 tumour was much lower than the uptake in the AR42J tumour. This was probably due to necrosis of the huge CA20948 tumour and higher GRP receptor density of the AR42J tumour.
The background radioactivity rapidly decreased after the first hour, which resulted in increasing tumour-to-background ratios. As 111In-Cmp 3 is excreted via the kidneys and the urinary system, radioactivity rapidly accumulated in the kidneys in the first hour after injection. At 4 h post injection, the radioactivity in the kidneys had decreased, and therefore the tumour-to-kidney ratio was more favourable at this time point.
In addition to the dynamic gamma camera imaging we also performed a microSPECT/CT imaging study with a nude mouse bearing the CA20948 tumour. The mouse was injected with 10 MBq/0.1 μg 111In-Cmp 3 and was scanned at 4 h post injection. Immediately after imaging at 4 h post injection, the mouse was sacrificed for determination of the in vivo biodistribution of the compound. Figure 5 shows the fused SPECT/CT images of the mouse. Biodistribution results are presented in the adjacent table. The sagittal, coronal and transaxial slices show a clear localisation of the CA20948 tumour on the right shoulder of the mouse, with no interfering background radioactivity. On the sagittal slice, bowel activity is visible which is probably caused by the GRP receptor-positive pancreas and intestines.
MicroSPECT/CT images of a Swiss nu/nu mouse bearing a CA20948 tumour on the right shoulder at 4 h post injection of 0.1 μg 111In-Cmp 3 (10 MBq). Biodistribution data obtained from this mouse immediately after imaging at 4 h post injection are shown in the adjacent table. The uptake values are presented as %ID/g tissue
The tumour uptake in the CA20948 tumour-bearing nude mouse was much higher than that found in the CA20948 tumour-bearing rats, which resulted in very high tumour-to-background ratios.
Discussion
The overexpression of peptide receptors in human tumours is of considerable clinical interest [46]. It has been shown that the overexpression of somatostatin receptors on human neuroendocrine tumours can be targeted successfully. On the one hand, long-term octreotide treatment of patients with somatostatin receptor-expressing tumours has been successful in relieving the symptoms related to excessive hormone production by these tumours [47]. On the other hand, the use of radiolabelled somatostatin analogues has permitted visualisation of in vivo neuroendocrine tumours and their metastases in patients [24]. In addition, 177Lu- and 90Y-labelled somatostatin analogues have been used in radionuclide therapy in this group of patients [48–55].
It has been reported that the GRP receptor is expressed in high densities on prostate cancers [11, 14, 18–22]. Based on this fact, and the experience with somatostatin receptors, we conclude that targeting the GRP receptor could be the molecular basis for clinical applications such as diagnostic scintigraphy and targeted radionuclide therapy of prostate tumours.
With its 27 amino acid sequence, the GRP peptide has poor in vivo stability and has not been found to be useful as a diagnostic or therapeutic agent. The carboxyl-terminal decapeptide of GRP is similar to that of the 14-amino acid peptide BN [56]. BN has a high affinity for the GRP receptor, which makes the development of optimised radiolabelled analogues of the BN peptide a very interesting goal.
As we previously reported, BN analogue [DTPA-Pro1,Tyr4]BN (Cmp 1) is capable of visualising GRP receptor-positive tumours in vivo. In this study we developed new DTPA-coupled BN analogues with shortened amino acid sequences containing non-natural amino acid derivatives to improve the receptor binding affinity of the compounds.
The results of the present study show that shortening the amino acid sequence and replacing amino acids by derivatives in the BN analogues improves the receptor affinity for both the rat and the human GRP receptor (Table 1). The new compounds internalised rapidly into GRP-receptor expressing rat and human tumour cells in vitro. In in vivo biodistribution studies, 111In-Cmp 3 showed a two to three times higher uptake in the GRP receptor-positive pancreas, CA20948 tumour (1.3 times for PC3) and also the whole gastro-intestinal tract compared with 111In-Cmp 1, with a good retention up to 72 h post injection. This good receptor-mediated tumour uptake and retention of 111In-Cmp 3 combined with its rapid clearance from the body (Table 2) resulted in good tumour-to-blood and tumour-to-muscle ratios ranging from 49 and 146 respectively at 4 h post injection up to 133 and 89 respectively at 72 h post injection in the CA20948 tumour-bearing rats. In the CA20948 mouse model the respective CA20948 tumour-to-blood and tumour-to-muscle ratios even reached 99 and 234 (Fig. 5). These ratios compare well with those reported for other radiolabelled BN analogues in the literature [26–31, 36, 40]. For example, the recently published 111In-BZH1 and 111In-BZH2 showed tumour-to-muscle ratios in AR42J tumour-bearing rats of 171 and 99 respectively, compared with 146 for 111In-Cmp 3 at 4 h post injection [26].
This study shows that shortening the amino acid sequence and replacing specific amino acids in the BN sequence can yield compounds with improved receptor affinity (Table 1). In preliminary studies we found that substitution of the native asparagine at position 6 with other amino acid derivatives did not always adversely affect receptor binding affinity, and the derivatives presented here are examples of modifications which were found to be beneficial. Additionally, not all shortened peptides have improved stability. For example, a peptide identical to Cmp 3, except with the aCMpip at position 5 deleted, has only 32.9% intact peptide left after 4-h incubation in human serum (data not shown), indicating that minor changes in the BN amino acid sequence can have a marked effect on the peptide’s stability. In the case of Cmp 3, the characteristic responsible for the increased internalisation in vitro and in vivo is the enhanced receptor affinity and not the serum stability, for the stability of this compound was only marginally improved compared with Cmp 1.
Gamma camera imaging of a CA20948 and AR42J tumour-bearing rat injected with 111In-Cmp 3 showed that the tumour could be visualised as early as at 1 min post injection (Fig. 4). MicroSPECT imaging of a CA20948 tumour-bearing nude mouse injected with the same compound resulted in clear tumour detection with very high tumour-to-background ratios (Fig. 5).
The dose-limiting organs in peptide receptor radionuclide therapy using somatostatin analogues are the kidneys, owing to the reabsorption and retention of these radiolabelled peptides. In this case, the kidneys can be protected by infusion of amino acids and by applying individual dosimetry in order to keep the kidney radiation dose below the maximum tolerated dose of 23–27 Gy [57]. However, the effect of amino acid infusion on kidney retention of BN analogues has not been published as yet. Nevertheless, compared with somatostatin analogues these BN analogues have an almost three times lower retention of radioactivity in the kidneys, which is very favourable for their possible use in radionuclide therapy.
It should be noticed that the in vitro and in vivo characteristics of some of the analogues obtained in the rat tumour models are sometimes the opposite of those found in the human tumour model. These findings are in agreement with the findings of Maina et al., who recently reported that there are interspecies differences in structure and pharmacology of human and animal GRP receptors [58]. This could be a major pitfall when using rat receptor models for selection of optimised analogues. Human receptor models should therefore always be used to verify the results obtained in rat receptor models. In this study, Cmp 3 has superior receptor binding and internalisation characteristics in both rat and human tumour models in vitro as well as in vivo. In addition, Cmp 3 also showed remarkable binding characteristics in primary human prostate cancer sections (IC50 = 1.3 nM, data not shown).
We plan to study further improvement of the tumour uptake of Cmp 3, e.g. by conjugating this BN analogue with the DOTA chelator. Breeman et al. reported an increased GRP receptor affinity and tumour uptake when replacing the DTPA chelator with the DOTA chelator coupled to the [Pro1,Tyr4]BN analogue (Cmp 1) [38]. Besides radiolabelling with 111In, this DOTA chelator enables radiolabelling with positron emitters like 68Ga and 64Cu for diagnostic PET imaging and also with β-emitters like 177Lu and 90Y; this may permit their use for radiotherapy to treat GRP receptor-positive tumours [26, 27, 33, 34, 36, 40], comparable to the use of 90Y-labelled octreotide and 177Lu-labelled octreotate to treat somatostatin receptor-expressing tumours [48–55]. Also increasing the receptor density on the tumours using gene therapy and, even more interestingly, delivering the radiolabelled analogue directly to the tumour using intratumoral injections to improve tumour uptake are to be studied.
In conclusion, the five new BN analogues were compared with analogue Cmp 1 concerning receptor binding affinity and in vitro internalisation into GRP receptor-expressing tumour cells. In addition, Cmp 3, with the best prospects based on the results of these studies, was further characterised in vivo for biodistribution and retention characteristics in tumour-bearing rats and mice. We found that 111In-Cmp 3 has good uptake in both rat and human GRP receptor-expressing tumours, which makes this analogue a good candidate for molecular imaging and therapy of GRP receptor-positive prostate tumours.
References
Erspamer V, Erpamer GF, Inselvini M. Some pharmacological actions of alytesin and bombesin. J Pharm Pharmacol 1970;22:875–6.
Kroog GS, Jensen RT, Battey JF. Mammalian bombesin receptors. Med Res Rev 1995;15:389–417.
Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol Endocrinol 1990;4:1956–63.
Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, et al. cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron 1991;6:421–30.
Nagalla SR, Barry BJ, Creswick KC, Eden P, Taylor JT, Spindel ER. Cloning of a receptor for amphibian [Phe13]bombesin distinct from the receptor for gastrin-releasing peptide: identification of a fourth bombesin receptor subtype (BB4). Proc Natl Acad Sci USA 1995;92:6205–9.
Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, et al. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem 1993;268:5979–84.
Rettenbacher M, Reubi JC. Localization and characterization of neuropeptide receptors in human colon. Naunyn-Schmiedeberg’s Arch Pharmacol 2001;364:291–304.
Ferris HA, Carroll RE, Lorimer DL, Benya RV. Location and characterization of the human GRP receptor expressed by gastrointestinal epithelial cells. Peptides 1997;18:663–72.
Halmos G, Wittliff JL, Schally AV. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res 1995;55:280–7.
Toi-Scott M, Jones CL, Kane MA. Clinical correlates of bombesin-like peptide receptor subtype expression in human lung cancer cells. Lung Cancer 1996;15:341–54.
Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate 2000;42:295–303.
Reubi C, Gugger M, Waser B. Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging 2002;29:855–62.
Qin Y, Ertl T, Cai RZ, Halmos G, Schally AV. Inhibitory effect of bombesin receptor antagonist RC-3095 on the growth of human pancreatic cancer cells in vivo and in vitro. Cancer Res 1994;54:1035–41.
Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res 1999;59:1152–9.
Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am J Pathol 1999;155:2067–76.
Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand 125I-[ d -TYR6, β-ALA11, PHE13, NLE14] bombesin(6–14). Clin Cancer Res 2002;8:1139–46.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106–30.
Aprikian AG, Han K, Chevalier S, Bazinet M, Viallet J. Bombesin specifically induces intracellular calcium mobilization via gastrin-releasing peptide receptors in human prostate cancer cells. J Mol Endocrinol 1996;16:297–306.
Bartholdi MF, Wu JM, Pu H, Troncoso P, Eden PA, Feldman RI. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int J Cancer 1998;79:82–90.
Reile H, Armatis PE, Schally AV. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate 1994;25:29–38.
Xiao D, Wang J, Hampton LL, Weber HC. The human gastrin-releasing peptide receptor gene structure, its tissue expression and promoter. Gene 2001;264:95–103.
Plonowski A, Nagy A, Schally AV, Sun B, Groot K, Halmos G. In vivo inhibition of PC-3 human androgen-independent prostate cancer by a targeted cytotoxic bombesin analogue, AN-215. Int J Cancer 2000;88:652–7.
Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med 2000;41:1704–13.
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716–31.
Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, et al. DOTATOC: a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med 1997;24:792–5.
Zhang H, Chen J, Waldherr C, Hinni K, Waser B, Reubi JC, et al. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor-expressing tumors. Cancer Res 2004;64:6707–15.
Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, et al. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med 2003;44:823–31.
La Bella R, Garcia-Garayoa E, Langer M, Blauenstein P, Beck-Sickinger AG, August Schubiger P. In vitro and in vivo evaluation of a 99mTc(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl Med Biol 2002;29:553–60.
Nock B, Nikolopoulou A, Chiotellis E, Loudos G, Maintas D, Reubi JC, et al. [99mTc]Demobesin 1, a novel potent bombesin analogue for GRP receptor-targeted tumour imaging. Eur J Nucl Med Mol Imaging 2003;30:247–58.
Nock BA, Nikolopoulou A, Galanis A, Cordopatis P, Waser B, Reubi JC, et al. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: a preclinical study. J Med Chem 2005;48:100–10.
Smith CJ, Sieckman GL, Owen NK, Hayes DL, Mazuru DG, Kannan R, et al. Radiochemical investigations of gastrin-releasing peptide receptor-specific [99mTc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and in vitro/in vivo studies where Dpr = 2,3-diaminopropionic acid and X = H2O or P(CH2OH)3. Cancer Res 2003;63:4082–8.
Van de Wiele C, Dumont F, Dierckx RA, Peers SH, Thornback JR, Slegers G, et al. Biodistribution and dosimetry of 99mTc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor-expressing malignancies. J Nucl Med 2001;42:1722–7.
Schuhmacher J, Zhang H, Doll J, Macke HR, Matys R, Hauser H, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6–14) analog. J Nucl Med 2005;46:691–9.
Rogers BE, Bigott HM, McCarthy DW, Della Manna D, Kim J, Sharp TL, et al. MicroPET imaging of a gastrin-releasing peptide receptor-positive tumor in a mouse model of human prostate cancer using a 64Cu-labeled bombesin analogue. Bioconjug Chem 2003;14:756–63.
Meyer GJ, Macke H, Schuhmacher J, Knapp WH, Hofmann M. 68Ga-labelled DOTA-derivatised peptide ligands. Eur J Nucl Med Mol Imaging 2004;31:1097–104.
Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, et al. microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med 2004;45:1390–7.
Breeman WA, De Jong M, Bernard BF, Kwekkeboom DJ, Srinivasan A, van der Pluijm ME, et al. Pre-clinical evaluation of [111In-DTPA-Pro1, Tyr4]bombesin, a new radioligand for bombesin-receptor scintigraphy. Int J Cancer 1999;83:657–63.
Breeman WA, de Jong M, Erion JL, Bugaj JE, Srinivasan A, Bernard BF, et al. Preclinical comparison of 111In-labeled DTPA- or DOTA-bombesin analogs for receptor-targeted scintigraphy and radionuclide therapy. J Nucl Med 2002;43:1650–6.
Breeman WA, Hofland LJ, de Jong M, Bernard BF, Srinivasan A, Kwekkeboom DJ, et al. Evaluation of radiolabelled bombesin analogues for receptor-targeted scintigraphy and radiotherapy. Int J Cancer 1999;81:658–65.
Smith CJ, Gali H, Sieckman GL, Hayes DL, Owen NK, Mazuru DG, et al. Radiochemical investigations of 177Lu-DOTA-8-Aoc-BBN[7–14]NH2: an in vitro/in vivo assessment of the targeting ability of this new radiopharmaceutical for PC-3 human prostate cancer cells. Nucl Med Biol 2003;30:101–9.
Bakker WH, Albert R, Bruns C, Breeman WA, Hofland LJ, Marbach P, et al. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: synthesis, radiolabeling and in vitro validation. Life Sci 1991;49:1583–91.
Bakker WH, Krenning EP, Reubi JC, Breeman WA, Setyono-Han B, de Jong M, et al. In vivo application of [111In-DTPA-D-Phe1]-octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci 1991;49:1593–601.
van Weerden WM, de Ridder CM, Verdaasdonk CL, Romijn JC, van der Kwast TH, Schroder FH, et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol 1996;149:1055–62.
Bernard BF, Krenning E, Breeman WA, Visser TJ, Bakker WH, Srinivasan A, et al. Use of the rat pancreatic CA20948 cell line for the comparison of radiolabelled peptides for receptor-targeted scintigraphy and radionuclide therapy. Nucl Med Common 2000;21:1079–85.
De Jong M, Bernard BF, De Bruin E, Van Gameren A, Bakker WH, Visser TJ, et al. Internalization of radiolabelled [DTPA0]octreotide and [DOTA0,Tyr3]octreotide: peptides for somatostatin receptor-targeted scintigraphy and radionuclide therapy. Nucl Med Common 1998;19:283–8.
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24:389–427.
Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 1991;12:450–82.
Otte A, Mueller-Brand J, Dellas S, Nitzsche EU, Herrmann R, Maecke HR. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 1998;351:417–8.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging 2003;30:207–16.
de Jong M, Kwekkeboom D, Valkema R, Krenning EP. Radiolabelled peptides for tumour therapy: current status and future directions. Plenary lecture at the EANM 2002. Eur J Nucl Med Mol Imaging 2003;30:463–9.
De Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med 2002;32:133–40.
Kwekkeboom DJ, Bakker WH, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging 2003;30:417–22.
Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, et al. [177Lu-DOTA0Tyr3]octreotate: comparison with [111In-DTPA0]octreotide in patients. Eur J Nucl Med 2001;28:1319–25.
Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001;12:941–5.
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med 2001;28:426–34.
Sunday ME, Kaplan LM, Motoyama E, Chin WW, Spindel ER. Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest 1988;59:5–24.
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22.
Maina T, Nock BA, Zhang H, Nikolopoulou A, Waser B, Reubi JC, et al. Species differences of bombesin analog interactions with GRP-R define the choice of animal models in the development of GRP-R-targeting drugs. J Nucl Med 2005;46:823–30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Visser, M., Bernard, H.F., Erion, J.L. et al. Novel 111In-labelled bombesin analogues for molecular imaging of prostate tumours. Eur J Nucl Med Mol Imaging 34, 1228–1238 (2007). https://doi.org/10.1007/s00259-006-0356-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0356-3