Abstract
A novel unsymmetrical diamine monomer 4-(2-(4-(4-aminophenoxy)phenyl)propan-2-yl)benzenamine (APPBA) containing –O– and –C(CH3)– groups was synthesized from 2-(4-aminophenyl)-2-(4-hydroxyphenyl)propane and 4-nitrofluorobenzene by the nucleophilic substitution reaction followed by catalytic reduction. Polycondensation of this diamine with aromatic dianhydrides through thermal solution imidization techniques afford a series of novel processable polyimides (PI). The structure of the monomer and polyimides were confirmed by FT-IR and 1H-NMR. The polyimides were characterized by XRD, TGA, DSC, electrical property, solution viscosity and solubility test. These polyimides had inherent viscosities in the range 0.48–0.61 dL/g. The X-ray diffraction measurements indicated that all these polyimides were amorphous. The polyimides exhibited excellent solubility in most of the organic solvents. The glass transition temperature (Tg) was observed in the range 242–282 °C. The polyimides displayed good thermal stability and the temperatures at which 10 % weight loss occurred in the range 475–504 °C. The dielectric constant of PIs was in the range of 2.82–3.55.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyimides [1–6] are a class of thermally stable polymers based on stiff aromatic back-bones. Polyimides have excellent thermal, mechanical and electrical properties as well as outstanding chemical resistance [7, 8]. Polyimides are used in many applications in the electrical, electronics, automotive and aerospace industries in the form of films, fibres, foams, plastics, adhesives and coatings [9, 10]. The polyimides exhibit low solubility and the melting points are far above their decomposition temperatures. So the processing of the polyimides is difficult which limits the applications. Therefore, many significant efforts have been made to chemically modify the structure of these polyimides with the aim of improving their processability. The main approach used for the structural modifications include, the introduction of flexible groups (–O–, –CO, –SO2–, –S– and –C(CF3)2–) into the polyimides backbone which reduce the chain stiffness, introduction of bulky side groups which hinders the molecular packing and crystallization, use of enlarged monomers containing flexible unit which separate the rigid imides groups [11, 12]. The solubility of the polyimides can be increased by the incorporation of a kink in the polyimides backbone through ortho or meta catenation instead of para catenation which would prevent ordering of molecule [13]. The processability of the polyimides can also be increased by incorporating unsymmetrical units in the polyimides chain which would inhibit chain-chain packing and increase the flexibility of the chain, which substantially increases the solubility [14, 15].
The properties of the polymers depend to a large extent on the monomer’s structure and hence the synthesize of novel monomers is of prime importance in the design of novel polymers. An unsymmetrical diamine monomer containing flexible –O– and –C(CH3)2– groups was synthesized. The structurally modified monomer was polymerized into polyimides through high temperature solution imidization technique by making use of commercially available aromatic dianhydrides having flexible groups (–CO, –O– and –C(CF3)2–). Since the molecular structure of the new diamine is asymmetric, the prepared polyimides should exhibit excellent solubility and retain other desirable properties. The flexible –CO, –O–, –C(CH3)2– and –C(CF3)2– groups in the polyimides chain will reduce the chain stiffness and gives flexibility, while the para phenylene units provide rigidity to the polyimides and the influence of these two opposing effects on properties of the polyimides are studied. The high temperature electrical insulation of polyimide film gains growing importance due to demand from various industrial and domestic insulation applications. The polyimides were studied for possible application as high temperature electrical insulation materials.
Experimental
Measurements
FT-IR spectra were obtained on Perkin Elmer Spectrum One and 1H-NMR spectra were recorded on a Bruker 300 MHz instrument. X-ray diffractograms were obtained on Bruker D8 FOCUS using CuKά radiation. Thermogravimetry (TG) analysis was carried out using the SDT-600 model of TA Instruments-USA at a heating rate of 10 °C / min in nitrogen atmosphere. Differential scanning calorimetric analysis was performed on DSC, Q20, TA instrument-USA at a heating rate of 10 °C / min in nitrogen atmosphere. Dielectric constant was determined by using HIOKI LCR HiTester 3532-50 at a frequency of 1 MHz. Inherent viscosity was determined at a concentration of 0.5 g /dL in NMP. The solubility of polyimides was determined at a 5 wt % concentration in various solvents.
Materials
Commercial bisphenol-A, aniline, 4-nitrofluorobenzene, pyromellitic dianhydride (PMDA), biphenyl tetracarboxylic dianhydride (BPDA), benzophenonetetracarboxylic dianhydride (BTDA), oxydiphthalic anhydride (ODPA) and hexafluoroisopropylidene diphthalic dianhydride (6FDA) were purchased from Aldrich Chemicals. The starting materials 2-(4-aminophenyl)-2-(4-hydroxyphenyl) propane was prepared in the laboratory from aniline hydrochloride and bisphenol-A as per the procedure reported in our previous publication [16]. All the solvent and reagents used were analytical grade.
Synthesis of unsymmetrical monomer
4-(2-(4-(4-nitrophenoxy) phenyl) propan-2-yl) benzenamine
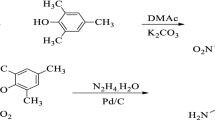
The nitro compound III was prepared by the nucleophilic substitution reaction [17–19]. 4-nitrofluorobenzene (II) (0.05 mol) was dissolved in 50 mL of dry NMP. To this 2-(4-aminophenyl)-2-(4-hydroxyphenyl) propane (I) (0.05 mol) and K2CO3 (0.12 mol) were added. Toluene (15 mL) was added into the mixture and heated at 120 °C for 6 h at nitrogen atmosphere with continuous stirring. The water formed was removed azeotropically using Dean-Stark trap. After complete dehydration, temperature was raised to 140 °C to remove the toluene and the reaction was continued for 6 h. The reaction mixture was cooled and poured into water. The gummy precipitate formed was filtered, washed with excess of 5 % NaOH solution and water. The 4-(2-(4-(4-nitrophenoxy) phenyl) propan-2-yl) benzenamine III collected was dried in vacuum oven. Colour: yellow, Yield: 98 %, IR (KBr): 1587 cm−1, 1342 cm−1 (–NO2 stretch), 2966 cm−1 (C-C stretch) 1248 cm−1 (C–O–C stretch), 3452 cm−1 and 3374 cm−1 (N-H stretch). 1H-NMR (300 MHz, DMSO-d6, ppm): δ 1.38(s,6 H,-CH3), δ 4.65 (s,2 H,-NH2), δ 6.25–6.27 (d, 2 H, Ar), δ 6.65–6.67 (d,2 H, Ar), δ 6.78–6.85 (m, 4 H, Ar), δ 7.02–7.06 (d,2 H,Ar), and δ 7.91–8.06 (d,2 H, Ar).
4-(2-(4-(4-aminophenoxy) phenyl) propan-2-yl) benzenamine (APPBA)
A mixture of 4-(2-(4-(4-nitrophenoxy)phenyl)propan-2-yl)benzenamine (0.04 mL), anhydrous “ Sn” metal ( 0.06 mol ) and ethanol (60 mL) was introduced into a round bottomed flask and stirred, into which 20 mL of HCl was added drop wise over a period of 15 min at ice cooled temperature [20–22]. After the addition of HCl was complete, the mixture was heated at reflux temperature for 6 h. After complete reduction of the nitro group, the reaction mixture was cooled and poured into water. The solution was neutralized by adding dilute NaOH solution, the precipated diamine was collected by filtration. The precipitate containing inorganic impurities was dissolved in acetone. Diamine alone dissolved in acetone leaving behind the inorganic impurities. The solution obtained was evaporated to collect the pure gummy solid 4-(2-(4-(4-aminophenoxy) phenyl) propan-2-yl) benzenamine (APPBA) IV. Colour: brown, Yield: 96 %, IR (KBr): 3538 cm−1 and 3414 cm−1 (N-H stretch), 2971 cm−1 (C–C aliphatic stretch) and 1250 cm−1 (C–O-C stretch). 1H-NMR (300 MHz, DMSO-d6, ppm): δ 1.51(s,6 H,-CH3), δ 4.90 (s,4 H, -NH2), δ 6.44–6.47(d, 2 H, Ar), δ 6.55–6.58 (d,2 H, Ar), δ 6.69–6.74 (m, 4 H, Ar), δ 6.83–6.86 (d,2 H,Ar) and δ 7.08–7.11(d,2 H, Ar).
Synthesis of polyimides-PIs
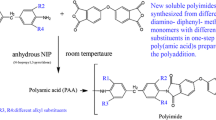
Polyimides PI-1 to PI-5 were prepared by the thermal solution imidization techniques [23, 24]. The typical procedure adopted was as follows. To a stirred solution of PMDA (Va)(0.006 mol) in NMP (25 mL), diamine APPBA (IV) (0.006 mol) was added under nitrogen atmosphere and stirred at room temperature for 12 h to get poly(amic acid). To this, azeotropic agent toluene (20 mL) was added and the mixture was refluxed at 160 °C for 6 h. The progress of the imidization reactions were observed by the collection of water by toluene–water azeotropic distillation making use of Dean-Stark trap. After complete imidization, the temperature was increased to remove the toluene. The reaction mixture was cooled, poured into water, and the precipitated polyimide PI-1 was filtered, washed with dilute HCl, water, methanol and dried. Polyimides PI-2 (BPDA/APPBA), PI-3 (BTDA/ APPBA), PI-4 (ODPA/ APPBA) and PI-5 (6FDA/ APPBA) were synthesized in the same procedures as adopted for the synthesize of PI-1.
Result and discussion
Characterization of 4-(2-(4-(4-nitrophenoxy)phenyl)propan-2-yl)benzenamine
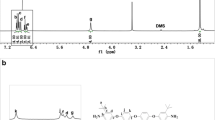
The nitro compound (III) was obtained by the nucleophilic substitution reaction of 4-nitrofluorobenzene (II) with 2-(4-aminophenyl)-2-(4-hydroxyphenyl)propane (I) as shown in Scheme 1. The IR spectrum of the nitro compound III showed a characteristic strong absorption band for the nitro group at 1587 cm−1 (symmetrical stretching), 1342 cm−1 (asymmetrical stretching), strong absorption band for –NH2 group at 3452 cm−1 and 3374 cm−1 (N-H stretch), 2966 cm−1 (C–C aromatic, stretch), and 1248 cm−1 (C–O–C stretch). The IR spectrum of the nitro compound III confirmed the formation of ether group between the 4-nitrofluorobenzene (II) and 2-(4-aminophenyl)-2-(4-hydroxyphenyl)propane(I). The 1H-NMR spectrum of the nitro compound III is given in Fig. 1. The two proton present in the benzene ring ortho and meta to the –NH2 group appeared as a doublet at 6.25–6.27 δ ppm and 6.65–6.67 δ ppm respectively. The six aliphatic protons appeared at 1.38 δ ppm as a singlet. The aromatic proton ortho to the isopropylidene group and lying between ether and isopropylidene group appeared as doublet at 7.02–7.06 δ ppm. The aromatic proton meta to the isopropylidene group and lying between ether and isopropylidene group and the aromatic proton meta to the nitro group designated by the symbol as “ f ” and “ g ” respectively merged and appeared as a multiplet at 6.78–6.85 δ ppm. The aromatic proton ortho to the nitro group appeared as a doublet at 7.91–8.06 δ ppm. The protons present in the two aromatic amino group appeared as a singlet at 4.65 δ ppm. The protons designated in the Fig. 1 as “ h ” appeared in the farthest downfield in the range 7.91–8.06 δ ppm was due to the electron withdrawing –NO2 group. The peaks at 2.5 δ ppm and at 3.4 δ ppm are due to DMSO and water in DMSO.
Characterization of 4-(2-(4-(4-aminophenoxy) phenyl) propan-2-yl) benzenamine
The unsymmetrical diamine monomer (IV) was successfully synthesized by reduction of the nitro compound (III) using Sn / HCl catalyst as shown in Scheme 1. The IR spectrum of the diamine IV showed a characteristic absorption band for the –NH2 group at 3538 cm−1 and 3414 cm−1 (NH stretch), 2971 cm−1 (C–C aliphatic stretch), and 1250 cm−1 (C–O–C stretch). The disappearance of strong absorption due to nitro group at 1587 cm−1& 1342 cm−1 that was present in the IR of the nitro compound III indicated the complete reduction of nitro group into amino group. The 1H-NMR spectrum of diamine is given in Fig. 2. The protons present in the benzene ring ortho to the –NH2 group and lying between –NH2 group and isopropylidene group appeared as a doublet at 6.55–6.58 δ ppm. The six aliphatic protons appeared at 1.51 δ ppm as a singlet. The protons present in the benzene ring at meta and ortho to the ether group and lying between ether and isopropylidene group designated by “e” and “f ” appeared as a doublet at 7.08–7.11 δ ppm and 6.83–6.86 δ ppm respectively. The proton ortho to the –NH2 group and lying between –NH2 group and ether group appeared as a doublet at 6.44–6.47 δ ppm. The proton present at meta to the –NH2 group and ortho to the isopropylidene group designated as “c” and the proton present meta to the –NH2 group and ortho to the ether group designated as “g” merged and appeared as a multiplet at 6.69–6.74 δ ppm. The amino protons originally present in the nitro compound-III and the newly formed amino protons merged and appeared as a singlet at 4.90 δ ppm. The displacement of the farthest downfield signal that was present in the 1H-NMR of the nitro compound III in the range 7.91–8.06 δ ppm (because of the electron withdrawing –NO2 group) and appearence of signal in the range of 6.44–6.47 δ ppm and also the increase in integral value doubly at 4.90 δ ppm (4 H,-NH2) confirmed the conversion of –NO2 group into –NH2 group. The peaks at 2.5 δ ppm and at 3.4 δ ppm are due to DMSO and water in DMSO.
Characterization of polyimides
The polyimides PI-1 to PI-5 were prepared by high temperature solution imidization techniques as shown in Scheme 2. The characteristics of the polyimides are given in Table 1. The IR spectra of PIs showed characteristic imides ring absorption at about 1776–1780 cm−1 (imide –CO symmetrical stretching), 1720–1725 cm−1 (imide –CO asymmetrical stretching),720 –740 cm−1 (imide ring deformation) and 1375–1378 (C-N stretching ) associated with imide structure indicated the formation of imide ring. The disappearance of strong absorption corresponding to amino group at 3538 cm−1 and 3414 cm−1 that was present in the IR of the diamine monomer and the appearance of absorption corresponds to imide group indicated the formation of imide ring between the diamine and the dianhydrides. The IR spectral data of polyimides PI-1 to PI-5 showed the formation of imide ring between the unsymmetrical diamine APIPA and the corresponding aromatic dianhydrides. 1H-NMR spectrum of PI-3 is given in Fig. 3. 1H-NMR spectrum of PI-3 showed absorption at 8.13–8.23 δ ppm, 7.31–7.47 δ ppm, 7.02–7.15 δ ppm and 1.61–1.64 δ ppm. The disappearance of high field signal that was present in the diamine monomer (APPBA) at 4.90 δ ppm corresponding to the amino group and the appearance of farthest downfield signal in the range 8.13–8.23 δ ppm due to electron withdrawing imide group indicated the formation of imide ring between the unsymmetrical diamine and BTDA. The observed 1H-NMR spectrum was broad and the peaks are merged. This was due to the fact that the polyimides PI-3 have three different structural isomers (Fig. 4) due to the non-symmetrical structure of diamine APPBA. The observed 1H-NMR spectrum was fully in agreement with the chemical structure of the polyimides PI-3. The polyimides were obtained in quantitative yield with the inherent viscosity in the range 0.48–0.61 dL/g indicating formation of polymers of moderate molecular weight.
Solubility characteristics polyimides
The solubility behavior of the polyimides (PI-1 to PI-5) was investigated in conventional organic solvents and the results are presented in the Table 2. All the polyimides were soluble in NMP and DMSO. All the polyimides except PI-1 were soluble in o-cresol and pyridine at room temperature. The polyimides PI-1 showed poor solubility in organic solvents due to presence of rigid imide rings based on PMDA. Among all, the polyimides PI-4 and PI-5 showed higher solubility due to the presence of bulky pendent –C(CF3)2–, –C(CH3)2– groups and flexible –O– groups in the backbone which disturb the ordering of molecule, thereby leading to increase in solubility. In general the higher solubility of polyimides indicated an amorphous character to the polyimides and was supported by the X-ray diffraction study, the diffraction patterns of the polyimides were broad. The solvent molecule diffuses easily through the amorphous polyimides and the polyimides chains get separated. The solubility of the polyimides was found to decrease in the following order PI-5 (6FDA/ APPBA) = PI-4 (ODPA/ APPBA) > PI-3 (BTDA/ APPBA) > PI-2 (BPDA/ APPBA) > PI-1 (PMDA/ APPBA). The higher solubility of the polyimides may be due to the presence of unsymmetrical diamine unit, the bulky –C(CH3)2– and –C(CF3)2– groups and the flexible –O– groups in the polymer chain. The asymmetric diamines led to the formation of twisted structures and configuration isomers of the repeat unit along the polymer backbone and cause the decrease in the intermolecular force and packing ability of the resulting polymers. These asymmetric twisted structures, bulky pendent –C(CF3)2– and –C(CH3)2– groups and flexible –O– groups of these polyimides loosen the polymer chain packing and subsequently led to increase in solubility.
Thermal stability of polyimides
Thermal stability of polyimides was investigated by thermogravimetric analysis (TGA) at a heating rate of 10 °C / min in nitrogen. The TG curves are shown in Fig. 5. The thermal analysis data is summarized in Table 3. The 10 % and 20 % weight loss occurred ranges from 475 to 504 °C and 510 to 533 °C respectively. The initial decomposition (Td) of the polyimides occurred in the temperature range of 220–245 °C. The char yield of the polyimides at 800 °C was in the range of 42–52 %. The higher char yield of these polyimides was due to the presence of rigid imide group in the backbone. The PI-5 (6FDA/ APPBA) showed comparatively higher thermal stability and higher char yield which can be attributed to strong C-F bond present in –C(CF3)2– unit in the polyimides chain.
The DTG curve indicated that the temperature at the maximum degradation (Tmax) of the polymides occurred in the range of 519–539 °C and is comparable to the maximum degradation temperature of other polyimides. Among all the polyimides the PI-5 (6FDA/ APPBA) showed higher Tmax ( 539 °C ) due to the presence of strong C-F bond present in –C(CF3)2– unit in the polyimides chain while the polyimides PI-3 (BTDA/ APPBA) and PI-4 (ODPA/ APPBA) have less Tmax (519 °C ) due the presence of –CO and –O– respectively in the polyimides chain.
Limited oxygen index - self extinguishing polymer
The limitied oxygen index (LOI) is the minimum concentration of oxygen expressed as a percentage that will support combustion of a polymer-the higher the LOI the lower the flammability. The LOI value can be used to evaluate the flame-retardancy of polymeric materials [25, 26]. Since air comprises about 21 % oxygen by volume, any material with a limiting oxygen index less than this will burn easily in air, while those with a higher LOI will tend not to burn. The higher the value of the LOI the safer the material. Theoretically, char yield can also be used for evaluating LOI of polymers materials. According to Van Krevelan–Hoflyzers equation LOI = 17.5 + 0.4 CR where CR is the char yield at 800 °C. The LOI values of PIs were in the range of 34–38. On the basis of the LOI values, such PIs can be classified as self extinguishing polymers.
Phase transitions of polyimides
The behavior of the PIs towards the heat was evaluated by DSC in nitrogen atmosphere at a heating rate of 10 °C/min. Representative DSC curve for the polyimides PI-2 is given in Fig. 6. The glass transition temperature was observed in the temperature range 242–282 °C. Endotherms corresponding to the crystalline melt temperature (Tm) were not observed for any of the polyimides, indicating that these polyimides were amorphous and was supported by X-ray diffraction studies. The higher Tg value was due to presence of rigid imide group in the polyimide chain.
Morphology of polyimides
The polyimides were structurally characterized by X-ray diffraction studies. The diffraction patterns were broad and no well defined peaks were observed in any of the polyimides, which revealed that all these polyimides were completely amorphous. The amorphous nature of these polyimides was well reflected in their excellent solubility, which is in agreement with the general rule that the solubility increases with decreasing crystalline character of the polymers. This amorphous morphology was due to the presence of unsymmetrical diamine, bulky hexafluoroisopropylidene and isopropylidene units and flexible ether and carbonyl groups in the polyimides chain. The para-phenylene units of the diamine try to pack the polyimides molecules, while the bulky and flexible unit decrease the inter chain interaction and loosen the polyimides chain packing and led to amorphous morphology. The solubility behavior was consistent with the results of X-ray diffraction studies.
Electrical properties of polyimides
The dielectric constant is an important parameter for selecting electrical insulation material [27, 28]. The dielectric constant of the PIs was determined at a frequency of 1 MHz. The dielectric constant of PIs was in the range of 2.82–3.55. The PIs have electrical insulation character and can be used in insulation of electrical items operating at elevated temperatures.
Conclusion
The objective of this study was the preparation of novel processable and thermally stable polyimides. An unsymmetrical diamine monomer was successfully synthesized and fully characterized. The PIs with moderate molecular weight were prepared from polycondensation reaction between the diamine monomer with various dianhydrides. Introduction of asymmetric monomer units, –C(CH3)2– , –C(CF3)2– and –O– groups along the polyimides backbone causes a decrease in intermolecular force and loosen the chain packing and the polyimides have balanced solubility and thermal stability. Thus, the new polyimides exhibited excellent solubility and thermal stability. Among the flexible unit, the –C(CF3)2– unit exhibited higher processability and higher thermally stability to the polyimides. The LOI values of PIs were in the range 34–38 and therefore can be used as self extinguishing polymers. The polyimides exhibited excellent dielectric properties and can be used as high temperature insulation materials in electric motors, generators, etc. These properties could make these PIs attractive for practical applications, such as processable high-performance engineering plastics.
References
Tsai P-F, Chyong-Fen W, Hsiao Y-C, Shau M-D (2009) Properties of novel polyimides containing bismaleimide and cyclic phosphine oxide. J Polym Res 16:673–680
Fang X, Wang Z, Yang Z, Gao L, Li Q, Ding M (2003) Novel polyimides derived from 2,3,3′,4′-benzophenonetetracarboxylic dianhydride. Polymer 44:2641–2646
Mallakpour S, Tirgir F, Sabzalian MR (2011) Synthesis and structural characterization of novel biologically active and thermally stable poly(ester-imide)s containing different natural amino acids linkages. J Polym Res 18:373–384
Vijay Kumar S, Yu H-C, Choi J, Kudo K, Jang Y-H, Chung C-M (2011) Structure–property relationships for partially aliphatic polyimides. J Polym Res 18:1111–1117
Perry RJ, Scott E, Tunny B, Wilson D (1996) Polyimides formation through the palladium-mediated carbonylation and coupling of Bis(o-iodo amides) and diamines. Macromolecules 29:1014–1020
Hsiao S-H, Guo W, Kung Y-C, Lee Y-J (2011) Redox-active and electrochromic aromatic poly(amide-imide)s with 2,4-dimethoxytriphenylamine chromophores. J Polym Res 18:1353–1364
Yang CP, Su YY (2003) Properties of organosoluble aromatic polyimides from 3′-trifluoromethyl-3,4′-oxydianiline. Polymer 44:6311–6322
Seyed M, Amini N, Mousa G (2011) Synthesis and characterization of new polyamides and polyimides containing dioxypyrimidine moiety in the main chain with bulky imidazole pendent group: solubility, thermal and photophysical properties. J Polym Res 18:1575–1586
Ree M (2006) High performance polyimides for applications in microelectronics and flat panel displays. Macromol Res 14:1–33
Koytepe S, Pasahan A, Ekinci E, Alıcı B, Seckin T (2008) Synthesis, characterization of phosphine oxide-containing polyimides and their use as selective membrane for dopamine. J Polym Res 15:249–257
Thiruvasagam P, Venkatesan D (2009) Synthesis and characterization of polyetherimides derived from AB monomers. J Macromol Sci Pure Appl Chem 46:419–424
Thiruvasagam P, Vijayan M (2012) Synthesis of new diacid monomers and poly(amideimide)s: study of structure-property relationship and applications. J Polym Res 19:9845
Thiruvasagam P, Shiva C, Sundararaman C, Venkatesan D (2011) Synthesis and characterization of new Diimide Diols and Processable Poly(esterimide)s derived there from. Polymer Polymer Compos 19:717–726
Li Y, Wang Z, Li G, Ding M, Yan J (2012) Synthesis and properties of polyimides based on isomeric (4,4′-methylenediphenoxyl) bis(phthalic anhydride)s (BPFDAs). J Polym Res 19:9772
Ghaemy M, Alizadeh R (2009) Synthesis of soluble and thermally stable polyimides from unsymmetrical diamine containing 2,4,5-triaryl imidazole pendent group. Eur Polym J 45:1681–1688
Thiruvasagam P, Venkatesan D (2010) Synthesis and characterization of processable aromatic polyimides. High Perform Polymer 22:682–693
Saeed Butt M, Akhter Z, Zafar-uz-Zaman M, Siddiqi HM (2008) Synthesis and characterization of some Schiff-base-containing polyimides. Colloid Polym Sci 286:1455–1461
Thiruvasagam P, Venkatesan D (2011) Synthesis and characterization of polyetherimides via nitrodisplacement reaction. High Perform Polymer 23:22–31
Nasab SMA, Ghaemy M (2011) Synthesis and characterization of new polyamides and polyimides containing dioxypyrimidine moiety in the main chain with bulky imidazole pendent group:solubility, thermal and photophysical properties. J Polym Res 18:1575–1586
Zhang S-J, Li Y-F, Wang X-L, Zhao X, Shao Y, Ma T (2007) Synthesis and characterization of soluble polyimides derived from the polycondensation of 2, 6-bis (4-amino-2- trifluoromethylphenoxy-4′-benzoyl)pyridine with some aromatic dianhydrides. High Perform Polymer 19:462–476
Chen J-C, Rajendran K, Huang S-W, Chang H-W (2011) Synthesis and characterization of aromatic polyamides derived from various derivatives of 4,4′-oxydianiline. J Polym Res 18:1693–1703
Chern Y-T, Twua J-T, Chen J-C (2009) High Tg and high organosolubility of novel polyimides containing twisted structures derived from 4-(4-amino-2-chlorophenyl)-1-(4-aminophenoxy)-2,6-di-tert-butylbenzene. Eur Polym J 45:1127–1138
Kim YP, Glass TE, Lyle GD, Mcgrath JE (1993) kinetics and mechanistic investigation of the formation of polyimides under homogeneous conditions. Macromolecules 36:1354–1358
Yilmaz T, Guclu H, Ozarslan O, Yildiz E, Kuyulu A, Ekinci E, Gungor A (1997) Kinetic investigations of formation of polyimides containing arylene sulfone ether linkages by potentiometric titration and their characterization. Polym Sci Part A Polym Chem 35:2981–2990
Mallakpour S, Soltanian S, Sabzalian MR (2011) Studies on synthesis and in vitro biodegradability of novel optically active nanostructure poly(ester-imide)s containing L-phenylalanine and L-isoleucine linkages. Colloid Polym Sci 289:93–100
Khalil F, Mohsen H, Meisam S (2010) New photosensitive and optically active organo-soluble poly(amide–imide)s from N, N′-(bicyclo[2, 2, 2]oct-7-enetetra carboxylic)-bis-L-amino acids and 1,5-bis(4-aminophenyl)penta-1,4-dien-3-one: synthesis and characterization. J Polym Res 17:379–390
Yang C-P, Chen Y-C, Hsiao S-H, Guo W, Wang H-M (2010) Optically transparent and colorless poly(ether-imide)s derived from a phenylhydroquinone bis(ether anhydride) and various trifluoromethyl-substituted bis(ether amine)s. J Polym Res 17:779–788
Purushothaman R, Mohammed Bilal I, Palanichamy M (2011) Effect of chemical structure of aromatic dianhydrides on the thermal, mechanical and electrical properties of their terpolyimides with 4,4′-oxydianiline. J Polym Res 18:1597–1604
Acknowledgments
The author expresses his thanks to SASTRA University for financial support (T.R. Rajagopalan fund).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiruvasagam, P. Synthesis of new unsymmetrical diamine and polyimides: structure–property relationship and applications of polyimides. J Polym Res 19, 9965 (2012). https://doi.org/10.1007/s10965-012-9965-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9965-7