Abstract
Poly(3-hydroxybutyrate-co-hydroxyvalerate) (PHBV) is a biodegradable polymer synthesized in microorganisms. The application of PHBV is limited by certain material disadvantages. Poly(ε-caprolactone) (PCL) possesses excellent thermodynamic and mechanical properties and was used to modify PHBV in the presence of triethyl citrate (TEC) and dicumyl peroxide (DCP), which was used as plasticizer and grafting agent, respectively. The effects of PCL and additive agents on the mechanical, thermal, amphipathic and degradability behaviors of the blends were investigated. The results showed that the mechanical properties of the PHBV blends improved by PCL incorporation and improved even further after TEC and DCP addition. The addition of DCP could not induce an increase in crystallization temperature but improved the crystallization degree of the blends. The presence of hydrophilic groups in TEC leads to an apparent increases in the hydrophilicity of the PHBV blends. A PHBV/PCL blend (40/60) with TEC (20 wt.%) and DCP (0.5 wt.%) was chosen for its good mechanical properties and hydrophilicity. The chosen ratio of the blends was also shown a preferable degradation activity by biodegradation assay using Pseudomonas mendocina. The addition of TEC and DCP has no conspicuous negative effect on the biodegradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are storage compounds accumulated by numerous bacteria as carbon and energy reserve materials under imbalanced growth conditions. Poly(3-hydroxybutyrate-co-hydroxyvalerate) (PHBV), a member of PHAs family, exhibits biodegradability, biocompatible characteristics and properties similar to traditional petrochemical plastic materials and is widely used in many branches of science and technology, such as plastic products, pharmaceutical carriers, and surgical suturing [1, 2]. Besides these advantages, however, the material also exhibits several shortcomings, such as poor thermal stability and fragility properties that narrow its utilization temperature range [3, 4]. Therefore, PHBV must be modified with other polymer materials to improve its comprehensive performance and expand its applications.
Several researchers have conducted simple physical blending modification to improve the properties of PHBV [5]. Nanofibrillated cellulose (NFC) has been added to PHBV via solution casting to increase tensile modulus and promote the crystallization of PHBV/NFC nanocomposites [6]. Certain plasticizers, including polyethylene glycol (PEG), triethyl citrate (TEC), epoxidized soybean oil and propylene glycol, have been used to promote PHBV blend flexibility and elongation [7, 8]. Chemical block copolymerization modification is a simple and effective approach to improve the mechanical properties of modification products. poly(butylene succinate), PEG, and poly (L-lactic acid) are used to modify PHBV via addition of dicumyl peroxide (DCP) to improve tensile toughness [9, 10]. Synthetic polymers and natural polymers, such as dextrans, have also bound together with PHBV as a backbone. Grafts with better thermal properties have been prepared by combining dextrans containing azide groups and PHBV oligomers containing an alkyne end group by click-chemistry [11].
Poly(ε-caprolactone) (PCL) is a semi-crystalline linear aliphatic polyester derived from the ring opening polymerization of ε-caprolactone. It is a biodegradable, biocompatible, and nontoxic polyester with excellent mechanical strength [12–14]. This work aims to develop a simple and efficient method to prepare functionalized polymer materials with superior mechanical and thermal properties. PHBV was modified with PCL by addition of a plasticizer and a crosslinking agent. The tensile properties, hydrophilic properties, crystallization and melting behaviors, thermal stability, and phase morphology of the polymer product were characterized to identify a suitable PHBV/PCL proportion. Finally, the biodegradation behavior of a suitable proportion of PHBV/PCL by Pseudomonas mendocina was investigated.
Materials and Methods
Materials
PHBV containing 12 mol. % hydroxyvalerate (HV) was obtained from Ningbo Tianan Biologic Material Co. (Ningbo, China). The number-average molecular weight of PHBV was 300,000–400,000. PCL grains with a melt index of 5 g/10 min, trademark CAPA 650, and reported M v of 70,000 were supplied by Solvay (Brussels, Belgium). DCP and TEC were purchased from Aladdin Industrial Inc. (Shanghai, China).
Sample Preparation
Two different methods were used for polymer blends. PHBV and PCL were directly mixed with different ratio (20/80, 40/60, 50/50, 60/40, and 80/20) at 180 °C and 32 rpm for 10 min by using a Rheocord 90 Haake rheometer. On the other hand, PHBV and TEC with 20 wt% content were dissolved in chloroform at 50 °C under stirring for 80 min. After chloroform was removed, the copolymers were also mixed with 20, 40, 50, 60, or 80 wt% PCL in the presence of DCP (0.5 wt%) at 180 °C and 32 rpm for 10 min by using a Rheocord 90 Haake rheometer. Blank samples with PHBV or PCL were also established.
Characterization of PHBV/PCL Blends
Tensile Testing
Tensile strength and elongation at break were determined according to ASTM D638-5 at a crosshead speed of 20 mm/min using an Instron 5500R Universal Testing Machine (Instron Corp., Canton, MA). Sample films were formed into rectangles with dimensions of 60 mm × 1 mm × 25 mm.
Thermogravimetry (TG)
The thermal behaviors of the modified films were investigated by thermogravimetric analysis (TGA; Pyris-1, Perkin-Elmer, Waltham, MA). All scans were carried out from room temperature to 600 °C at a heating rate of 20 °C min−1 to 600 °C under a nitrogen atmosphere (40 mL min−1).
Differential Scanning Calorimetry (DSC)
The thermal properties of the samples were analyzed by using a differential scanning calorimeter (DSC; Diamond, Perkin-Elmer). Duplicates of specimens weighing approximately 5 mg were used for the DSC study. All of the samples were heated from room temperature to 200 °C at a rate of 10 °C min−1, cooled at the same rate to −50 °C, and then re-heated to 200 °C at 10 °C min−1 under nitrogen flowing at a rate of 50 mL min−1.
Polarizing Optical Microscope (POM)
Spherulite morphologies were characterized by a polarizing microscope (BX51 POM, Olympus) with a hot stage (LTS420, Linkam); here, the PHBV blends were heated to 190 °C at a rate of 30 °C min−1, maintained at this temperature for 2 min, subsequently cooled to room temperature, and then heated to 190 °C at different rates.
Scanning Electron Microscopy (SEM)
The fracture morphology of PHBV blend films were observed by SEM (XL30 ESEM-FEG, FEI Co., Netherlands) at an acceleration voltage of 4 keV.
Water Contact Angle
The hydrophilic properties of samples were assayed through micro-optical angle measurement (OCA40, Data Physics Co., Germany). Here, the static liquid injection speed was 0.6 μL s−1, and the injection volume was 3.0 μL. Each sample was tested thrice and spaced at 1 cm intervals. The final results were obtained by averaging.
Biodegradation of PHBV/PCL Blends
P. mendocina DS04-T, which can degrade both PHBV and PCL, was grown in mineral medium containing 0.15 % (w/v) PHBV with or without agar. The mineral medium consisted of Na2HPO4·12H2O, KH2PO4, NH4Cl, MgSO4·7H2O,CaCl2·2H2O and the pH was adjusted to 8.
P. mendocina DS04-T strains were inoculated into liquid mineral medium containing the sample films and incubated at 37 °C. Samples were collected at different incubation times (i.e., 12, 24, 36, 48, 60, 72, 84, or 96 h), and a blank sample was prepared. Each film was collected and thoroughly washed with double-distilled water and EtOH to remove media components or bacterial cells present on the film surface. Then, the films were dried at 60 °C until a constant weight was achieved. Degradation was determined by the difference in sample weight before and after incubation. The weight loss and degradation rates of the sample degraded by P. mendocina DS04-T were measured and calculated.
Changes in the surface morphology of the sample film after degradation were studied by using an SEM (XL30 ESEM-FEG) with an acceleration voltage of 4 keV.
Results and Discussion
Mechanical Behaviors of PHBV/PCL Blends
Mechanical behaviors are key parameters of performance of polymers in various engineering applications. The results of elongation and tensile strength are shown in Figs. 1 and 2. As shown in Fig. 1, the tensile strength of PHBV/PCL blends with TEC and DCP is lower than that of without TEC or DCP for the same ratio. The lowest tensile strength of the blends are both approximately 40 MPa at a ratio of 40:60 (PHBV: PCL) whether or not adding TEC and DCP. The tensile strength of the blends appears to decrease and then increase with increased content of PHBV. The decreasing of tensile strength is because that PHBV (dispersed phase) is scattered in the PCL (continuous phase) when PHBV content is lower. Then with further increasing of PHBV, PCL was changed to disperse phase and scattered into PHBV. It damaged the plasticity of PCL and leaded to the increase of tensile strength.
In Fig. 2, the elongation of PHBV/PCL blends with TEC and DCP is evidently higher than that of without TEC and DCP. Along with the increase of PHBV ratio, the elongation of the blends with or without TEC and DCP appear to increase firstly and then decrease. The elongation of blends with TEC and DCP also reached maximum levels at a ratio of 40:60 (PHBV: PCL), and it is about three times higher than that of without TEC and DCP.
The results are better than that of blending PHBV with PEG and DCP in previous report [15]. The improvements observed in this work may be attributed to the good plasticity of PCL offsetting the brittleness of PHBV, thereby minimizing the overall tensile strength and enhancing flexibility. On the other hand, because the TEC were plugged into the PHBV chain, the intermolecular forces of the blends were weakened. So the blends at a ratio of 40:60 (PHBV: PCL) exhibited the better mechanical properties in the presence of TEC and DCP.
Thermal Stability and Crystalline Behavior of the PHBV/PCL Blends
Thermal stability is an important issue in the research and applications of PHBV blends. The thermal degradation kinetics of the blends was investigated by TGA, as shown in Fig. 3. Degradation of pure PHBV and PCL, which occurs in one step, begins at approximately 269 and 345 °C, respectively, and is completed at 298 °C and 438 °C, respectively. The TG curves of the blank samples (Fig. 3a) show that the weight loss of PHBV/PCL undergoes two-step processes, with initial and final decomposition temperatures similar to those of pure PHBV and PCL. The samples added TEC and DCP have the same tendency (Fig. 3b) with blank samples.
TGA results showed that the weight loss transition temperature of the PHBV/PCL blends with TEC and DCP shifted toward slightly lower values compared with those of blank samples. Addition of DCP induced grafting of PHBV with PCL and destroyed high-energy functional groups to form new functional groups with low energy. Thus, the TGA results in this study are similar to previously reported changes in PHBV blends [16]. TGA revealed a decrease in transition temperature in blends with TEC and DCP; the DSC curves obtained showed the same results [17].
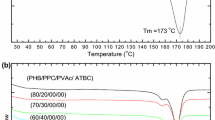
The brittleness of pure PHBV is caused by its high crystallinity and heterogeneous crystallization [18]. The crystallization behavior of the PHBV blends was studied by using DSC. Figure 4a is the heating curve of PHBV/PCL without addition of TEC and DCP. According to Fig. 4a, the pure PHBV and PCL begin to crystallize at 98 and 33 °C and the peaks appear at 85 and 29 °C, respectively. A non-interference phenomenon between PHBV and PCL during crystallization was also observed in Fig. 4a. The heating curve of PHBV/PCL with TEC and DCP (Fig. 4b) shows that TEC and DCP play important roles in lowering the crystallization temperature of PHBV. By contrast, the crystallization temperatures of blends with TEC and DCP increase by 25 °C at most with 80 % PCL content. PHBV and PCL functioned instead as a block copolymer, and the significant crystallization property of PHBV offset that of PCL. Therefore, PHBV/PCL blends with TEC and DCP crystallized at lower temperatures and the degree of crystallinity was decreased [19]. As DCP increased with PCL, the crystallization temperature of the PHBV/PCL (20/80) blends exceeded those of the others.
POM micrographs of PHBV blends are shown in Fig. 5. These micrographs provide an easy means of studying the crystallization process of the two components. Figure 5 reveals significant differences among the PHBV blends. In PHBV, spherulites presents a Maltese cross and dense distribution. First, nucleus growth occurs over the central region and then spreads until the crystals cover the entire field of vision. These highly distinctive morphologies may be attributed to the presence of DCP and TEC, as shown in Figs. 5a–c. The difference between the PHBV blends does not involve the crystal form but the process of nucleation growth: spherulites with indefinite shapes of the PHBV/PCL blends with TEC and DCP at a ratio of 40:60 develop rapidly, and the crystal nucleus grows over the entire region; by contrast, two types of spherulites are observed in the blank. The spherulite of PHBV (bright area) appears first, followed by that of PCL (dark area). As seen in Figs. 5d–f, the crystal nuclei of PCL are extremely small, indicating the low crystallinity of the component. The crystallization temperatures of blends with TEC and DCP in POM behave in accordance with the results obtained from DSC. Figures 5g–l reveal that additives perform an important function in the grafting process, consistent with a previous study [20, 21]. DCP grafts PHBV with PCL, so the crystalline form of PHBV was destroyed by PCL in blends with TEC and DCP (40:60). In the blank samples, however, PHBV and low-crystallinity PCL dispersed in the polymer because of the mutual immiscibility of both materials.
Morphological Characterization of PHBV/PCL Blends
The surface morphology of PHBV blends was studied via SEM (Fig. 6). No phase boundary can be seen in blends with TEC and DCP. Figure 6 shows that addition of DCP and TEC induces PHBV and PCL to react with each other and produce grafting products. In the blank samples, the uneven phases in Figs. 6c, e and g represent etched PCL, whereas flat, sporadically porous phases denote PHBV. In addition, a sharp phase boundary between PHBV and PCL is observed. PHBV blends with TEC and DCP at all ratios exist as a single phase (Fig. 6d, f, h, j and i); by contrast, the blank shows a two-phase morphology. The result verifies the previous reports [22–24] and is in agreement with the result in above mentioned. The crosslinking action of DCP is also verified.
Fracture surface of PHB/PCL blends films observed by SEM [a, PHBV; b, PCL; c, PHBV/PCL (80/20); d, PHBV/PCL (80/20) added TEC and DCP; e, PHBV/PCL (60/40); f, PHBV/PCL (60/40) added TEC and DCP; g, PHBV/PCL (50/50); h, PHBV/PCL (50/50) added TEC and DCP; i, PHBV/PCL (40/60); j, PHBV/PCL (40/60) added TEC and DCP; k, PHBV/PCL (20/80); l, PHBV/PCL (20/80) added TEC and DCP]
Hydrophilicity of the PHBV/PCL Blends
Measurement of static water contact angles can be used to assess the hydrophilicity of the outermost few angstroms of polymer films. The static water contact angles of the original PHBV and PCL were 75.77° and 64.40°, respectively. As can be seen from Fig. 7, regardless of the presence or absence of TEC and DCP, the static water contact angle of the modified materials is smaller than that of pure PHBV. The trend of the water contact angles of the two blends is close to type V. The water contact angle of the PHBV blends with identical PHBV segments changed with increasing PCL segment content. This finding indicates that surface hydrophilicity is enhanced through incorporation of PCL. A similar conclusion was obtained through PEG blocking of PHBV [12, 25]. However, because of the presence of TEC and DCP, the contact angle of the blends with TEC and DCP was smaller than that of the blank. It is because that TEC contains hydrophilic hydroxyl groups and DCP makes PHBV react with PCL to produce some functional groups with better hydrophilicity than pure PHBV. In particular, the hydrophobicity of blends with additive agents was highest at a ratio of 40:60. It can be observed from Fig. 8 that a PHBV: PCL ratio of 40:60 yields optimal hydrophilic properties in the blends (with TEC and DCP) and the blank under similar conditions. These results demonstrate that the surface hydrophilicity of the PHBV blends improves to the maximum degree by introduction of TEC and DCP, as expected.
Biodegradation of the PHBV/PCL Blends
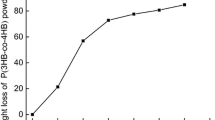
Based on the characterization results described above as well as performance considerations, the PHBV/PCL blend with agents at a ratio of 40:60 was selected for degradation by P. mendocina DS04-T. Figure 8 shows the weight loss curves of PHBV blends with TEC and DCP at 40:60 as degraded by P. mendocina DS04-T with increasing cultivation time. The biodegradation rates of PHBV blends reach 45 % after 96 h of incubation, and the weight loss progress displays three significant stages: the preparative stage (0 to 24 h), the rapid weight loss stage (24 to 84 h), and the gradual weight loss stage (84 to 96 h).
Figure 9 shows changes in the appearance of the PHBV/PCL blend (40:60) with DCP and TEC during biodegradation. The modified blends films present a smooth surface before degradation (Fig. 9a). In the initial stages of degradation (Fig. 9b), the modified blends films began to be degraded and appear holes. Along with the increase of the degradation time, the holes appeared larger and larger (Fig. 9c–h). After degraded 96 h (Fig. 9i), the PHBV blends decompose extensively and leave behind several channel structures. According our previous reports [26, 27], P. mendocina DS04-T showed a remarkable degradation ability of PHBV. In this work, the PHBV portion of the blends degraded completely by P. mendocina DS04-T, and the PCL portion was remained. The biodegradation of PHBV produced an abundance of holes and channels. Further study of porous materials via the proposed method is feasible.
Conclusion
In this work, PCL with great thermodynamic and mechanical properties was used to modify PHBV, and TEC and DCP were also mixed in the blends to promote mechanical behavior. According to the assay of tensile testing and water contact angle, a PHBV/PCL blend (40/60) with TEC (20 wt%) and DCP (0.5 wt%) was chosen for its good mechanical properties and hydrophilicity. The results of characterization indicate that DCP can make PHBV and PCL graft, while PHBV and PCL are immiscible without DCP. Compared with the blends without TEC and DCP, PHBV blends added TEC and DCP have higher breaking elongation and lower tensile strength. The addition of DCP can rise the crystallization degree of the blends and decrease the crystallization temperature. The hydrophilicity of PHBV blends with TEC is also better than those of without TEC. Meanwhile, TEC and DCP have no conspicuous negative effect on biodegradation by P. mendocina. In order to get PHBV blends with greater application potential, the works utilizing another component or crossing agent to modify PHBV is studied currently in progress.
References
Carolina L, Betina MPF, Eliana ARD, Arnaldo RSJ, Paulo PJ (2008) J Mater Sci Mater Med 19:635–643
Meng W, Xing ZC, Jung KH, Kim SY, Yuan J, Kang IK, Sung CY, Hong ISJ (2008) J Mater Sci Mater Med 19:2799–2807
Avella M, Martuscelli E, Raimo M (2000) J Mater Sci 35:523–545
Zhang KY, Amar KM, Misra M (2012) ACS Appl Mater Interfaces 4:3091–3101
Wang BJ, Zhang YJ, Zhang JQ, Gou QT, Wang ZB, Chen P, Gu Q (2013) Chin J Polym Sci 3:670–678
Yottha S, Thomas E, Jun P, Ronald S, Craig C, Turng LS, Srikanth P (2013) Polym Degrad Stab 98:1439–1449
Jost V, Langowski HC (2015) Eur Polym J 68:302–312
Li X, Loh XJ, Wang K, He CB, Li J (2015) Biomacromolecules 6:2740–2747
Ma PM, Denka GHB, Lemstra PJ, Zhang Y, Wang SF (2012) Macromol Mater Eng 297:402–410
Wang S, Ma PM, Wang RY, Wang SF, Zhang Y, Zhang YX (2008) Polym Degrad Stab 93:1364–1369
Pierre L, Estelle R, Jean G, Christelle SC, Valerie L (2012) React Funct Polym 72:487–494
Hala FN, Mohamed SAA, Sherif MS, Gamal RS (2011) J Polym Res 18:1217–1227
Khodaverdi E, Heidari Z, Tabassi SAS, Tafaghodi M, Alibolandi M, Tekie FSM, Khameneh B, Hadizadeh F (2015) AAPS Pharm Sci Tech 16:140–149
Li Y, Xin S, Bian Y, Xu K, Han C, Dong L (2016) Int J Biol Macromol 85:63–73
Liu QS, Zhu MF, Chen YM (2010) Polym Int 59:842–550
Li X, Loh XJ, Wang K, He CB, Li J (2005) Biomacromolecules 6:2740–2747
Jia L, Yin LZ, Li Y, Li QB, Yang J, Yu JY, Shi Z, Fang Q, Cao AM (2005) Macromol Biosci 5:526–538
Dekoning GJM, Scheeren AHC, Lemstra PJ, Peeters M, Reynaers H (1994) Polymer 35:4598–4605
Francisco REC, Suzan AC, Jose AMA (2013) J Polym Environ 21:789–794
Liu QS, Shyr TW, Tung CH, Zhu MF, Deng BY (2011) Macromol Res 19:1220–1223
Xiang HX, Wang SC, Wang RL, Zhou Z, Peng C, Zhu MF (2013) Sci China Chem 56:716–723
Zhao HB, Cui ZX, Wang XF, Turng LS, Peng XF (2013) Compos B 51:79–91
Nagarajan V, Misra M, Mohanty AK (2013) Ind Crop Prod 42:461–468
Guan Q, Naguib HE (2014) J Polym Environ 22:119–130
Badia JD, Kittikorn T, Strömberg E, Santonja-Blasco L, Martínez-Felipe A, Ribes-Greus A, Ek M, Karlsson S (2014) Polym Degrad Stab 108:166–174
Mao HL, Jiang HS, Su TT, Wang ZY (2013) Biotechnol Lett 35:1919–1924
Wang ZY, Lin XD, An J, Ren C, Yan X (2013) Polym Plast Technol Eng 52:195–199
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 31100099 and 31570097) and Program for Liaoning Excellent Talents in University (Grant No. LJQ2014040).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Gao, Z., Hu, X. et al. Blending Modification of PHBV/PCL and its Biodegradation by Pseudomonas mendocina . J Polym Environ 25, 156–164 (2017). https://doi.org/10.1007/s10924-016-0795-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0795-2