Abstract
A series of blends of biodegradable PHB and PPC with and without additives were prepared in different ratios by the casting method. The effect of additives on miscibility, morphology and melting of PHB and PPC blends was investigated by differential scanning calorimetry (DSC), polarized optical microscopy (POM), Fourier transform infrared and wide angle X-ray diffraction (WAXD). The immiscibility between the PHB and PPC blends has been shown using DSC and POM. Acetyl triethyl citrate/polyvinyl acetate (PVAc) as plasticizer and compatibilizer was used to improve the miscibility between the PHB and PPC blends. FT-IR confirmed that the miscibility can be improved by additives, therefore the crystallinity and melting temperature of PHB is depression with increasing PVAc content, the OH group broadened and the ester band C=O shifted in blends 7, 8 and 9, due to hydrogen-bonding interaction. The peak position of C=O of blend 1, 2 and 3 remains constant without any change, i.e. PHB and PPC do not form any intact due to their incompatibility. WAXD showed reduced crystallinity and peak broadening in the patterns of the blends. Electrospun fiber of blend 9 has been manufactured and the morphology was investigated by scanning electron microscopy. Electrospun fibers are homogenous and their diameters were affected by solution concentration. It was found that the elongation at the break of electrospun fibers of PHB/PPC blends increased by adding additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most consumer products are used in the packaging of plastic from petrochemicals and produced in large quantities to meet the needs of the market. However, it causes problems for the pollution of the environment when discarded. Burning plastic from petrochemical waste causes an environmental problem in developing countries for air quality, as a result, causes the earth’s temperature to rise because of the emission of large amounts of carbon dioxide (CO2) in the atmosphere. These harmful effects give reason and motivation for governments and scientists to produce biodegradable materials. In addition, the usage of CO2 gas helps to manufacture bio-materials, to reduce pollution and to overcome environmental problems [1]. PHB is a semi crystalline thermoplastic aliphatic polyester that is 100 % biodegradable and biocompatible. PHB has not been used extensively until now because of the disadvantages such as high price, brittleness and low crystallization rate [2, 3]. PHB has very poor processability and is very sensitive to higher processing temperature, i.e. after it reaches its melting temperature, there is an occurrence of the thermal degradation, therefore the crystallization grow very slow. In addition, a second crystallization of PHB occurs during storage at room temperature because the crystallization process is incomplete by fast cooling during manufacturing [2, 3], therefore the material becomes extra brittle. PHB can be used in a number of industrial applications such as packaging, food packaging and medicine.
PPC is a new class from biopolymer. It is produced by polymerization of propylene oxide and carbon dioxide. However, it has low mechanical strength and poor thermal stability. PPC is a biodegradable aliphatic polycarbonate that can be degraded to H2O and CO2 [4]. Blending of PHB with PPC allows reduction of cost and enhancement of its biodegradability by reducing the crystallization and making the material rubbery and strong. Many scientists have attempted to improve the mechanical properties of PHB by blending with other polymers such as poly(lactic acid) (PLlA) [5, 6], poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(propylene carbonate) blends [7–10], poly(e-caprolactone) (PCL) [11, 12] and poly propylene carbonate (PPC) [13, 14]. It is known that the addition of a small percentage of PVAc improved miscibility of PC and PMMA blends [15] as well as PHB and PLLA blends [6]. The mechanical properties of the polymer blend depend on the interactions between the two PHB and PPC are weak, i.e. two phases and immiscible, the result will be poor adhesion between them, consequently having poor mechanical properties. On the other hand, if the interaction between the two PHB and PPC with additives is strong (see FT-IR), the result would enhance the interaction between PHB, PPC and additives, this leads to improving the elongation at break of PHB.
Electrospinning is a very simple process by which it can obtain electrospun fibers with different diameters from nano to micrometers. With adding the additives to PHB and PPC, we have succeeded in improving the elongation at break in compared with PHB and PPC. Therefore, the electrospun fibers can be applied as filter for air and water, drug delivery and wound dressing, surgical suture and mat fiber used in pampers for children. The novelty in this paper is adding the plasticizer, to help the connection together in blends 7, 8 and 9 as hydrogen bonds, followed to improve the elongation at break than pure PHB and PPC. In this paper is used The polarized optical microscope (POM) to study the miscibility in PHB and PPC blends.
The objective of this paper is to study the miscibility of PHB/PPC/PVAc/plasticizer blend by POM, DSC, WAXD, FT-IR and to manufacture electrospun fiber by electrospinning from this blends.
Experimental
Materials
PHB crystallinity 60 % (Mw = 2.3 × 105 g mol−1). PPC (MW = 1.3 × 105 g mol−1), acetyl triethyl citrate (ATEC) and PVAc (MW = 0.51 × 105 g mol−1) were purchased from Sigma-Aldrich Chemicals Ltd.
Preparation of Blends
The compositions of PHB/PPC/PVAc/ATEC blends were prepared with different weight ratios as follows: blend 1 (80/20/0/0), blend 2 (75/25/0/0), blend 3 (70/30/0/0), blend 4 (60/40/0/0), blend 5 (50/50/0/0), blend 6 (49/25/06/20), blend 7 (47/25/08/20), blend 8 (45/25/10/20) and blend 9 (43/25/12/20). All blends were prepared by dissolving components together in hot chloroform at 50 °C, and then the solution was cast in a petri dish to prepare the casting films. The samples were dried at 60 °C for 24 h to remove any residual solvent completely. The blends casting films were cut into small pieces and a compression molded between two sheets of aluminum folders in a hydraulically heated press between 170 and 180 °C for 2 min without pressure and 1 min with pressure of 20 kN. After molding, the samples were cooled between two metal plates. At this temperature, thermal decomposition cannot occur. All samples are melted again at a temperature equal to Tm + 20 and at this temperature, no thermal decomposition does not happen.
Methods
Differential Scanning Calorimetry (DSC)
DSC is an important technique to study melting and crystallization behavior of polymers. A thermal analysis was carried out with a differential scanning calorimeter (Schimadzu-DSC 50, Japan). All samples of 5 ± 0.1 mg was sealed in an aluminum sample pan for DSC. Samples were kept under a dry nitrogen atmosphere. DSC analysis was carried out from room temperature to 200 °C at heating and cooling rates of 10 °C min−1. Besides, the analysis of DSC curves was carried out for the second heating run data to examine, the melting temperature (Tm) and the cold crystallization temperature (Tcc).
Polarized Optical Microscopy (POM)
The evolution of microstructure for all blends was examined using POM [Nikon polarizing microscope (Nikon Eclipse E600) equipped with hot-stage (Instec STC200)]. Small amount of polymer is placed between two microscopy glass slides as sandwich and inserted to hot stage and melted at 200 °C. After melting, thin film was obtained by applied small pressing on top of the cover glass (the thin film was approximately 0.05–0.1 mm in thickness). The blends samples were heated on the hot stage from room temperature to 200 °C and then kept at 200 °C for 3 min to erase their thermal history and cooled from 200 °C to a temperature where the growing of spherulites are started.
Wide Angle X-ray Diffraction (WAXD)
The crystalline phases were analyzed by wide-angle x-ray diffraction (WAXD) measured with A X’Pert Pro X-ray diffractometer (Phillips, Netherlands) with Cu Kα radiation (λ = 1.5406 Å) at a generator voltage of 40 kV and a current of 40 mA was used to analyze the crystallinity properties of PHB and PPC with and without additives at room temperature (~25 °C), the scan rate of (2°) 2θ min−1. Film samples were cut into rectangular pieces (4 cm2) and mounted on the matrix prior to analysis.
FT-IR Spectrometer
Infrared spectra were recorded with a Fourier Transform FT-IR 6100 Jasco spectrometer in the wavenumber range 550–4000 cm−1. All spectra are recorded at room temperature. The films of the samples are cut into rectangular pieces (4 cm2).
Electrospinning Equipment
For electrospinning experiments, PHB and PPC blends without and with additives dissolved in different solvents in a mixture of chloroform (90 % CF) and dichloromethane (10 % DMF) and at different concentrations 20, 25 and 35 wt %. Electrospinning experimental consists of a high voltage power supply from USA (model NO. ES60P-20W), a programmable syringe pump (No. BS-9000-USA), syringe needle with an inner diameter (ID) of 1.3 mm and a grounded collector.
Blend 9 dissolved in different solvents at different concentrations and taken in a plastic syringe (5 mL) that connected to a needle with an inner diameter (ID) of 1.3 mm. The flow rate of 0.185 mL/h, the distance between needle and target and applied voltage 20 kV. The fibers were collected on a grounded aluminum foil.
Scanning Electron Microscopy (SEM)
The surface morphology of the sample 9 with different concentration was observed using a scanning electron microscope (JSM-6360LA, JEOL Co., Japan) at an accelerating voltage of 15 kV. The samples were sputter-coated with gold for 120 s to a thickness of 2–3 nm using a sputter coater (EMITECH K550X, England). Images of sample fibers were obtained using SEM to measure the fiber diameter. The samples are coated with a thin layer of gold to investigate by SEM.
Mechanical Properties of Electrospun Mats of Pure PHB and Their Blends
Mechanical properties of electrospun of samples 1 and 9. All samples for testing in the tensile test device were cut in a dumb-bell shape (Dumb Bell Ltd SDL-100 11221-13, Japan). Sample thickness was measured using a micrometer. Tensile tests were performed at room temperature, at a crosshead speed of 1 mm/min−1, using a Shimadzu universal testing machine equipped with a 10-kN load cell and interfaced with a computer. Five specimens of each formulation were tested and the average values are reported. From the correlation between stress σ (in Pa) and elongation ε (in %), ε = (L0 − L)/L, L0 = original length, L = length after for electrospinning experiments, PHB + PPC blends with additives dissolved in different solvents in a mixture of chloroform and dichloromethane (75/25, v/v) at different concentrations 20 and 25 wt% electrospinning experimental consists of a high voltage power supply from USA (model NO. ES60P-20W), a programmable syringe pump (No. BS-9000-USA), syringe needle with an inner diameter (ID) of 1.3 mm and a grounded collector. PHB and its blends dissolved in different solvents at different concentrations and taken in a plastic syringe (5 mL) that connected to a needle with an inner diameter (ID) of 1.3 mm. The flow rate of 0.185 mL/h, the distance between needle and target and applied voltage 20 kV. The fibers were collected on a grounded aluminum foil.
Results and Discussion
Differential Scanning Calorimeter (DSC) Analysis
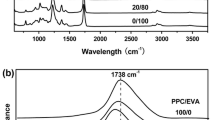
DSC analysis is used to investigate the miscibility behavior in polymer blend. Figure 1a shows the DSC curves of the melting of the PHB and PPC blend. It was found that the melting temperature (Tm) of blends slightly decreased with increasing PPC content, it showed an impressed crystallinity of PHB.
Figure 1b shows the DSC second heating curves of PHB and PPC blend with and without additives. It is known that PHB is immiscible with PPC [14], by adding a small amount of PVAc (6–12 %) to both immiscible (PHB/PPC) blends. It can be seen by adding PVAc and plasticizer to PHB/PPC blend that the Tm shifts towards lower temperature. The Tm of PHB blends with additives is shifted from 175 to 150 °C, respectively. The depressions in Tm in the blends indicate that there is a strong intermolecular interaction between PHB and PPC with additives. We fixed both the PPC and plasticizer and change the value of PVAc by a adding small amount, which makes a compatibilizer to improve the miscibility between PHB and PPC which leads to improve the elongation at break of PHB.
Morphological Studies
Morphological structure of the polymer blend is a very important factor to improve the mechanical properties. Optical polarized microscopy was also used to investigate the miscibility and crystallization behavior of PHB/PPC blend. PHB is not miscible with PPC for all ratios. It observed by POM with—and without polarized in Fig. 2 (a, a′, b, b′ and c, c′) that the phase separation occurred at all ratio of PHB/PPC 80/20, 75/25, 70/30. It is shown in Fig. 2b that small and large domains of PPC inside and outside the PHB spherulite grow at 80 °C. The shape and size of dispersed PPC domains is large and irregularly circular. With adding additives to (PHB/PPC) blend, the PPC domains will be uniformly dispersed in PHB matrix, i.e. by increasing the ratio of blend 9. The size of dispersed domains of PPC shows many finer uniform spherical phase structures (Fig. 3d). The PPC domains were well dispersed in PHB matrix with very small domains but similar shaped, these domains were strongly stuck to each other, and the additives were well embedded into PHB matrix. These interpretations suggested that the additives affected the splitting and spreading of PPC to a very small domain in the PHB matrices. This leads to make a strong adhesion interface between PHB/PPC, these results are consistent with the results FT-IR.
Wide Angle X-ray Diffraction Analysis
Figure 4 a shows the WAXS of (PHB and PPC) blends with different ratios (80/20, 70/30, 60/40). For the blends of (PHB and PPC) show the four strong diffraction peaks at 2θ of 12.9°, 16.4°, 19.1° and 22.1°, which are similar to those of PHB homo polymer in our previous work [16]. This suggests that blends have the same crystalline structures as PHB. The peaks at 2θ = 12.9°, 16.4°, and 22.1° ascribed to 020, 110, and 121 reflections. The peaks at 2θ = 16.4 of α-crystal form of PHB decreased with the increase of PPC, and α-crystal form appears very small when the amount of PPC was increasing and the peak at 2θ = 17.7 increased with boarding (halos). With increasing PPC in blend, the intensities of (111) the crystal plane becomes lower than that of (110) the crystal plane; demonstrating that the addition of additives may effect the crystallographic orientation for the formation of the strong intermolecular interactions between PHB and PPC. Figure 4b shows the blends of PHB/PPC/PVAc/ATBC. They show that the change is relatively intensive of the (110) crystal plane to (021 and 111). The crystal plane is attributed to the strong intermolecular interaction between PHB and amorphous PPC. The intensity of the diffraction peaks of PHB decreases as additives are added to the blends. As shown in Fig. 4c, the WAXD patterns of PHB and PPC with additives are the same of those of pure PHB and PPC. It shows three diffraction peaks at 2θ = 12.9°, 16.4°, and 22.1° ascribed to 020, 110, and 121 reflections.
FT-IR Analysis
FTIR is a suitable device for the investigation of the miscibility, compatibility and phase behaviors of a polymer blend, and as a consequence, the bands shift to a lower or a higher wavelength. Figures 5 and 6 is showing the FTIR spectrum of pure PHB, pure PPC and their blends without additives in the 1600–600 cm−1 regions. Characteristic peaks of symmetric –C–O–C– stretching vibration were present from 800 to 975 cm−1 and the antisymmetric –C–O–C– stretching vibrations is placed in 1060, 1150 and 1277 cm−1. The band around 1375 cm−1 agrees to the symmetrical of methylene groups (CH2) and the band at 1453 cm−1 for asymmetric deformation of CH3, the intensity of both the peaks is simple for crystallinity. The bands at 976, 1224, 1277 and 1720 cm−1 are crystalline regions, but the bands at 1740, 1453, 1183 cm−1 belong to amorphous regions. The peak at 1224 cm−1 is the conformational band of crystalline helical molecular chains. The FTIR spectrum of pure PHB, pure PPC and their blends with additives in the 1600–4000 cm−1 region is showing in Figs. 5 and 6. The ester group C=O band of PHB is existing at 1720 cm−1 for crystalline structure and small shoulder at 1740 cm−1 for the amorphous region of PHB. The amorphous of C=O groups for PPC are placed at 1740 cm−1 [13]. It can be seen that the peak position of C=O of PHB/PPC blend remains constant without any change. This indicates that PHB/PPC blend is immiscible. But the peak position of C=O of PHB/PPC with additives is shifted towards a higher wave number. The hydroxyl groups (OH) of blends in region 3500–3700 cm−1 with additives became broader due to the hydrogen-bonding interaction. These changes are observable than those of PHB/PPC blend without additives. The peak at 2925 and 2854 cm−1 correspond, to asymmetric and symmetric C–H stretching of in CH3 group of PHB. The band at 1277 cm−1 is approved to the vibration of CH2 group of PHB. It has been observed from Fig. 6b the stretching vibration of carbonyl groups band C=O of blends is shifted from 1729 to 1733 cm−1, which characteristics to addition of PVAc, in blends 7, 8 and 9. This shift in C=O peaks confirms that the strong interaction takes place between the PHB, PPC, PVAc and plasticizer. The band at 1183 cm−1 is indicator to measure the crystallinity of PHB/PPC blends with additives and without additives. This band remains constant in PHB PPC blends in blends 1, 2 and 3. It was observable that the crystallinity of PHB/PPC blends decreased with increasing contents of PVAc in blends 7, 8 and 9. The amorphous band at 1183 cm−1 showed overlap with increasing PVAc contents in blends. FTIR and DSC results showed that the crystallinity of the blends with additives decreased with increasing of PVAc contents. The C=O band is useful for analyzing the hydrogen bonds and the crystallization behavior of PHB. It was demonstrating that the interaction of blend 7, 8 and 9 was stronger than blends 1, 2 and 3, because some peaks showed shifting in position and broadening band. Also, the compatibility between PHB/PPC is enhanced by adding the PVAc.
Effect of Fiber Diameter on Tensile Behavior
It is well known that plasticizers lead to increase the deformation of polymers and flexibility, as well as leading to increase the elongation at break and reduce the tensile strength of polymers. These plasticizers must be non-toxic and biodegradable. Electrospun fibers were manufactured and the electrospun fibers were cylindrical, uniform and without beads. The diameter of the electrospun fibers can be improved by controlling the concentration of the polymer in the electrospinning solution. During the process of preparation, the fiber of blends 1 and 9 is injected through a small nozzle (1.3 μm) with applied higher voltage at 20 kV and flow rate 0.185 μm h−1. Increase of electrostatic charges on the surface of the liquid drops at the needle lead to the formation of a jet [17]. The jet spreads to form an unbroken fiber. The solvent evaporates before reaching the target and the solid fiber collection on aluminum foil.
SEM images the electrospun fibers of blend 9 at concentration 25 % is seen in Fig. 7. It is found from these images that the fibers are uniformed and their surfaces are smoothed. The diameter of fiber is between 2 and 790 nm. Figure 8 is showing the electrospun fiber of blend 9 at a concentration 35 %. It is observed at this concentration that the number of pores on the surface is greater in comparison within the fiber at a concentration of 25 % with the same resolution. We can conclude that the concentration ratio has affected the fibers’ diameter, these electrospun fibers are smaller in comparison with a concentration of 35 % in the same blend 9 (Fig. 9).
The mechanical behavior generally depends on the fiber diameter. Higher magnification imaging of the electrospun fiber mats after cold drawing showed that all the electrospun fibers deformed and plastic deformation before break. The strength of load cell 10 kN is very larger than the section area of fiber (thickness), therefore for this reason noise appear. But it does not have a smaller load cell than of 10 kN. It is important to test the electrospun fiber to determine its applications.
Figure 10 shows the tensile properties of the blends 1 and 9 with different concentration (25 and 35 %). It is found that the best improvement of the mechanical properties of the PHB/PPC blend with additives because hydrogen bonds are formed (see FT-IR) and it makes PPC to small particles in PHB matrix (see POM). The mechanical behavior of blend 1 showed lower values of tensile strength of 16 MPa, elongation at break of 50 %. But the blend 9 with different concentration is more flexible, and the values of tensile strength is of 22–18 Mpa, with an elongation at break of 335–475 %. It can be concluded that the blends of PHB and PPC with additives can be an effective and very suitable route for several applications as biodegradable materials.
Conclusion
Finally, physical cross linking blend is a useful method to obtain new materials with improved physical properties with lower cost than chemical cross linking or synthesis of copolymers. The miscibility, thermal behavior, morphology, and mechanical properties of PHB/PPC blends with and without additives were investigated by using DSC, FTIR, WAXD, POM, tensile tester and SEM. It found no miscibility of PHB/PPC blend without additives at all ratios, but partial miscibility of PHB/PPC blend with additives. The melting temperatures, Tm, from DSC measurement also showed a depression trends with the increase of PPC contents. The Tm of PHB decreased from 178 to 153 °C with the increase of additives content. POM results demonstrated the stronger compatibility between PHB and PPC with additives than those of PHB/PPC blends. The optimal mechanical of PHB/PPC with additives could be realized in blend 9, due to well dispersion of PPC with PVAc in PHB matrix. FTIR technique was confirmed that there are the strong intermolecular interactions between PHB and PPC with additives. It is noted the concentration of blend 9 has influence on the diameter of the electrospun fiber. The elongation at break of PHB and PPC with additives was improved.
References
Hopewell J, Dvorak R, Kosior E (2009) Philos Trans R Soc B Biol Sci 364:2115
El-Hadi A, Schnabel R, Straube E, Mueller G, Riemschneider M (2002) Macromol Mater Eng 278:363
El-Hadi A, Schnabel R, Straube E, Mueller G, Henning S (2002) Polym Test 21:665
Inoue S, Koinuma H, Tsuruta TJ (1969) J Polym Sci Part B Polym Lett 7:28
El-Hadi AM (2014) Poly Eng Sci 54:1394
El-Hadi AM (2011) Poly Eng Sci 51:2191
Peng SW, An YX, Chen C, Fei B, Zhuang YG, Dong L (2003) J Appl Polym Sci 90:4054
Li J, Sun CR, Zhang XQ (2012) Preparation, thermal properties, and morphology of graft copolymers in reactive blends of PHBV and PPC. Polym Compos 33:1737–1749
Li J, Lai MF, Liu JJ (2005) J Appl Polym Sci 98:1427
Tao J, Song C, Cao M, Hu D, Liu L, Liu N, Wang S (2009) Polym Degrad Stab 94:575
Chun YS, Kim WN, Chun YS, Kim WN (2000) Polymer 41:2305
Hirotsu T, Ketelaars AA, Akayama KN (2000) Polym Degrad Stab 68:311
Zhang S, Sun X, Ren Z, Li H, Yan S (2015) Phys Chem Chem Phys 17:32225
Wang X, Peng S, Dong L (2005) Colloid Polym Sci 284:167
Li Y, Shimizu H (2009) ACS Appl Mater Interfaces 1:1650
El-Hadi AM (2014) Polym Bull 71:1449
El-hadi AM, Al-Jabri FY (2016) Polymers 8:97
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Hadi, A.M. Improvement of the Miscibility by Combination of Poly(3-hydroxy butyrate) PHB and Poly(propylene carbonate) PPC with Additives. J Polym Environ 25, 728–738 (2017). https://doi.org/10.1007/s10924-016-0863-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0863-7