Abstract
Wood mice of the genus Apodemus are widely distributed in Eurasia, with the Eastern Mediterranean being considered as a hotspot. Indeed, numerous species have been documented in Iran, including A. witherbyi, A. hyrcanicus, A. uralensis, A. avicennicus, A. hermonensis, and A. arianus. In this study, 129 specimens were collected from different Iranian localities and two specimens from Afghanistan. The animals were identified taxonomically and their phylogenetic relationships were investigated using cytochrome b mitochondrial DNA sequences. Five species of the genus Apodemus were identified in Iran, including A. hyrcanicus, A. witherbyi, A. cf. ponticus, A. uralensis, and A. mystacinus, beside, A. pallipes from Afghanistan. This study found no evidence of A. flavicollis or A. sylvaticus in Iran, despite their occurrence in Turkey, shedding doubt on the status of A. flavicollis in Iran, Asia Minor, and the Levant. Phylogenetic analyses imply that A. witherbyi has priority over A. avicennicus, A. hermonensis, and A. iconicus. Estimation of the divergence time for these taxa suggests a separation at around 7.2 Ma for the subgenera Karstomys (including A. mystacinus and A. epimelas) and Sylvaemus (including A. flavicollis, A. sylvaticus, A. uralensis, A. pallipes, A. hyrcanicus, A. witherbyi, and A. cf. ponticus). Within the subgenus Karstomys, the divergence times for A. mystacinus and A. epimelas were between 3.0 and 6.1 Ma, and divergence times within the subgenus Sylvaemus were between 5.2 and 6.9 Ma for A. witherbyi and other species in this subgenus. It is postulated that vicariance events including the uplifting of the Zagros Mountains and Anatolian Plateau in the middle Miocene and climate oscillations during the Messinian Salinity Crisis besides formation of the Hyrcanian tertiary forests during the Neogene probably played substantial roles in the radiation and distribution of the genus Apodemus in the Eastern Mediterranean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paleontological data have identified the Eastern Mediterranean as a crossroads for many taxa from the early Miocene (Koufos et al. 2005). This area shows high endemicity in the Palearctic Region (Kryštufek et al. 2009). Its current geographical aspect was formed between the middle Miocene and the present (Macey et al. 1998; Koufos et al. 2005), and the distributions of many taxa in this area are tightly related to geological events that occurred during the Neogene (Macey et al. 1998). From the middle Miocene about 15–5 Ma (million years ago), a counter-clockwise movement of the Arabian platform and its collision with Eurasia led to the uplifting of the Zagros and Alborz Mountains, considered as parts of the Alpine-Himalayan mountain chain (Dercourt et al. 1993; Sengor and Natalin 1996; Mouthereau 2011). The Alborz range was subsequently compressed and folded (Guest et al. 2007) and a deep depression was formed between the south Caspian Sea and the Alborz Mountains during the Neogene, while the south Caspian microcontinent moved northwards and reached its maximum subsidence during the late Miocene and Quaternary (Golonka 2004). These tectonic and orogenic activities create some barriers and corridors in the Iranian Plateau for gene flow (Macey et al. 1998; Ahmadzadeh et al. 2013; Rajaei Sh. et al. 2013). In the Near East, the central Anatolian Plateau was also formed as a result of volcanic activity during the Neogene (8 Ma) (Yılmaz et al. 1998; Aydar et al. 2013). In addition, in the late Miocene, the Mediterranean Sea underwent desiccation during the Messinian Salinity Crisis (5.96–5.33 Ma), which also impacted on the Near East (Clauzon et al. 1996; Krijgsman et al. 1999). The onset of the Praetiglian cold stage in Europe (2.5–2.2 Ma) was then preceded by the formation of ice sheets and glaciers at the early Pliocene/late Pliocene boundary (about 3 Ma) (Ruddiman and Raymo 1988), resulting in the retreat of forests and the prevalence of cold, dry habitats in the Mediterranean region. These climatic oscillations following geographical events formed the biological aspects of the Circa-Mediterranean regions.
Wood mice of the genus Apodemus are widely distributed in the Eastern Mediterranean and are adapted to different types of habitats. In total, 20 species of the genus Apodemus attributed to three subgenera (Apodemus, Karstomys, Sylvaemus) are known in Eurasia (Michaux et al. 2002; Bellinvia 2004; Musser and Carleton 2005; Suzuki et al. 2008), six of which are supposed to exist in Iran, including A. witherbyi, A. hyrcanicus, A. uralensis, A. flavicollis, A. ponticus, and A. mystacinus (Musser and Carleton 2005; Kryštufek and Hutterer 2006; Darvish et al. 2014; Mohammadi et al. 2014). Five species were attributed to the subgenus Sylvaemus, which ranges through Europe, Near East, and Middle East, and one (A. mystacinus) was included in the subgenus Karstomys (Musser and Carleton 2005; Kryštufek and Hutterer 2006).
In Iran, habitats used by Apodemus species range from the Hyrcanian forests of the northern Alborz Mountains and the steppes of the southern slopes of the Alborz to farmlands and gardens in mountainous ranges, as well as grasslands beside shrub lands and scrub in the Zagros range. This genus was considered to be absent from the Persian Gulf Basin, Lut Desert, and the Kavir Plain in the central part of Iran, as well as from the Sistan depression in the southeast of Iran. However, Blanford (1875) described a new taxon from Kuhrud, Isfahan, as Mus erythronotus, which was subsequently recognized as a junior name for A. flavicollis (Kryštufek 2002), since when this genus has undergone numerous revisions (Darvish et al. 2014).
Goodwin (1940) recognized two different morphs of the genus Apodemus in northeastern Iran, from where specimens captured from deciduous forest were identified as A. arianus and were later treated as A. hyrcanicus (Musser and Carleton 2005). Conversely, specimens collected from higher elevations were considered to be A. sylvaticus chorassanicus, but were later were attributed to A. witherbyi (Musser and Carleton 2005). Further, Lay (1967) misidentified and reported A. sylvaticus from the northern parts of Iran. A biochemical study of the genus Apodemus demonstrated a different allozymic pattern in specimens from central north Iran compared with A. sylvaticus and A. flavicollis (Darviche et al. 1979).
Macholán et al. (2001) reported A. hyrcanicus and A. flavicollis from the north and west parts of Iran, respectively. The dental and cranial characteristics of A. witherbyi (under the name of A. hermonensis) and A. hyrcanicus of the northern parts of Iran were investigated by Javidkar et al. (2005). Geometric morphometric comparisons of the specimens from Fakhrabad-Yazd with A. witherbyi and A. hyrcanicus led to the description of A. avicennicus as a new species from the Central Iranian Plateau (Darvish et al. 2006; Siahsarvie and Darvish 2008), and its status was confirmed by random-amplified polymorphic DNA–restriction fragment length polymorphism (RAPD-RFLP) analysis (Naseri et al. 2006). Darvish et al. (2010) conducted the first multivariate analysis of morphometric characters, combined with RFLP, for three species of the genus Apodemus in Iran. Moreover, A. witherbyi from the north of Iran was investigated using molecular and geometric morphometric analyses (Pour Feizi et al. 2009; Jangjoo et al. 2011). Finally, Mohammadi et al. (2012) investigated the karyotype of this genus in Iran. However, controversies remain because of overlaps in identification keys, the lack of discriminant characteristics between species, and misidentifications. Apodemus hyrcanicus and A. ponticus have rarely been included in phylogenetic analyses, and their phylogenetic status relative to other species of the genus Apodemus remains vague.
In this study, we performed phylogenetic analyses using the cytochrome b gene (cyt b) to: (i) clarify the species composition and the distributions of the various species of the genus Apodemus in Iran; (ii) address the enigmatic taxonomic situation of A. ponticus; and (iii) reconstruct the evolutionary history of Apodemus spp. in the Eastern Mediterranean.
Materials and Methods
Sampling
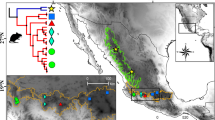
We collected 129 specimens from different Iranian localities and two additional specimens were collected in Afghanistan from 2010 to 2013 (Table 1 and Fig. 1). The materials were identified using available identification keys (Corbet 1978; Kryštufek and Vohralik 2009). Taxonomic names followed Musser and Carleton (2005), except for the subgenus ranks, which are based on Michaux et al. (2002). Phylogenetic relationship of five species of the subgenus Sylvaemus including A. hyrcanicus, A. cf. ponticus, A. uralensis, A. witherbyi from Iran, as well as A. pallipes from Afghanistan and A. mystacinus attributed to subgenus Karstomys were investigated. All specimens were deposited in the Zoology Museum of Ferdowsi University of Mashhad (ZMUFM), Mashhad, Iran.
In addition to the cyt b sequences resulting from this study, 59 additional sequences of the genus Apodemus, retrieved from GenBank, were included in our phylogenetic analyses. It comprised the species A. flavicollis, A. uralensis, A. witherbyi, A. pallipes, A. ponticus, A. sylvaticus, A. mystacinus, and A.epimelas. Finally, sequences of Rattus rattus and Tokudaia osimensis were used as outgroups.
DNA Extraction, PCR Amplification and Sequencing
Tissue samples were available from 131 specimens belonging to the genus Apodemus and one Mus musculus (Table 1, Fig. 1), and comprised muscles preserved in 90 % ethanol. Total genomic DNA for polymerase chain reaction (PCR) was extracted using a standard salt extraction method (Bruford et al. 1992). Double-stranded DNA amplifications of the whole cytochrome b gene (cyt b) were performed with the primer pair cyt b L7 (5′-ACT AAT GAC ATG AAA AATCAT CGT T-3′)/ cyt b H6 (5′-TCT TCA TTT TTG GTT TAC AAGAC-3′; Montgelard et al. 2002) following the protocol of Chevret et al. (2005). The sequences were deposited in Genbank database under the accession numbers that are given in Table 1.
Phylogenetic Analyses
The nucleotide sequences were aligned using ClustalW (Thompson et al. 1994) as implemented in the software Bioedit, ver. 7.0.9 (Hall 1999). Genetic distance was calculated with MEGA v4.0 (Tamura et al. 2007) and the Kimura 2-parameter (K2P) model (Kimura 1980). We used PAUP* 4.0b 10 software (Swofford 2000) to perform maximum parsimony (MP) and maximum likelihood (ML) analyses. Bayesian analyses (BA) were conducted using MrBayes ver. 3.2.2 (Huelsenbeck et al. 2011). The model best fitting our data for the ML and BA analyses was determined using the Akaike information criterion, as implemented in Modeltest (Posada and Crandall 1998). Modeltest proposed a general time-reversible substitution model (GTR+I+G), with a proportion of invariable sites I = 0.5478, variable sites G = 1.8152, empirical base frequencies (A: 0.3223; C: 0.2946; G: 0.1098; T: 0.2733), and substitution rates (rate [A–C] 1.9917, rate [A–G] 7.5046, rate [A–T] 3.1752, rate [C–G] 0.6178, rate [C–T] 24.5240, rate [G-T] 1.0000) estimated from the data set. These parameters were used for the ML analyses.
ML analyses were carried out under heuristic tree search with ten random addition sequence replicates, and tree bisection reconnection (TBR) branch swapping. Parsimony analyses were performed using heuristic searches with tree-bisection-reconnection (TBR) branch swapping and random addition sequence with 1000 replicates. To assess support for internal nodes, we ran nonparametric bootstrapping (500 and 5000 replicates) (Felsenstein 1985) under ML and MP, respectively, with a single random addition sequence replicate per bootstrap replicate.
Bayesian inference (BI) reconstructions were performed with MrBayes 3.2.2 (Huelsenbeck et al. 2011) using a GTR model. Four simultaneous Markov chain Monte Carlo (MCMC) chains with incremental heating temperature of 0.2 were run 6,000,000 generations and sampled every 100 generations. The burn-in size was determined by checking the convergence of −log likelihood (−InL) values, and the first 5000 generations were discarded as burn-in. The Bayesian posterior probability (BPP) was evaluated from the remaining trees. Haplotypes were estimated using the program DnaSP v5 (Librado and Rozas 2009).
Divergence Time
We performed dating analyses using Beast 2.1.3 (Bouckaert et al. 2014) with an uncorrelated lognormal relaxed clock and a yule branching process as a tree prior. This tree prior is the most suitable for trees describing the relationships between individuals from different species. The coefficient of variation frequency histogram did not abut against zero, meaning that there was among-branch rate heterogeneity within our data (Drummond et al. 2007). Consequently, as suggested by Drummond et al. (2007), we used a relaxed molecular clock. The analysis was performed with two independent chains and 60 million generations; chains were sampled every 1000 generations with a burn-in of three million generations.
We used one calibration point derived from fossil records, i.e., the split between the genus Rattus and Mus, which occurred 12 Ma (see e.g., Michaux et al. 2003), and two secondary calibration points from Michaux et al. (2005b), i.e., the split between A. sylvaticus and A. flavicollis (4 Ma) and the split between A. mystacinus and A. flavicollis-A. sylvaticus (7 Ma).
Results
Haplotype Selection
A total of 61 unique haplotypes were identified from the 190 Apodemus cyt b sequences from Genbank and this study. Within the Iranian samples, a total of 28 haplotypes were detected among the five studied species and 131 specimens (129 belong to this study and two obtained from Michaux et al. 2004), i.e., four in A. uralensis, three in A. hyrcanicus, 16 in A. witherbyi, two in A. mystacinus, and three in A. cf. ponticus. Additionally, a haplotype belonging to A. pallipes from Afghanistan was identified. The analyzed alignment (1076 bp) showed 403 variable sites; 327 of them were parsimony-informative. Gaps were missing and no ambiguity remained in our final dataset.
Phylogenetic Relationships
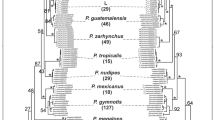
The ML, MP, and BA analyses showed very similar relationships and our phylogeny was well resolved (Fig. 2). Indeed, the branching orders of the deeper clades were similar and only small differences were observed at the intraspecific level. Maximum likelihood analysis revealed nine lineages (A to I) that corresponded to the species A. flavicolis, A. cf. ponticus, A. sylvaticus, A. uralensis, A. pallipes, A. hyrcanicus, A. witherbyi, A. mystacinus, and A. epimelas (Fig. 2). Kimura 2-parameter distances among the nine clusters ranged from 6.4 % (A. pallipes versus A. uralensis) to 19.2 % (A. cf. ponticus versus A. epimelas). Distances within each of the nine lineages range from 0.8 % (within A. hyrcanicus) to 2.2 % (within A. mystacinus; Table 2). Support for higher-level relationships between Karstomys and Sylvaemus subgenera were of 72, 79, and 1.0 for MP, ML, and BA analyses, respectively. The supports for the Karstomys group, including A. epimelas and A. mystacinus, were of 100, 100, and 1.0 and for all Sylvaemus species of 91, 96, and 1.0 for MP, ML, and BA, respectively (supports will be given in the same order below). Finally, A. witherbyi had a basal position within the members of the Sylvaemus group (for more details see Fig. 2).
Maximum likelihood phylogenetic reconstruction of Apodemus (based on haplotypes of cytb gene sequences). Numbers represent MP and ML bootstrap values (MP/ML) (500/5000 replicates). The posterior probability values from the Bayesian analysis are indicated at the >99 % (**) and >95 % (*) significance levels
Lineage (A) included haplotypes previously identified as A. flavicollis from Turkey and the Levant besides haplotypes from the western part of Iran (which were identified as A. cf. ponticus), as well as a haplotype of A. ponticus from Russia (support of 100, 99, and 1.0). Lineage (B) consisted of haplotypes of A. flavicollis from Russia, Turkey, and Greece (support of 100, 93, and 1.0). Lineage (C) included the only A. sylvaticus haplotype from Turkey, (D) haplotypes of A. uralensis (99, 97, and 1.0), (E) haplotypes of A. pallipes (100, 84, 1.0), and (F) haplotypes of A. hyrcanicus (100, 100, and 1.0), this last being basal to A. cf. ponticus, A. flavicollis, A. sylvaticus, A. uralensis, and A. pallipes. Lineage (G) included haplotypes of all the specimens of A. witherbyi from different regions of Iran, besides published sequence of A. witherbyi, A. iconicus, and A. hermonensis from Genbank. Lineage (H) (100, 99, and 1.0) included haplotypes of A. mystacinus from the northwest of Iran, Syria, the Levant, and Turkey, and finally lineage (I) (100, 100, and 1.0) consisted of haplotypes of A. epimelas.
Divergence Time
Molecular dating based on cyt b revealed that the split between the subgenus Karstomys and Sylvaemus occurred approximately 7.2 Ma (95 % CI: 6.7–7.8; Fig. 3). Within the subgenus Karstomys, the split between A. mystacinus (clade H) and A. epimelas (clade I) occurred 4.5 Ma (95 % CI: 3.0–6.1). Within the subgenus Sylvaemus, the split between A.witherbyi and the remaining species including (A. hyrcanicus, A. cf. ponticus, A. uralensis, A. flavicollis, A. pallipes, and A. sylvaticus) occurred 6 Ma (95 % CI: 5.2–6.9). The split between A. hyrcanicus (clade F) and the other clades (A, B, C, D, and E) occurred 5.2 Ma (95 % CI: 4.4–6.1), and the split between A. pallipes (clade E) and the other species (A. hyrcanicus, A. cf. ponticus, A. uralensis, A. flavicollis, and A. sylvaticus) occurred 4.6 Ma (95 % CI: 3.9–5.6). The split between A. pallipes (clade E) and A. uralensis (clade D) occurred 2.9 Ma (95 % CI: 1.9–4.0), whereas the splits between A. sylvaticus and the other two species (A. cf. ponticus and A. flavicollis) occurred between 3.8 Ma (95 % CI: 3.3–4.7). The split between the A. cf. ponticus and A. flavicollis occurred 2.6 Ma (95 % CI: 1.9–3.6) (Fig. 3). These finding were entirely congruent with the order of the analysed species in our phylogenetic tree, considering that the older clades took place as basal positions and the newest ones were arranged as terminal clades (Fig. 2).
Discussion
Taxonomy
Despite its important diversity, data on the different species of the genus Apodemus from Iran are scarce. The results of this study thus provide important new information on these species. To date, the west of the Iranian Plateau was supposed to be inhabited by A. witherbyi, reported as A. sylvaticus arianus by Ellerman and Morrison-Scott (1951), with A. uralensis in the northwest and northeast of Iran (Kryštufek and Hutterer 2006; Darvish et al. 2010). Additionally, A. flavicollis was the only species of the genus Apodemus identified from the Zagros Mountains in western Iran (Etemad 1978; Kryštufek 2002).
The current study detected a high diversity of wood mice in Iran. Different lineages of the genus Apodemus belonging to five species were identified, four of which (A. hyrcanicus, A. witherbyi, A. cf. ponticus, A. uralensis) belonged to Sylvaemus and one (A. mystacinus) was included in the subgenus Karstomys. This study provided no evidence to support the presence of A. flavicollis or A. sylvaticus in Iran, despite their occurrence in Turkey.
The results of this study also shed light on the enigmatic situation of the taxon A. avicennicus, which was previously described as a new species based on morphological, morphometric, and RFLP analyses. Haplotypes of A. witherbyi, A. avicennicus, A. hermonensis, and A. iconicus constitute a monophyletic clade characterized by a mean genetic distance of only 1.1 %. This suggests that all three of these taxa, i.e., A. avicennicus, A. hermonensis, and A. iconicus, should be considered as synonyms of A. witherbyi. The priority of A. witherbyi over A. hermonensis and A. iconicus was previously affirmed by several authors (Filippucci et al. 1989; Kryštufek 2002; Kryštufek and Mozetic Francky 2005; Hoofer et al. 2007).
Haplotypes of lineage (A) include two specimens from Khoramabad, western Iran, as well as haplotypes from the Levant and Turkey (except Thrace), which were previously recognized as A. flavicollis (Michaux et al. 2004) and haplotypes from Kordestan province, western Iran. Interestingly, this lineage is genetically distant (6.8 %) from lineage (B) including haplotypes of A. flavicollis. Hence, the status of A. flavicollis in Iran, Asia Minor, and Levant is questionable. Differences between eastern and western populations of A. flavicollis have previously been reported, but were attributed to the small sample size (Macholán et al. 2001). Michaux et al. (2004) demonstrated high genetic divergence between the western and eastern clades of A. flavicollis in relation to their origins from different Pleistocene refugia.
The Kordestan haplotypes in clade (A) were compared with European A. flavicollis mentioned in the literature (Reutter et al. 1999; Barčiová and Macholán 2009; Kryštufek and Vohralik 2009) and were identified as A. cf. ponticus based on differences in coloration (smaller yellow spot on their chest), body and skull size (smaller mean), and pattern of the occlusal surface of the molars. This study thus provided good evidence for verifying A. ponticus as a distinct species, but closely related to A. flavicollis. In accordance with Suzuki et al. (2008), a sister relationship was confirmed between A. ponticus and A. flavicollis.
Furthermore, A. uralensis and A. pallipes from Afghanistan were also identified as sister taxa. Despite the low genetic distance between the lineages (E) representing A. pallipes and (D) A. uralensis, there were significant differences in their morphometric characteristics. Additionally, in agreement with Bellinvia (2004) and Michaux et al. (2002), the subgenus Karstomys was identified as a sister taxon to the Sylvaemus group.
Geographic Distribution
Apodemus witherbyi was identified as the most widespread species of the genus Apodemus in Iran, occupying a range of habitats, except for deciduous forests of the south Caspian Sea, Persian Gulf shores, the central deserts, and southeast of Iran. This species inhabits juniper and coniferous forests on the northern slopes of the Alborz Mountains in the north and northeast Iran (Ghorbani et al. 2010), and sparse wild oak and pistachio forests in the Zagros Mountains, where it is sympatric with A. cf. ponticus (Mohammadi et al. 2014). Apodemus witherbyi also inhabits drier habitats on the southern slopes of the Alborz Mountains and in the highlands of Yazd near Shirkouh, central Iran, which is surrounded by the central deserts of Iran.
However, other species have more restricted ranges. For instance, A. hyrcanicus and A. uralensis are dense-forest dwellers and distributed sympatrically around the southern margin of the Caspian Sea (Ghorbani et al. 2010) in tertiary relict broad-leaf forests, so called Euxino-hyrcanian forests, on the northern slopes of the Alborz (Akhani et al. 2010). In addition, A. cf. ponticus is considered to be the least common species in Iran, with a restricted distributional range. This species is sympatric and syntopic with A. witherbyi in the thickets of the northwest Zagros Mountains (Mohammadi et al. 2014).
Apodemus mystacinus has been found in the high-altitude oak forests of the Zagros Mountains, where it was recently recorded in oak forests in Marivan (Darvish et al. 2014). However, Firouz (2008) also noted its presence in northwestern Iran, in the Alborz range. Apodemus mystacinus is sympatric with A. cf. ponticus on the western slopes of the Zagros, but these two species were not captured at the same elevation; A. mystacinus lives at higher elevations, i.e., above 1400 m above sea level. The western slopes of the Zagros Mountains in Iran are thought to be the easternmost limit of A. mystacinus.
The south Caspian Sea depression formed during the Neogene (Golonka 2004), after which the region was gradually covered with late Tertiary deciduous forests and played a role as a refugium for many taxa during the Pleistocene. We can hypothesize that the south Caspian refugium had a profound impact on the speciation and divergence of A. hyrcanicus. Additionally, some refugial areas have been defined in the Zagros range (Djamali 2008; Ahmadzadeh et al. 2013; Rajaei Sh. et al. 2013) and could have had similarly essential roles in the genetic divergence of the population of A. mystacinus from Iran.
Phylogeography of the Genus Apodemus in the Eastern Mediterranean
The orogenic activities in the Eastern Mediterranean and the squeezing of the Arabian and Turkish plates into the Iranian plate, which led to the uplifting of the High Zagros Mountains and the Anatolian Plateau from the middle and late Miocene (Dercourt et al. 1993; Sengor and Natalin 1996; Yılmaz et al. 1998), hampered biotic exchange between the central Iranian Plateau and the Near East. Further, the establishment of a marine barrier between Anatolia and the Balkan region, in addition to the uplifting of the Carpathians in the Tortonian age (11.62–7.2 Ma) (Rogl 1999), caused climate change and increased seasonality in the Eastern Mediterranean (Brachert et al. 2006). This phase was characterized by a gradual change from a subtropical to a warm temperate climate, and alteration of the vegetation from mixed mesophytic mountain forest to open habitats and savannah in Anatolia and the Middle East (Akgün et al. 2007; Pound et al. 2011). These tectonic events and the consequent climatic configuration of the late Miocene probably strongly reduced gene flow between different lineages of the genus Apodemus, resulting in diversification of the subgenus Karstomys in the Eastern Mediterranean (about 7.3 Ma). Interestingly, the split between the rodent clades Spalax/Nannospalax (Hadid et al. 2012) and different salamander lineages Mertensiella luschani (Weisrock et al. 2001), as well as the eastern/western Mediterranean clades of the plant genus Haplophyllum, occurred during the same period (Manafzadeh et al. 2013).
The estimated divergence times imply speciation of A. witherbyi at the Miocene/Pliocene transition (about 6 Ma). The late Miocene was characterized by extended savannah, called the Greco-Iranian Province, from the Balkan Peninsula to Iran and Afghanistan (De Bonis et al. 1993). During the late Miocene, seasonal rainfall led to the establishment of grasslands with small trees and replacement of the woodland by more open habitats (Koufos and Konidaris 2011). In turn, extension of the steppe area was responsible for a change in mammal diets from C3 plants to C4, leading to a replacement of forest dwellers by open-adapted mammals in western Asia (Barry et al. 1985). This transition occurred in Pakistan between about 7.8 and 6 Ma (Cerling et al. 1997). Moreover, Koufos et al. (2005) suggested that extinction and immigration of woodland-dependent mammals at the Miocene/Pliocene boundary provided some unoccupied ecological niches for taxa such as Apodemus and Parapodemus in the Eastern Mediterranean. Additionally, Wessel (1955) proposed that southwestern Asia was a cradle for the evolution of different clades of rodents, as a result of their adaptation to new habitats. Based on our results, dispersal and the occupation of new habitats resulted in the radiation of the genus Apodemus to a new lineage (A. witherbyi) in the Eastern Mediterranean (6 Ma).
The split between A. mystacinus and A. epimelas (4.5 Ma) occurred after the Messinian Salinity Crisis (5.96–5.33 Ma), characterized by drought and cold climate (Hsu et al. 1977). During the Messinian Salinity Crisis (5.96–5.33 Ma) in the late Miocene, uplifting of Spain and Morocco closed the seaway between the Atlantic Ocean and the Mediterranean (Clauzon et al. 1996; Krijgsman et al. 1999). It is postulated that desiccation of Circa-Mediterranean regions trapped A. epimelas in islands (Mljet, Korcula) and restricted this taxon to Europe, i.e., the Balkan region, Macedonia, and Thrace, where the climate was still relatively humid about 5 Ma (Fortelius et al. 2006). In contrast, A. mystacinus occupied more southeastern regions (Anatolia, the Levant, some Aegean islands, and the western Zagros), which were drier under the effect of a north–south polarization of humidity (Fortelius et al. 2006). The results corroborate the diversification of two different clades of the subgenus Karstomys under the impact of late Pliocene climatic fluctuation, associated with the Messinian crisis (Michaux et al. 2005a).
The split between A. sylvaticus and A. flavicollis lineages (3.8 Ma) is thought to be related to the Zanclean phase (5.33–3.6 Ma), after the refilling of the Mediterranean Sea. Circa-Mediterranean regions were affected by a warm, humid climate during the early Pliocene and provided forest habitats in at least some parts of the Italo-Balkan/Near East regions (Michaux et al. 2004; Koufos et al. 2005; Kovar-Eder et al. 2006). This type of habitat supported the distribution of the ancestral A. flavicollis in the Italo-Balkan Peninsulas and the Near East, while the population of A. sylvaticus was restricted to the Iberian Peninsula (Michaux et al. 2003, 2004). This scenario is supported by the fact that the present seaway between the Black and Mediterranean seas (the Bosphorus Strait), which creates a barrier to terrestrial exchange, was not opened until the end of the Pliocene (Cheylan 1995; Dubey et al. 2007). This view was confirmed by the preference of A. flavicollis for forested habitats (Michaux et al. 2004) and of A. sylvaticus for bushy habitats (Kryštufek and Vohralik 2009). The current distributions of A. sylvaticus in the northern part of the Danube River (Bugarski-Stanojevic et al. 2008) and A. flavicollis in the southern parts further support this hypothesis (Kryštufek and Vohralik 2009).
Divergence between the clades including A. flavicollis and A. cf. ponticus dates to 2.6 Ma, which is congruent with the starting of the Praetiglian cold stage in Europe, and forest withdrawal (Ruddiman and Raymo 1988). These events caused the formation of steppe habitats (Fauquette et al. 1998, 1999), resulting in the contraction and bounding of the ancestral populations of A. flavicollis and A. cf. ponticus to different refuge areas: one (A. flavicollis) in the south Balkans and another (A. cf. ponticus) in the Eastern Mediterranean, which was likely still covered with forests at that time. As noted above, our results are in concordance with those of Michaux et al. (2004), who recognized two different clades of A. flavicollis (Oriental and European ones) originating from different refugia during the Tertiary/Quaternary transition. (The oriental clade was recognized as A. cf. ponticus in our study).
The Hyrcanian forests were considered to act as refugia for many Arcto-Tertiary relict species (Zohary 1973; Leestmans 2005). From the middle late Miocene, the region had a humid, closed landscape (Mirzaie Ataabadi 2007), providing a suitable climate for expansion of the Pontic forests in the early Pliocene (5–2 Ma; Fortelius et al. 2006). These kinds of broad-leaf forest habitats remained stable during the Pleistocene (Weiss and Ferrand 2007), supporting the existence of forest-dwelling mammals. In this respect, the divergence of A. hyrcanicus (5.2 Ma) is postulated to have occurred after the formation of the Hyrcanian forests over the Caucasus and southern Caspian Sea at the Miocene/Pliocene boundary.
Conclusions
The uplifting of the Zagros Mountains and Anatolian Plateau in the middle Miocene, the Messinian Salinity Crisis, and the formation of the Hyrcanian tertiary forests during the Neogene probably all played substantial roles in the radiation and subsequent distribution patterns of members of the genus Apodemus in the Eastern Mediterranean region. Alternative phases of climate fluctuations led to the formation and expansion of savannah, characterized by open-adapted mammals, and forest habitats in the Eastern Mediterranean, further driving the diversification of the genus Apodemus.
References
Ahmadzadeh F, Carretero MA, Rödder D, Harris DJ, Freitas SN, Perera A, Böhme W (2013) Inferring the effects of past climate fluctuations on the distribution pattern of Iranolacerta (Reptilia, Lacertidae): evidence from mitochondrial DNA and species distribution models. Zool Anz 252:141–148
Akgün F, Kayseri MS, Akkiraz MS (2007) Palaeoclimatic evolution and vegetational changes during the late Oligocene–Miocene period in Western and Central Anatolia (Turkey). Palaeogeogr Palaeoclimatol Palaeoecol 253:56–90
Akhani H, Djamali M, Ghorbanalizadeh A, Ramezani E (2010) Plant biodiversity of Hyrcanian relict forests, N Iran: an overview of the flora, vegetation, palaeoecology and conservation. Pak J Bot 42:231–258
Aydar E, Çubukçu HE, Şen E, Akin L (2013) Central Anatolian Plateau, Turkey: incision and paleoaltimetry recorded from volcanic rocks. Turkish J Earth Sci 22:739–746
Balakirev AE, Baskevich MI, Gmyl AP, Okulova NM, Andreeva TA, Sokolenko OV, Malygin VM, Khlyap LA, Oparin ML, Orlov VN (2007) On the taxonomic rank of ciscaucasicus and its relationships with the pygmy wood mouse Sylvaemus uralensis inferred from the mtDNA cytochrome b gene sequence. Genetika 43:1651–1666
Barčiová L, Macholán M (2009) Morphometric key for the discrimination of two wood mice species, Apodemus sylvaticus and A. flavicollis. Acta Zool Acad Sci Hung 55:31–38
Barry JC, Johnson NM, Raza SM, Jacobs LL (1985) Neogene mammalian faunal change in southern Asia: correlations with climatic, tectonic, and eustatic events. Geology 13:637–640
Bellinvia E (2004) A phylogenetic study of the genus Apodemus by sequencing the mitochondrial DNA control region. J Zool Syst Evol Res 42:289–297
Blanford WT (1875) Descriptions of new Mammalia from Persia and Baluchistan. Ann Mag Nat Hist London, 4th Series, 16:309–313
Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput Biol 10: e1003537
Brachert TC, Reuter M, Felis T, Kroeger KF, Lohmann G, Micheels A, Fassoulas C (2006) Porites corals from Crete (Greece) open a window into late Miocene (10 Ma) seasonal and interannual climate variability. Earth Planet Sci Lett 245:81–94
Bruford MW, Hanotte O, Brokfield JFY, Burke T (1992) Single-locus and multilocus DNA fingerprinting. In: Hoelzel AR (ed) Molecular Genetic Analysis of Populations: A Practical Approach. Oxford University Press, New York, pp 225–269
Bugarski-Stanojevic V, Blagojevic J, Adna–devic T, Jojic V, Vujosevic M (2008) Molecular phylogeny and distribution of three Apodemus species (Muridae, Rodentia) in Serbia. J Zool Syst Evol Res 46:278–286
Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR (1997) Global vegetation change through the Miocene/Pliocene boundary. Nature 389:153–158
Chelomina GN, Atopkin DM (2010) Molecular genetic evidence of a deep phylogenetic discontinuity between the Asian and European races of pygmy wood mouse based on the mitochondrial cytochrome b gene variation. Mol Biol 44:699–708
Chevret P, Veyrunes F, Britton-Davidian J (2005) Molecular phylogeny of the genus Mus (Rodentia: Muridae) based on mitochondrial and nuclear data. Biol J Linn Soc 84:417–427
Cheylan M (1995) Les reptiles du Pale´artique occidental. Diversite´ et Conservation. Doctoral dissertation (unpublished), Laboratoire de Bioge´ographie et Ecologie des Verte´bre´ s, Montpellier, pp 339
Clauzon G, Suc JP, Gautier F, Berger A, Loutre MR (1996) Alternate interpretation of the Messinian salinity crisis: controversy resolved? Geology 24:363–366
Corbet GB (1978) The Mammals of the Palaearctic Region, A Taxonomic Review. British Museum (Natural History), London
Darviche D, Benmehdi F, Britton-Davidian J, Thaler L (1979) Donnees preliminaries sur la systematique biochimique des genres Mus et Apodemus en Iran. Mammalia 43:427–430
Darvish J, Akbary Rad S, Siahsarvieh R, HosseinPour Feizi MA, Ghorbani F (2010) New record on pigmy field mouse (Muridae, Rodentia) from Northeast Iran. Hystrix 21:115–126
Darvish J, Javidkar M, Siahsarvie R (2006) New species of wood mouse of genus Apodemus (Rodentia: Muridae) from Iran. Zool Middle East 38:5–16
Darvish J, Mohammadi Z, Ghorbani F, Mostafavi E (2014) Morphological morphometric characterisation of the eastern broad-toothed field mouse Apodemus mystacinus (Rodentia: Muridae) from Zagros Mountains, north-western Iran. Acta zool bulg 66:461–468
De Bonis L, Brunet M, Heintz E, Sen S (1993) La province Greco-irano-afgane et la repartition des faunes mammaliennes au Miocene superieur. Paleontol Evol 24–25:96–106
Dercourt J, Ricou LE, Vrielynck B (1993) Atlas Tethys Paleoenvironmental Maps. Gauthiers-Villars, Paris
Djamali M (2008) Palaeoenvironmental changes in Iran during the last two climatic cycles (vegetation-climate-anthropisation). PhD thesis, Université Paul Cézanne (Aix-Marseille), Faculté des Sciences et Techniques
Drummond AJ, Ho SYW, Rawlence N, Rambaut A (2007) A rough guide to BEAST 1.4. Available at: http://beast.bio.ed.ac.uk
Dubey S, Cosson JF, Vohralik V, Kryštufek B, Diker E, Vogel P (2007) Molecular evidence of Pleistocene bidirectional faunal exchange between Europe and the Near East: the case of the bicoloured shrew (Crocidura leucodon, Soricidae). J Evol Biol 20:1799–1808
Ellerman JR, Morrison-Scott TCS (1951) Checklist of Palaearctic and Indian Mammals 1758 to 1946. Trustees of the British Museum (Natural History), London
Etemad E (1978) The Mammals of Iran. Rodents and Key to Their Classification (Vol. 1). National Society for Protection of Natural Resources and Human Environment, Teheran (in Farsi, with a summary in English)
Fauquette S, Clauzon G, Suc JP, Zheng Z (1999) A new approach for palaeoaltitude estimates based on pollen records: example of the Mercantour massif (southeastern France) at the earliest Pliocene. Earth Planet Sci Lett 170:35–47
Fauquette S, Guiot J, Suc JP (1998) A method for climatic reconstruction of the Mediterranean Pliocene using pollen data. Palaeogeogr Palaeoclimatol Palaeoecol 144:183–201
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Filippucci MG, Simson S, Nevo E (1989) Evolutionary biology of the genus Apodemus Kaup, 1829 in Israel. Allozymic and biometric analyses with description of a new species: Apodemus hermonensis (Rodentia, Muridae). Monit Zool 56:361–376
Firouz E (2008) A Guide to the Fauna of Iran (Vertebrates). Iran University Press, Tehran (in Farsi)
Fortelius M, Eronen J, Liu L, Pushkina D, Tesakov A, Vislobokova I, Zhang Z (2006) Late Miocene and Pliocene large land mammals and climatic changes in Eurasia. Palaeogeogr Palaeoclimatol Palaeoecol 238:219–227
Ghorbani F, Darvish J, Kami HG, Mirshamsi O (2010) Rodent fauna of the western Golestan Province in northeast Iran. IJAB 6:37–48
Golonka J (2004) Plate tectonic evolution of the southern margin of Eurasia in the Mesozoic and Cenozoic. Tectonophysics 381:235–273
Goodwin GG (1940) Mammals collected by the Legendre 1938 Iran expedition. American Museum Novitates 1082:1–17
Guest B, Guest A, Axen G (2007) Late Tertiary tectonic evolution of northern Iran: a case for simple crustal folding. Global Planet Change 58:435–453
Hadid Y, Nemeth A, Snir S, Pavlıcek T, Csorba G, Kazmer M, Major A, Mezhzherin S, Rusin M, Coskun Y, Nevo E (2012) Is evolution of blind mole rats determined by climate oscillations? PLoS ONE 7:e30043
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95⁄98⁄NT. Nucleic Acids SympSer 41:95–98
Hoofer SR, Gaschak S, Dunina-Barkovskaya Y, Makluk J, Meeks HN, Wickliffe JK, Baker RJ (2007) New information for systematics, taxonomy, and phylogeography of the rodent genus Apodemus (Sylvaemus) in Ukraine. J Mammal 88:330–342
Hsu KJ, Montadert L, Bernoulli D, Cita MB, Erickson A, Garrison RE, Kidd RB, Melieres F, Muller C, Wright R (1977) History of the Mediterranean salinity crisis. Nature 267:399–403
Huelsenbeck JP, Ronquist FR, Teslenko M (2011) MRBAYES: Bayesian inference of phylogeny. http://mrbayes.sourceforge.net/authors.php
Jangjoo M, Darvish J, Vigne JD (2011) Application of outline analysis on fossil and modern specimens of Apodemus. IJAB 7:143–155
Javidkar M, Darvish J, RiahiBakhtiari A (2005) Discriminate analysis of dental and cranial characteristics in Apodemus hyrcanicus and A. hermonensis (Rodentia, Muridae) from Iran. Zool Middle East 35:5–12
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Mol Evol 15:111–120
Koufos GD, Konidaris GE (2011) Late Miocene carnivores of the Greco-Iranian Province: composition, guild structure and palaeoecology. Palaeogeogr Palaeoclimatol Palaeoecol 305:215–226
Koufos GD, Kostopoulos DS, Vlachou TD (2005) Neogene/Quaternary mammalian migrations in Eastern Mediterranean. Belg J Zool 135:181–190
Kovar-Eder J, Kvaček Z, Martinetto E, Roiron P (2006) Late Miocene to early Pliocene vegetation of southern Europe (7–4Ma) as reflected in the megafossil plant record. Palaeogeogr Palaeoclimatol Palaeoecol 238:321–339
Krijgsman W, Hilgen FJ, Raffi I, Sierro FJ, Wilson DS (1999) Chronology, causes and progression of the Messinian salinity crisis. Nature 400:652–655
Kryštufek B (2002) Identity of four Apodemus (Sylvaemus) types from the Eastern Mediterranean and the Middle East. Mammalia 66:43–51
Kryštufek B, Hutterer R (2006) The Ural field mouse Apodemus uralensis–a mammal species new to Iran. Zool Middle East 38:111–112
Kryštufek B, Mozetic Francky B (2005) Mt. Hermon field mouse Apodemus iconicus is a member of the European mammal fauna. Folia Zool 54:69–74
Kryštufek B, Vohralik V (2009) Mammals of Turkey and Cyprus. Rodentia II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae. Knjižnica Annales Majora
Kryštufek B, Vohralík V, Obuch J (2009) Endemism, vulnerability and conservation issues for small terrestrial mammals from the Balkans and Anatolia. Folia Zool 58: 291–302
Lay DM (1967) A study of the mammals of Iran resulting from the Street Expedition of 1962–63. Fieldiana Zool 54:1–282
Leestmans R (2005) Le refuge caspiens et son importance en biogéographie. Linn Belg 20:97–102
Librado P, Rozas J (2009) DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Macey JR, Schulte JA, Ananjeva NB, Larson A, Rastegar-Pouyani N, Shammakov SM, Papenfuss TJ (1998) Phylogenetic relationships among agamid lizards of the Laudakia caucasia species group: testing hypotheses of biogeographic fragmentation and an area cladogram for the Iranian Plateau. Mol Phylogenet Evol 10:118–131
Macholán M, Filippucci MG, Benda P, Frynta D, Sádlová J (2001) Allozyme variation and systematics of the genus Apodemus (Rodentia: Muridae) in Asia Minor and Iran. J Mammal 82:799–813
Manafzadeh S, Salvo G, Conti E (2013) A tale of migrations from east to west: the Irano-Turanian floristic region as a source of Mediterranean xerophytes. J Biogeogr doi:10.1111/jbi.12185
Michaux J, Bellinvia E, Lymberakis P (2005a) Taxonomy, evolutionary history and biogeography of the broad-toothed field mouse (Apodemus mystacinus) in the Eastern Mediterranean area based on mitochondrial and nuclear genes. Biol J Linn Soc 85:53–63
Michaux J, Chevret P, Filippucci MG, Macholán M (2002) Phylogeny of the genus Apodemus with a special emphasis on the subgenus Sylvaemus using the nuclear IRBP gene and two mitochondrial markers: Cytochrome b and 12S rRNA. Mol Phylogenet Evol 23:123–136
Michaux JR, Libois R, Filipucci MG (2005b) So close and so different: comparative phylogeography of two small mammal species, the yellow-necked field mouse (Apodemus flavicollis) and the wood mouse (Apodemus sylvaticus) in the western Palearctic region. Heredity 94:52–63
Michaux JR, Libois R, Paradis E, Filippucci MG (2004) Phylogeographic history of the yellow-necked field mouse (Apodemus flavicollis) in Europe and in the Near and Middle East. Mol Phylogenet Evol 32:788–798
Michaux JR, Magnanou E, Paradis E, Nieberding C, Libois R (2003) Mitochondrial phylogeography of the wood mouse (Apodemus sylvaticus) in the western Palearctic region. Mol Ecol 12:685–697
Mirzaie Ataabadi M (2007) Cenozoic mammal footprints of Iran and their significance. Cenozoic Vertebrate Tracks and Traces. Bull New Mexico Mus Nat Hist Sci 42:251–260
Mohammadi Z, Darvish J, Ghorbani F, Mostafavi E (2014) First record of the Caucasus field mouse Apodemus ponticus Sviridenko, 1936 (Rodentia Muridae) from Iran. Biodiversity Journal 5:475–480
Mohammadi Z, Darvish J, Haddad F, Ghorbani F (2012) A karyological study of some murid rodents (Rodentia: Muridae) of Iran. Prog Biol Sci 2:30–39
Montgelard C, Bentz S, Tirard C, Verneau O, Catzeflis FM (2002) Molecular systematic of Sciurognathi (Rodentia): the mitochondrial cytochrome b and 12S rRNA genes support the Anomaluroidea (Peptidae and Anomaluridae). Mol Phylogenet Evol 22:220–233
Mouthereau F (2011) Timing of uplift in the Zagros belt/Iranian Plateau and accommodation of late Cenozoic Arabia–Eurasia convergence. Geol Mag 148:726–738
Musser GG, Carleton MD (2005) Superfamily Muroidea. In: Wilson DE, Reeder DM (eds) Mammal Species of the World: A Taqxonomic and Geographic Reference, 3rd edn. The Johns Hopkins University Press, Baltimore, pp 894–1531
Naseri Z, Jalal R, Darvish J (2006) Genetic study on Apodemus avicennicus and Apodemus witherbyi by RAPD-PCR. IJAB 2:77–81
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Pound MJ, Haywood AM, Salzmann U, Riding JB, Lunt DJ, Hunter SJ (2011) A Tortonian (late Miocene, 11.61-7.25 Ma) global vegetation reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 300:29–45
Pour Feizi MH, Darvish J, Pouladi N, Akbari Rad S, Siahsarvie R (2009) Biosystematics study of steppe field mouse Apodemus witherbyi (Rodentia: Muridae) from North-West Iran. IJAB 5:47–58
Rajaei Sh H, Rödder D, Weigand AM, Dambach J, Raupach MJ, Wägele JW (2013) Quaternary refugia in southwestern Iran: insights from two sympatric moth species (Insecta, Lepidoptera). Org Divers Evol 13:409–423
Reutter BA, Hausser J, Vogel P (1999) Discriminant analysis of skull morphometric characters in Apodemus sylvaticus, A. flavicollis, and A. alpicola (Mammlia; Rodentia) from the Alps. Acta Theriol 44:299–308
Rogl F (1999) Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geol Carpath 50:339–349
Ruddiman WF, Raymo ME (1988) Northern hemisphere climate regimes during the past 3 ma: possible tectonic connections. Phil Trans R Soc London B 318:411–430
Sengor AMC, Natalin BA (1996) Paleotectonics of Asia: fragment of a synthesis. In: Yin A, Harrison TM (ed) The Tectonics of Asia. Cambridge University Press, New York, pp 486–640
Siahsarvie R, Darvish J (2008) Geometric morphometric analysis of Iranian wood mice of the genus Apodemus (Rodentia, Muridae). Mammalia 72:109–115
Suzuki H, Filippucci MG, Chelomina GN, Sato JJ, Serizawa K, Nevo E (2008) A biogeographic view of Apodemus in Asia and Europe inferred from nuclear and mitochondrial gene sequences. Biochem Genet 46:329–346
Suzuki H, Sato JJ, Tsuchiya K, Luo J, Zhang Y-P, Wang Y-X, Jiang X-L (2003) Molecular phylogeny of wood mice (Apodemus, Muridae) in East Asia. Biol J Linn Soc 80:469–481
Suzuki H, Tsuchiya K, Takezaki N (2000) A molecular phylogenetic framework for the Ryuku endemic rodents Tokudaia osimensis and Diplothrix legata. Mol Phylogenet Evol 5:15–24
Swofford DL (2000) PAUP: Phylogenetic analysis using parsimony (*and other methods). Version 4.0b4a
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal-W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Weisrock DW, Macey RJ, Ugurtas IH, Larson A, Papenfuss TJ (2001) Molecular phylogenetics and historical biogeography among salamandrids of the “true” salamander clade: rapid branching of numerous highly divergent lineages in Mertensiella luschani associated with the rise of Anatolia. Mol Phylogenet Evol 18:434–448
Weiss S, Ferrand N (2007) Phylogeography of Southern European Refugia. University of Porto, Portugal
Wessels W (1955) Miocene rodent evolution and migration (Muroidea from Pakistan, Turkey and Northern Africa). Faculty of Geosciences, Utrecht University
Yılmaz Y, Guner Y, Saroglu F (1998) Geology of the Quaternary volcanic centers of the east Anatolia. J Volcanol Geotherm Res 85:173–210
Zohary M (1973) Geobotanical Foundation of the Middle East. Gustav Fische Verlag Stuttgart, Amsterdam
Acknowledgments
This research was supported by grants from Pasteur Institute of Iran under project (No 92–242), Department of Environment of Yazd Province, Iran (No.121-32217), and Vector-borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran (No. 1.19727). The authors are indebted to Hitoshi Suzuki for providing two sequences (HS1102 and Mys 2) and Johan Michaux for his kind consultation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jamshid Darvish and Zeinolabedin Mohammadi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Darvish, J., Mohammadi, Z., Ghorbani, F. et al. Phylogenetic Relationships of Apodemus Kaup, 1829 (Rodentia: Muridae) Species in the Eastern Mediterranean Inferred from Mitochondrial DNA, with Emphasis on Iranian Species. J Mammal Evol 22, 583–595 (2015). https://doi.org/10.1007/s10914-015-9294-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-015-9294-9