Abstract
Alternating glacial and interglacial periods led to range shifts (contractions and expansions), persistence in distinct glacial refugia and extinction events in various temperate organisms. Today, the integrative analysis of molecular markers and spatial distribution models conducted for multiple taxa allows the detection of phylogeographical patterns, thus reconstructing major biogeographical events in their shared evolutionary history. In this study, the effects of past climate change on the evolutionary history of two sympatric moth species (Gnopharmia colchidaria s.l. and G. kasrunensis) and their host plants (Prunus scoparia and P. fenzliana) were inferred for the largely neglected biodiversity hot spot Iran. We complementarily analyzed the population structure of both moth species (187 specimens, based on COI) in congruence with batched species distribution models (SDMs) for all four taxa and for the times of the Last Glacial Maximum (21 ky BP), 6 ky BP and today. Coincidence of SDMs and the distribution of haplotype lineages indicated a shared refugium for the southwestern Zagros Mountains and potential species-specific refugial areas in the southern Caucasus and the Kope-Dagh Mountains. Both moth species experienced past population expansion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climatic oscillations during the Quaternary glaciation had a crucial influence on the formation of biota in the northern hemisphere. Organisms were forced to local extinction, to range contractions with survival in refugial areas and to inter- or postglacial (re-)colonization (e.g., Avise 2000; Hewitt 2000). Contemporary species population genetic structure thus reflects responses to past climatic oscillations of the Pleistocene period (Hewitt 1996, 2000; Petit et al. 2003; Stewart et al. 2010).
Refugial survival promoted population isolation and accompanied with limited gene flow and genetic drift led to the formation of endemic haplotypes, lineages and/or species (Weir and Schluter 2004). After the Last Glacial Maximum (LGM, 23,000-18,000 years before present; Proven and Bennett 2008), refugial populations were able to (re-)colonize the surrounding areas, giving rise to the observed extant distribution patterns. Hereby, postglacial population expansions may have occurred in several ways and be distinguished by their resulting spatial-genetic patterns. On the one hand, following rapid events of (re-)colonization, a few individuals may establish founder populations that are prone to population bottlenecks. Due to a small effective population size and genetic drift, these are likely for fixation of newly arisen haplotypes (Hartl and Clark 1997). Moreover, founder populations frequently demonstrate low genetic diversity (Avise 2000). On the other hand, slow but steady population expansions can maintain a high amount of their ancestral genetic diversity (Hartl and Clark 1997). Previously isolated refugial lineages may expand and form zones of secondary contact, which are characterized by a local increase in genetic diversity (Hewitt 2001). These effects have been well studied for European and North American biota. Several LGM refugia have been reconstructed based on different methodological approaches and for various taxonomic groups (e.g., Avise 2000; Petit et al. 2003; Habel and Assmann 2009; Stewart et al. 2010).
Despite the plethora of phylogeographical studies focusing on North America and Europe, other parts of the world are less well surveyed. The Middle East in general and Iran in particular have been largely neglected in past phylogeographical studies (Ahmadzadeh et al. 2012). Climatic and tectonic events of the Quaternary period are scarcely documented, and only very limited knowledge exists about the responses of organisms to changing environmental conditions (El-Moslimany 1986). Nevertheless, glacial remains are still visible in the high mountain areas of Azerbaijan, Alborz and Zagros and in the Central Iranian massifs such as Kerman and Shirkuh (e.g., Wright 1961; Kuhle 2008). Despite different methodological aspects and many ambiguities, most of the available paleoecological records confirmed the marked effects of Quaternary climate oscillations in Iran, characterizing the glacial periods and especially the LGM with a cooler and more arid climate than today (e.g., van Zeist and Wright 1963; van Zeist and Bottema 1977; El-Moslimany 1986; Kelts and Shahrabi 1986; Djamali 2008; Djamali et al. 2011). The direct effects of these climatic oscillations on organisms have been documented in a few pollen records (van Zeist and Bottema 1977; Djamali 2008). Furthermore, Djamali et al. (2010) postulated that global and regional climatic changes might have indirectly affected the dynamics of the Zagros Oak woodlands during the Quaternary period by changes in climate seasonality (see also El-Moslimany 1986; Stevens et al. 2001). More recently, palynological study results by Djamali et al. (2011) suggested grassland and arboreal vegetation types probably with scattered oak trees for north and northwestern Zagros at the Quaternary glacial intervals.
In this study we performed a comparative phylogeographical approach to contribute to a further understanding of the impact of the Pleistocene glacial cycles on the biodiversity hot spot of Iran. The two widespread and sympatric moth species Gnopharmia colchidaria s.l. and G. kasrunensis (Geometridae) were analyzed for their population structure in a spatial-genetic context. Distribution ranges and the taxonomy of these two species have been recently revised (Rajaei Sh et al. 2012). G. colchidaria s.l. with its three subspecies occurs mainly from southern Iran to the Caucasus in the northwest, and to the Kopet-Dagh, Afghanistan and Pakistan in the east. Gnopharmia kasrunensis is distributed only in south and west Iran (Rajaei Sh et al. 2012). Due to these characteristics (sympatry, well-known distribution range and taxonomy), we take Gnopharmia moths as candidate organisms for tracing paleo-events in the region of Iran. As both species are monophagous, successful population foundations depend on the presence of their host plants, Prunus scoparia and P. fenzliana (Rajaei Sh 2010; Rajaei Sh et al. 2012). Depending on this biological interaction, congruent patterns may indicate, but simultaneously cross check, the evidence for shared events during their evolutionary history. We used species distribution modeling (Gnopharmia + Prunus) in congruence with the analysis of the population genetic structure (Gnopharmia) to find evidence for (1) the existence and location of LGM refugia in Iran and (2) postglacial population expansion dynamics.
Material and methods
Sampling and species identification
Sampling sites and species identifications were retrieved from Rajaei Sh et al. (2012). In total, we genetically analyzed 187 individuals (see Table 2, supplement data) comprising 123 specimens of G. colchidaria s.l. from 39 sampling sites and 64 of G. kasrunensis from 16 sampling sites. Sampling sites of less than 50 km distance were pooled into a single geographic region, resulting in 11 regions for G. colchidaria s.l. and 5 for G. kasrunensis (Fig. 1).
Phylogeographic analyses
DNA extractions, as well as amplification and sequencing of the cytochrome c oxidase subunit I (COI) mtDNA, were performed at the Centre for Molecular Biodiversity Research (ZMB), Bonn, Germany, and at the Canadian Centre for DNA Barcoding (CCDB), Guelph, Canada. All steps were conducted according to Ivanova et al. (2006) without modifications. Sequences were edited in BioEdit 7.1.3 (Hall 1999), aligned with MAFFT 6 (Katoh et al. 2002) by using default settings and deposited in GenBank (see Table 2, supplementary data for collecting data and GenBank accession numbers).
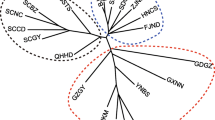
Statistical parsimony networks for each species were reconstructed with TCS v.1.3 (Clement et al. 2000). With this analysis, sequences are separated into a network of closely related haplotype groups with connected branches with less than 95 % probability. A nested clade analysis (Templeton 1998) was conducted to identify separately evolving haplotype lineages (Fig. 1). For comparison of the population genetic structure, overall and regional haplotype diversity (h) and nucleotide diversity (π) (Nei and Miller 1990) of both Gnopharmia species were calculated using Arlequin 3.5.1.2 (Excoffier and Lischer 2010) (Table 1).
For testing the possibility of a past population expansion event with effects on the recent demographic structure of genetic diversity, neutrality tests and pairwise mismatch distributions between haplotypes were conducted with Arlequin 3.5 (Excoffier and Lischer 2010) and DnaSP 5.10 (Libradi and Rozas 2009). Parameters of the demographic model expansion [i.e., tau (τ), theta 0 and theta 1) were obtained from Arlequin 3.5 analyses. Tajima’s D (Tajima 1989) and Fu’s FS (Fu 1997) estimates to test for deviations from the neutral theory model at equilibrium between genetic drift and mutation were performed. A significantly negative deviation from zero can be interpreted as a result of past population expansion and/or purifying selection, whereas a significantly positive value can result from balancing selection and/or a decrease in population size. The goodness of fit of observed data to a simulated model of population expansion was assessed with the Harpending’s raggedness index (Hri, Harpending 1994) and the sum of squared deviations (SSD). A significantly high value of Hri (p < 0.05) or high estimates of SSD mean a non-fitting deviation and thus a rejection of the null hypothesis of a population expansion model.
Species distribution modeling
In recent years, the application of species distribution models (SDMs) has become very popular. Correlative SDMs are commonly based on information on the target species’ current distribution and associated environmental conditions, which may comprise both bioclimatic conditions and cover information (e.g., Guisan and Zimmermann 2000; Elith and Leathwick 2009). Depending on the type of SDM algorithm applied, the target species’ realized or potential distribution is approximated, which can be subsequently projected into geographic space (Jiménez-Valverde et al. 2011). Projections of this estimated niche envelope through space and/or time allow an estimation of the species’ potential distribution in other geographic areas or time slices, e.g., during the last glacial maximum (Waltari et al. 2007; Svenning et al. 2011; Roberts and Hamann 2012). A detailed comparison of biogeographic patterns derived from population genetics and SDMs can provide independent but complementary lines of evidence (Chan et al. 2011).
A total of 137 presence records of G. colchidaria s.l. and 45 for G. kasrunensis were compiled for SDM development following the taxonomic assignments proposed by Rajaei Sh et al. (2012). The populations of both species are monophagous. Therefore, we additionally developed SDMs for the host plants based on 81 records of Prunus scoparia and 65 for Prunus fenzliana (distribution data from Browicz and Zieliński 1984). Coordinates of moth records were either directly taken from the specimen’s labels or georeferenced using Google Earth (www.google.com/earth/index.html). The “Road Atlas of Iran” (2005) was used for controlling the local names. The final data sets were checked for geographic plausibility in DIVA-GIS 7.4.0 (Hijmans et al. 2005).
A set of 19 bioclimatic variables was downloaded from the WorldClim database (Hijmans et al. 2005, www.worldclim.org) as environmental predictors that comprehensively describe the climatic conditions of the study area. This set of predictor variables has been frequently suggested to be suitable for SDM development (Busby 1991; Beaumont et al. 2005). Herein, we used all 19 bioclimatic variables with a spatial resolution of 2.5 arc min matching the spatial resolution of the species records. Computing pairwise Pearson’s correlation coefficients in Cran R 14.0 was used to assess collinearity among the variables. Out of highly correlated pairs of variables with R2 > 0.75, the putatively biologically most relevant variable was retained. The final set of predictors comprised 12 bioclimatic variables (bio 2-3, 6-10, 15-19) (see Table 3, supplemental data, for definition of these variables).
For SDM development, the Maxent algorithm was used applying the default settings (Phillips et al. 2006; Phillips and Dudík 2008; Elith et al. 2011). The training area of an SDM ideally comprises those environmental conditions that are accessible for the target species (Barve et al. 2011; Phillips et al. 2009). Therefore, we restricted the training range to an area defined by a circular buffer of 200 km enclosing all species records. In 100 iterations, the species records were randomly split into 70 % used for model building and 30 % used for model evaluation via the area under the receiver-operating characteristic curve (AUC, Swets 1988) and the point biserial correlation (COR, Elith et al. 2006). Finally, the average predictions per grid cell were used for further processing.
To assess possible changes of the species’ potential distribution during the past, we projected the SDMs developed under current conditions onto environmental conditions for 6 ky BP and 21 ky BP. We used paleoenvironmental conditions as suggested by two different global circulation models (GCM): the Community Climate System Model (CCSM, Kiehl and Gent 2004) and the Model for Interdisciplinary Research on Climate (MIROC, http://www.ccsr.u-tokyo.ac.jp/∼hasumi/MIROC/). Raw GCM outputs for 6 ky BP were downloaded from the PMIP 2 homepage (Braconnot et al. 2007, available through http://pmip2.lsce.ipsl.fr/) and spatially downscaled as described by Peterson and Nyári (2007). LGM scenarios for 21 ky BP, which were downscaled using the same technique, were obtained from the WorldClim database.
Any projection of a SDM onto conditions exceeding those within the training range of the model may negatively affect its reliability (Elith et al. 2010; Rocchini et al. 2011). Therefore, in all projections, we identified those areas outside of the training range using multivariate environmental similarity surfaces (Elith et al. 2010).
Results
Haplotype diversity and phylogenetic relationships of haplotypes
In total, 654 bp of COI mtDNA was sequenced. The COI fragment contained 35 variable sites for G. colchidaria s.l. and 29 for G. kasrunensis with 32 and 25 haplotypes revealed, respectively (Fig. 2a, b). The region of Fars exhibited the highest number of different haplotypes in both species (11 haplotypes for G. colchidaria s.l. and 15 for G. kasrunensis). Overall haplotype diversity in both species was high (h > 0.8, Table 1). A comparatively low haplotype diversity for the former species was found at Hormozgan, Lorestan and Kordestan as well as in Kerman for the latter (Fig. 3a, b). The highest haplotype diversity was found in Esfahan and Semnan (G. colchidaria), as well as in Fars and Hormozgan (G. kasrunensis). Haplotypes formed three (G. colchidaria) and two separately evolving lineages (G. kasrunensis) following the results of a third level nesting (Fig. 1). Haplotypes with high frequency (and a central position in the network; e.g., G. colchidaria: HP1, HP6, HP12, HP13; G. kasrunensis: Hp8, Hp14) and haplotypes demonstrating a tip position with low frequency were present in our data set. Localities of G. colchidaria s.l. comprising haplotypes from more than one nested clade were found in Kordestan, Dena, southern Fars (clades 3-1 + 3-2), Semnan, Khorasan and Pakistan (clades 3-1 + 3-3). The two nested clades revealed for G. kasrunensis were also geographically separated (Fig. 1).
Genetic diversity, population differentiation and population expansion
Genetic diversity of G. colchidaria s.l. was considerably higher compared to G. kasrunensis (Table 1, Fig. 2c, d). Spatial distribution of genetic diversity is visualized in Fig. 3c, d. The localities of Semnan, Khorasan and Pakistan demonstrated the highest genetic diversity in G. colchidaria. Remarkably, these three regions also possessed the largest genetic distance to almost all other populations (Fig. 2e). In the case of G. kasrunensis, the populations found at Yazd, Fars and Hormozagan had the highest genetic diversity. Individuals found at Hormozagan were strongly differentiated from all other specimens (Fig. 2f).
Both data sets demonstrated evidence for deviation from a model of selective neutrality (Fig. 4). Statistical tests of neutrality were significantly negative in the case of G. colchidaria s.l. (only Fu’s FS) and G. kasrunensis (both Fu’s FS and Tajima’s D) (Fig. 4). Hri and SDD values for both species were low, implying that the observed mismatch distribution was not significantly different from a distribution pattern expected after past population expansion (Fig. 4).
Mismatch distribution and tests of selective neutrality. Dotted line: The observed distribution; solid line: the expected distribution after past population expansion. For the neutrality tests, P-values are provided in brackets. Significant results are marked in bold. Hri: Harpending’s raggedness index; SDD: sum of squared deviations
Based on pairwise FST estimates for G. colchidaria s.l. (Fig. 2g, h), populations of Pakistan, Khorasan and Hormozgan were each clearly differentiated from all remaining populations. Western populations, on the contrary, shared various haplotypes and probably exhibited frequent gene flow with each other. The population of G. kasrunensis found at the Hormozgan population was clearly isolated from all remaining localities.
Species distribution modeling
For both species, the suggested suitable climate regions during glacial times (21 ky BP) were only partly congruent between the model scenarios CCSM and MIROC. For G. colchidaria s.l., the more conservative CCSM 21k model output suggested distribution areas in the northeastern and northwestern Alborz Mountains (entering Azerbaijan) as well as in the central Zagros Mountains. However, the MIROC model additionally suggested the Zagros Mountain range and the coastal area of the Gulf of Oman, demonstrating high habitat suitability (Figs. 5, 6, 7 and 8). The CCSM 6k and MIROC 6k model results were relatively similar: Wide regions within the Alborz Mountains and the Zagros Mountains could have provided suitable climatic conditions for G. colchidaria (Fig. 5). Similarly, the present distribution of this species is confined to those regions. Despite the potential refugial areas within the mountain ranges of Iran, long-term suitability of climatic conditions was revealed for northeastern Pakistan, eastern Afghanistan, Uzbekistan and Syria/Iraq.
Both SDM projections (CCSM 21k and MIROC 21k) for G. kasrunensis and the time of the LGM suggested suitable habitats in the Central Zagros Mountains (Fig. 6). Similar to the case of G. colchidaria, the CCSM 21k model for G. kasrunensis was more conservative than the MIROC 21k. The latter predicted suitable climatic conditions at the coast of the Gulf of Oman and in large parts of the west and central Zagros Mountains. High habitat suitability in those two regions was also suggested for the Mid-Holocene. Moreover, they matched the present day distribution of G. kasrunensis. Outside of Iran, the north and south of Afghanistan and northern Iraq have most probably possessed permanently suitable climatic conditions since the LGM. However, no record of G. kasrunensis has been reported from outside of Iran (Rajaei Sh et al. 2012).
In agreement with SDM results obtained for the two moth species, the SDMs for the widespread and shared host-plant P. scoparia suggested permanently suitable climatic conditions in the Zagros Mountain range (for CCSM 21k only in the southern Zagros Mountains), at the northern coasts of the Gulf of Oman and in the western Alborz Mountains (Fig. 7). Although the vast majority of presence records used for model building in P. scoparia originated from the area of Iran, regions of high climatic suitability were also revealed for south Afghanistan, eastern Turkmenistan and at the border between Syria and Turkey. Model results for P. fenzliana, the host plant of the designated subspecies G. colchidaria colchidaria (see Rajaei Sh et al. 2012), differed between the SDM models. For the LGM, the MIROC 21k model predicted a potential refugial area in the eastern Alborz Mountains, whereas the CCSM 21k model revealed the southern Caucasus, although with lower habitat suitability. Both models for the Mid-Holocene, CCSM 6k and MIROC 6k revealed the southern Caucasus and to some extent the Alborz Mountain ranges as climatically suitable areas. However, the current distribution of P. fenzliana is restricted to the southern Caucasus (Fig. 8).
Discussion
A congruent pattern displayed among different taxa that occur in the same biogeographic region is evidence that a shared event played an important role in driving evolution. After studying independent but complementary sources of data (i.e., genetic and paleo-bioclimatic data) for two mostly sympatric moth species (Gnopharmia kasrunensis and G. colchidaria) and their shared host-plant (Prunus scoparia), we found evidence for the existence and location of LGM refugia in Iran. Moreover, we revealed an impact of the glacial-interglacial periods on the population structures of both moth species, which were formed by separately evolving haplotype lineages and demonstrated signs of past population expansions. Based on the genetic results and on hind-casting species distribution models for both moth species and their shared host plant, we inferred potential refugial areas in the southwest Zagros Mountains (with the center of Dena) and potentially in the Kope-Dagh Mountains and the southern Caucasus.
Due to long-term isolation, the Zagros Mountains are characterized as an area of endemism (Rechinger and Lack 1991; Akhani 2004; Noroozi et al. 2008). High haplotype diversity in the western populations of G. colchidaria and G. kasrunensis support this point of view. The presence of a wide refugial area in the southeastern Zagros Mountains can be expected. Consequently, this region could well have served as the source population for the detected postglacial expansion events in both moth species. Species distribution models for the LGM revealed high habitat suitability in the south and western Zagros Mountains and in southeastern Iran stretching along the coast of the Persian Gulf. Besides the suggested distribution areas of G. colchidaria in the southwestern Zagros Mountains and eastern Alborz Mountains, LGM and Mid-Holocene paleo-reconstructions of species distributions indicate regions with high habitat suitability in the southern Caucasus and the southern Kope-Dagh. Conclusively, these models (especially under the CCSM) favor the existence of three distinct refugial areas for G. colchidaria. Previously, the Kope-Dagh mountain range with its highly complex biodiversity was identified as the region comprising source populations for many floral elements of adjacent regions, e.g., the eastern forests of Arborz (Kryzhanovsky and Atamuradov 1994; Akhani 1998).
From a genetic perspective, we find further evidence for refugial areas distinct to the Zagros Mountains. Three clearly separated haplotype lineages were revealed for G. colchidaria s.l., one being geographically restricted to the northeast of Iran (Kope-Dagh Mountains) and the Pakistan population. The latter could have been colonized after a postglacial expansion event with origin in the Kope-Dagh Mountains. Similarly, haplotypes of G. colchidaria from Semnan scattered in different haplotype lineages. The Semnan population could comprise a zone of secondary contact or present a corridor for this species resulting from postglacial population expansions from the east and west.
The population of G. kasrunensis found in the eastern lowlands of Hormozgan at the coast of the Persian Gulf is genetically clearly separated. Paleoclimatic models for the period of the LGM point to a bioclimatic barrier between this area and the west and north of Hormozgan. Thus, differing bioclimatic conditions between the northern coasts of the Persian Gulf and the Zagros Mountains may have played a prominent role leading to population isolation and genetic diversification in the long run. Based on the differentiation of phyto-geographical regions, Djamali et al. (2012) came up with a similar conclusion. Many floral elements at the northern coasts of the Persian Gulf are shared with the Mesopotamian flora and highly differentiated from the floral elements of the Zagros Mountains and Central Iran (Zohary 1973; White and Léonard 1991; Akhani 2007). Moreover, several faunal elements of the coasts of the Persian Gulf are closely related to the Afro-Arabian zone, e.g., birds (Evans 1994), reptiles (Anderson 1999) and lepidopterans (Naumann 1987). Due to its specific climatic condition and biodiversity, the eastern coasts of the Persian Gulf could well have served as a separate refugial area distinct to the southwestern Zagros Mountains.
The inferred LGM distribution patterns of G. kasrunensis and G. colchidaria in southern Iran fit well with the potential distribution of their shared host plant P. scoparia. The presence of the host plant may thus control the foundation of moth populations affecting both the dispersal potential and distribution range of the species. The latter hypothesis, i.e., a limited distribution range of host-specific Lepidoptera defined by their host plant(s), finds further support by Kunte (2008), but was rejected by Schweiger et al. (2008). Considering the trophic niche of the monophagous Gnopharmia populations, it is most reasonable that the distribution of host plants has a profound effect on confining the investigated moths to shared refugia. Such host-specific moth species may track the contractions and expansions of their host plants during ice age oscillations. However, both the documented presence of host plants and suitable climatic conditions showed congruent patterns.
During the LGM, Prunus as well as other trees and shrubs (e.g., Crataegus, Cerasus, Amygdalus, Pistacia) were rare in the northern parts of the Zagros Mountains (El-Moslimany 1986; Djamali et al. 2011). However, Prunus probably tolerated the colder climate conditions with a higher rate of precipitation during the early Paleocene (at least for the time of the LGM). The last glacial conditions may have confined the host plant distribution to the southern parts of the Zagros Mountains. Palynological studies in Lake Zeribar and Lake Mirabad provided evidence for a late establishment of deciduous forests in west Iran (van Zeist and Wright 1963; van Zeist and Bottema 1977). Several studies have shown the effects of dry and cold conditions during the early Holocene together with anthropogenic influences on slowing the rate of dispersal of these forest trees in the northern Zagros Mountains (Hillman 1996; Roberts 2002; Roberts and Wright 1993; van Zeist and Bottema 1991).
Based on morphological data, the Caucasus populations of G. colchidaria have been treated as a distinct subspecies (G.c. colchidaria, Rajaei Sh et al. 2012). At present, no genetic data exist for this region. However, LGM reconstructions, especially under the CCSM, indicate a potential refugial area in the Caucasus. As an independent source of data, we used presence records of its suggested host plant (Prunus fenzliana) to compare the inferred distribution patterns of the moth and host plant. The CCSM model for the LGM and both Mid-Holocene models reconstructed suitable climatic conditions for this plant at the south of the Caucasus. We assume that northern populations of G. colchidaria have adapted to the colder and more humid climate of the southern Caucasus, thus enabling them to co-occur with their host plant. The localization of a glacial refugium in the region of the southern Caucasus is further supported by high regional biodiversity (Volodicheva 2002; Seddon et al. 2002; Ahmadzadeh et al. 2012).
Conclusion
We combined molecular genetic and paleoclimatic data of two mostly sympatric moth species of the genus Gnopharmia to localize potential Last Glacial Maximum refugial areas for the biodiversity hot spot of Iran. Both species possessed a population genetic structure formed by three (G. colchidaria s.l.) and two (G. kasrunensis) distinct haplotype lineages, probably resulting from population differentiation in isolated refugial areas. As such, the southwestern Zagros Mountains comprised a shared LGM refugium. Moreover, potential species-specific refugia in the southern Caucasus, along the coast of the Persian Gulf and in the southern Kope-Dagh mountains were revealed. Paleodistributions of their shared host plant Prunus demonstrated congruent patterns, thus providing further evidence. Other phylogeographic studies are needed to shed more light on the largely neglected biogeographical history of Iran.
References
Ahmadzadeh, F., Carretero, M. A., Rödder, D., Harris, D. J., Freitas, S. N., Perera, A., et al. (2012). Inferring the effects of past climate fluctuations on the distribution pattern of Iranolacerta (Reptilia, Lacertidae): evidence from mitochondrial DNA and species distribution models. Zoologischer Anzeiger. doi:10.1016/j.jcz.2012.05.002.
Akhani, H. (1998). Plant biodiversity of Golestan National Park, Iran. Stapfia, 53, 1–411.
Akhani, H. (2004). A new spiny, cushion-like Euphorbia (Euphorbiaceae) from south-west Iran with special reference to the phytogeographic importance of local endemic species. Botanical Journal of the Linnean Society, 146, 107–121.
Akhani, H. (2007). Diversity, biogeography, and photosynthetic pathways of Argusia and Heliotropium (Boraginaceae) in South-West Asia with an analysis of phytogeographic units. Botanical Journal of Linnean Society, 155, 401–425.
Anderson, S. C. (1999). The Lizards of Iran. 442 pp. Ithaca (Society for the Study of Amphibians and Reptiles). ISBN 0-916984-49-4
Avise, J. C. (2000). Phylogeography: The history and formation of species. Cambridge: Harvard University Press.
Barve, N., Barve, V., Jiménez-Valverde, A., Lira-Noriega, A., Maher, S. P., Peterson, A. T., et al. (2011). The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling, 222(11), 1810–1819.
Beaumont, L. J., Hughes, L., & Poulsen, M. (2005). Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecological Modelling, 186, 250–269.
Braconnot, P., Otto-Bliesner, B., Harrison, S., Joussaume, S., Peterchmitt, J.-Y., Abe-Ouchi, A., et al. (2007). Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum – Part 2: feedbacks with emphasis on the location of the ITCZ and mid- and high latitudes heat budget. Climate of the Past, 3(2), 279–296.
Browicz, K., & Zieliński, J. (1984). Chorology of trees and shrubs in south-west Asia and adjacent regions Vol. 4. Warszawa: Polish Scientific Publishers.
Busby, J. R. (1991). BIOCLIM - A bioclimatic analysis and prediction system. In C. R. Margules & M. P. Austin (Eds.), Nature conservation: Cost effective biological surveys and data analysis (pp. 64–68). Melbourne: CSIRO.
Chan, L. M. L., Brown, J. L., & Yoder, A. D. (2011). Integrating statistical genetic and geospatial methods brings new power to phylogeography. Molecular Phylogenetics and Evolution, 59(2), 523–537.
Clement, M., Posada, D., & Crandall, K. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9(10), 1657–1660.
Djamali, M. (2008). Palaeoenvironmental changes in Iran during the last two climatic cycles (vegetation-climate-anthropisation). PhD thesis in Universite Paul Cezanne (AIX-Marseille III).
Djamali, M., Akhani, H., Andrieu-Ponel, V., Braconnot, P., Brewer, S., de Beaulieu, J. L., et al. (2010). Indian Summer Monsoon variations could have affected the early-Holocene woodland expansion in the Near East. The Holocene, 20, 813–820.
Djamali, M., Biglari, F., Abdi, K., Andrieu-Ponel, V., de Beaulieu, J. L., Mashkour, M., et al. (2011). Pollen analysis of coprolites from a late PleistoceneeHolocene cave deposit (Wezmeh Cave, west Iran): insights into the late Pleistocene and late Holocene vegetation and flora of the central Zagros Mountains. Journal of Archaeological Science, 38, 3394–3401.
Djamali, M., Brewer, S., Breckle, S. W., & Jackson, S. T. (2012). Climatic determinism in phytogeographic regionalization: a test from the Irano-Turanian region, SW and Central Asia. Flora, 207, 237–249.
Elith, J., & Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annual Reviews in Ecology, Evolution and Systematics, 40, 677–697.
Elith, J., Graham, C. H., Anderson, R. P., Dudík, M., Ferrier, S., Guisan, A., et al. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29(2), 129–151.
Elith, J., Kearney, M., & Phillips, S. (2010). The art of modelling range-shifting species. Methods in Ecology and Evolution, 1(4), 330–342.
Elith, J., Phillips, S. J., & Hastie, T. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17(1), 43–57.
El-Moslimany, A. P. (1986). Ecology and late-Quaternary history of the Kurdo-Zagrosian oak forest near Lake Zeribar, western Iran. Vegetatio, 68, 55–63.
Evans, M. I. (1994) Important bird areas in the Middle East. Bird Life International Publication. 410 pp.
Excoffier, L., & Lischer, H. E. L. (2010). Arlequin ver. 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10, 564–567.
Fu, Y. (1997). Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics, 147, 915–925.
Guisan, A., & Zimmermann, N. (2000). Predictive habitat distribution models in ecology. Ecological Modelling, 135, 147–186.
Habel, J. C., & Assmann, T. (2009). Relict species: Phylogeography and conservation biology. Berlin: Springer.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Harpending, H. C. (1994). Signature of ancient population growth in a lowresolution mitochondrial DNA mismatch distribution. Human Biology, 66, 591–600.
Hartl, D. L., & Clark, A. G. (1997). Principles of population genetics (3rd ed.). Sunderland: Sinauer Associates Inc.
Hewitt, G. M. (1996). Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society, 58, 247–276.
Hewitt, G. M. (2000). The genetic legacy of the Quaternary ice ages. Nature, 405, 907–913.
Hewitt, G. M. (2001). Speciation, hybrid zones and phylogeography — or seeing genes in space and time. Molecular Ecology, 10, 537–549.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978.
Hillman, G. (1996). Late Pleistocene changes in wild plant-foods available to hunter-gatherers of the northern Fertile Crescent: Possible preludes to cereal cultivation. In D. R. Harris (Ed.), The origins and spread of agriculture and pastoralism in Eurasia (pp. 159–203). UCL Press.
Ivanova, N. V., deWaard, J. R., & Hebert, P. D. N. (2006). An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes, 6, 998–1002.
Jiménez-Valverde, A., Peterson, A. T., Soberón, J., Overton, J. M., Aragón, P., & Lobo, J. M. (2011). Use of niche models in invasive species risk assessments. Biological Invasions, 13(12), 2785–2797.
Katoh, K., Misawa, K., Kuma, K., & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res., 30, 3059–3066.
Kelts, K., & Shahrabi, M. (1986). Holocene sedimentology of hypersaline Lake Urmia, northwestern Iran. Palaeogeography, Palaeoclimatology, Palaeoecology, 54, 105–130.
Kiehl, J. T., & Gent, P. R. (2004). The community climate system model, version 2. Journal of Climate, 17, 1666–1669.
Kryzhanovsky, O. L., & Atamuradov, K. I. (1994). Zoogeography of Coleoptera in Turkmenistan. In V. Fet (Ed.), Biogeography and ecology of Turkmenistan (pp. 403–418). Boston: Dordrecht. 616 p.
Kuhle, M. (2008). The Pleistocene Glaciation (LGP and pre-LGP, pre-LGM) of SE Iranian mountains Exemplified by the Kuh-i-Jupar, Kuh-i-Lalezar and Kuh-i-Hezar Massifs in the Zagros. Polarforschung, 77, 71–88.
Kunte, K. (2008). Competition and species diversity: removal of dominant species increases diversity in Costa Rican butterfly communities. Oikos, 117, 69–76.
Libradi, P., & Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25, 1451–1452.
Naumann, C. M. (1987). Distribution patterns of Zygaena moths in the Near and Middle East. In F. Krupp, W. Schneider & R. Kinzelbach (Eds.), Proceedings of the Symposium on the Fauna and Zoogeography of the Middle East: Beihefte zum TAVO A, 28, 200-212.
Nei, M., & Miller, S. C. (1990). A simple method for estimating average number of nucleotide substitutions within and between populations from restriction data. Genetics, 125, 873–879.
Noroozi, J., Akhani, H., & Breckle, S. W. (2008). Biodiversity and phytogeography of the alpine flora of Iran. Biodiversity and Conservation, 17(3), 493–521.
Peterson, A. T., & Nyári, Á. S. (2007). Ecological niche conservatism and pleistocene refugia in the Thrush-like Mourner, Shiffornis sp., in the Neotropics. Evolution, 62, 173–183.
Petit, R. J., Aguinagalde, I., de Beaulieu, J.-L., Bittkau, C., Brewer, S., Cheddadi, R., et al. (2003). Glacial refugia: hotspots but not melting pots of genetic diversity. Science, 300, 1563–1565.
Phillips, S. J., & Dudík, M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography, 31, 161–175.
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259.
Phillips, S. J., Dudík, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., et al. (2009). Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecological Applications, 19(1), 181–197.
Proven, J., & Bennett K. D. (2008). Phylogeographic insights into cryptic glacial refugia, Cell press, doi:101016/j.tree.2008.6.10.
Rajaei Sh, H. (2010). Life history of Gnopharmia kasrunensis Wehrli, 1939 and G. colchidaria Lederer, 1870 (Geometridae, Ennominae) and their distribution in Iran, with first host-plant records for the genus. Bonn Zoological Bulletin, 57(1), 65–73.
Rajaei Sh, H., Stüning, D., & Trusch, R. (2012). Taxonomic revision and zoogeographical patterns of the species of Gnopharmia Staudinger, 1892 (Geometridae, Ennominae). Zootaxa, 3360, 1–52.
Rechinger, K. H., & Lack, W. (1991). Dipsacaceae. In K. H. Rechinger (Ed.), Flora Iranica 168. Graz: Akademische Druck-u.-Verlagsanstalt.
Roberts, N. (2002). Did prehistoric landscape management retard the post-glacial spread of woodland in Southwest Asia? Antiquity, 76, 1002–1010.
Roberts, D. R., & Hamann, A. (2012). Predicting potential climate change impacts with bioclimate envelope models: a palaeoecological perspective. Global Ecology and Biogeography, 21(2), 121–133.
Roberts, N., & Wright Jr., H. E. (1993). Vegetational, lake-level and climatic history of the Near East and Southwest Asia. In H. E. Wright Jr., J. E. Kutzbach, T. Webb III, W. F. Ruddiman, F. A. Street-Perrott, & P. J. Bartlein (Eds.), Global climates since the last glacial maximum (pp. 194–220). University of Minnesota Press.
Rocchini, D., Hortal, J., Lengyel, S., Lobo, J. M., Jimenez-Valverde, A., Ricotta, C., et al. (2011). Accounting for uncertainty when mapping species distributions: the need for maps of ignorance. Progress in Physical Geography, 35(2), 211–226.
Schweiger, O., Settele, J., Kudrna, O., Klotz, S., & Kühn, I. (2008). Climate change can cause spatial mismatch of trophically interacting species. Ecology, 89(12), 3472–3479.
Seddon, J. M., Santucci, F., Reeve, N., & Hewitt, G. M. (2002). Caucasus Moun-tains divide postulated postglacial colonization routes in the white-breasted hedgehog, Erinaceus concolor. Journal of Evolutionary Biology, 15, 463–467.
Stevens, L. R., Wright, H. E., Jr., & Ito, E. (2001). Proposed changes in seasonality of climate during the Late-glacial and Holocene at Lake Zeribar, Iran. The Holocene, 11, 747–756.
Stewart, J. R., Lister, A. M., Barnes, I., & Dalén, L. (2010). Refugia revisited: individualistic responses of species in space and time. Proceedings of the Royal Society B, 277(1682), 661–671.
Svenning, J. C., Fløjgaard, C., Marske, K. A., Nógues-Bravo, D., & Normand, S. (2011). Applications of species distribution modeling to paleobiology. Quaternary Science Reviews, 30(21–22), 2930–2947.
Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science, 240, 1285–1293.
Tajima, F. (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics, 123, 585–595.
Templeton, A. R. (1998). Nested clade analyses of phylogeographic data: testing hypotheses about gene flow and population history. Molecular Ecology, 7, 381–397.
van Zeist, W., & Bottema, S. (1977). Palynological investigations in western Iran. Palaeohistoria, 19, 19–85.
van Zeist, W., & Bottema, S. (1991). Late quaternary vegetation of the near East. Dr Ludwig Reichert Verlag.
van Zeist, W., & Wright, H. E. (1963). Preliminary pollen studies at Lake Zeribar, Zagros Mountains, southwestern Iran. Science, 140, 65–67.
Volodicheva, N. (2002). The Caucasus. In M. Shahgedanova (Ed.), The physical geography of Northern Eurasia (pp. 350–376). UK: Oxford University Press.
Waltari, E., Hijmans, R. J., Peterson, A. T., Nyári, A. S., Perkins, S. L., & Guralnick, R. P. (2007). Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS ONE, 7(e563), 1–11.
Weir, J. T., & Schluter, D. (2004). Ice sheets promote speciation in boreal birds. Proceedings of the Royal Society of London, Biological Science, 271, 1881–1887.
White, F., & Léonard, J. (1991). Phytogeographical links between Africa and Southwest Asia. Fl Veg Mundi, 9, 229–246.
Wright, H. E. (1961). Pleistocene glaciation in Kurdistan. Eiszeitalter und Gegenwart, 12, 131–164.
Zohary, M. (1973). Geobotanical foundations of the Middle East (Vol. 2). Stuttgart: Fischer.
Acknowledgments
We thank Thomas Schmitt (Trier, Germany) for his advice on the preliminary steps of this project. Special thanks go to Morteza Djamali (France) for sharing his knowledge on the paleoecology and climatology of Iran. Paul Hebert (Biodiversity Institute of Ontario, Guelph, Canada) and Axel Hausmann supported the main part of DNA sequencing in the framework of the International Barcode of Life (iBOL) project that is run by Genome Canada, the Ontario Ministry of Research and Innovation and Natural Science and Engineering Research Council of Canada (NSERC). The second part of DNA sequencing was provided by the ZMB (Center of Molecular Biodiversity Research, ZFMK, Bonn).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 2 and 3
(DOC 470 kb)
Rights and permissions
About this article
Cite this article
Rajaei Sh, H., Rödder, D., Weigand, A.M. et al. Quaternary refugia in southwestern Iran: insights from two sympatric moth species (Insecta, Lepidoptera). Org Divers Evol 13, 409–423 (2013). https://doi.org/10.1007/s13127-013-0126-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-013-0126-6