Abstract
Chilecomadia valdiviana (Philippi) (Lepidoptera: Cossidae) is an insect native to Chile. The larval stages feed on the wood of economically important fruit tree species such as apple, pear, olive, cherry, and avocado, and also on eucalyptus. This causes weakening and, in case of severe infestation, death of the tree. We report identification of the sex pheromone produced by females of this species. Hexane extracts of the abdominal glands of virgin females were analyzed by gas chromatography (GC) with electroantennographic detection, GC coupled with mass spectrometry, and GC coupled to infrared spectroscopy. The major pheromone component was identified as (7Z,10Z)-7,10-hexadecadienal (Z7,Z10–16:Ald), and minor components present in the extracts were (Z)-7-hexadecenal and (Z)-9-hexadecenal, hexadecanal, and (9Z,12Z)-9,12-octadecadienal. Structural assignments were carried out by comparison of analytical data of the natural products and their dimethyl disulfide adducts with those of authentic reference samples. In field tests, traps baited with Z7,Z10–16:Ald captured significantly more males than control traps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chilecomadia valdiviana (Philippi) (Lepidoptera: Cossidae) is native to Chile and Argentina, and attacks trees of economic importance in Chile (Artigas 1994; Cerda et al. 2000; Ripa and Larral 2008). It has long been known to be associated with native trees and bushes, such as Nothofagus spp. (Angulo and Olivares 1991, 2008; Gentili 1988; Petersen 1988). However, in the early 1990s C. valdiviana was detected attacking commercial Eucalyptus nitens plantations (Cerda and Lewis 1993) in southern Chile. Since this report, the insect has not only established itself, but has expanded its presence in eucalyptus. In addition to eucalyptus, C. valdiviana attacks economically important fruit trees such as avocado, apple, cherry, olive, and pear (Artigas 1994; Prado 1991), and infestations have been increasing over the last years in these crops, particularly in apple orchards in central Chile. Despite the rather slow dispersal of the pest, host-plant shifts do occur and are causing concern. The host-plant shift towards Eucalyptus nitens, reported for C. valdiviana in Chile about 20 years ago, was independently observed in South Africa for another cossid species, Coryphodema tristis (Gebeyehu et al. 2005). This coincidence is intriguing because these two species do not share a close phylogenetic relationship (Degefu et al. 2013; Schoorl 1990), and emphasises the potential risk these insects present for commercial tree plantations in the medium- to long-term, not only in Chile.
Upon feeding on the wood of their host species, the larvae bore galleries of considerable diameter (up to 1 cm) and length (up to 25 cm), causing the tree to lose static balance and become prone to breakage. Additionally, the galleries provide entry for microorganisms, further affecting plant health and reducing the wood value (Kliejunas et al. 2001). Apart from the impact on local production, exports also are affected by the presence of C. valdiviana. The United States Agricultural Department (USDA) assigned the species a high pest risk potential in the importation of eucalyptus logs and chips from South America, indicating that “specific phytosanitary measures may be required to ensure the quarantine safety of proposed importations” (Kliejunas et al. 2001).
Larvae are present throughout the year in all stages, pupae are found mainly in winter (July–September), and adults usually fly in spring and summer (September–February). The duration of the life cycle is not known with certainty and has been estimated to be of one, two, or even three years. As adults show a long emergence period and the larvae develop inside the tree branches and trunks, the control of C. valdiviana is difficult. According to Ripa and Larral (2008), no effective control methods are known, and the only alternative is removal of damaged wood. A well-established approach for the monitoring and/or control of lepidopteran pests is the use of pheromones. In view of the increasing number of infestations and the lack of effective control methods, we identified the sex pheromone of C. valdiviana, and this may be used to develop monitoring or control strategies for this pest.
Methods and Materials
Insects
Pupae were collected near Talca (Maule Region, Chile) from infested apple tree logs during late winter, sexed based on external observation of the terminalia (ventral side of 8th and 9th abdominal segments), and maintained individually in 50 ml polystyrene containers under laboratory conditions (20 °C, 60–70 % r.h., 12 L:12D) until emergence of adults. After experimental studies, abdominal tips were excised and their genitalia prepared for taxonomic identification according to the keys available in Gentili (1989) and Olivares et al. (2010) and for comparison with authentic reference specimens from the Museum of Zoology, Universidad de Concepción (MZUC-UCCC).
Preparation of Gland Extracts
Observations indicated that females displayed calling behavior ca. 3–4 h before the start of the photophase. At that time, virgin females (1–3 d-old) were freeze-killed at −20 °C for 20 min, and the pheromone gland was extruded by gently pressing the abdominal tips, excised, and immersed in 50 μl of hexane (SupraSolv, Merck, Darmstadt, Germany). After 10 min, the hexane was carefully removed, and the sample was either analyzed immediately or stored at −20 °C until use. Glands were extracted and extracts analyzed individually.
Chemical Analyses
Gas chromatography with electroantennograpic detection (GC/EAD) was carried out using a Shimadzu GC-2014 AFSC gas chromatograph (Shimadzu, Kyoto, Japan) coupled to an electroantennographic detector (Syntech, Kirchzarten, Germany). The column effluent was split in two equal parts, with one part going to the flame ionization detector (FID) and the other through a heated transfer line into a humidified airstream (400 ml min−1) that was directed to the male antennal preparation. Antennae were prepared by decapitating the insect (1–3 d-old males) and connecting the base of the head and the tips of both of the antennae to the two electrodes (Syntech probe) covered with conducting gel (Spectra 360 Electrode Gel, Parker Laboratories, Fairfield, NY, USA). The signal was acquired with the signal acquisition interface IDAC-2 (Syntech) and recorded and processed using the software GC-EAD 2010 v1.2.2 (Syntech). The GC was equipped with a fused silica RTX-5 capillary column (30 m × 0.25 mm id, 0.25 mm film, Restek, Bellefonte, PA, USA). The oven was programmed from 50 °C for 5 min and then at 8 °C min−1 to 270 °C. The GC was operated in split/splitless mode (30 s sampling time) with an injector temperature of 250 °C. Helium was used as the carrier gas.
Gas chromatography coupled with mass spectrometry (GC/MS) was carried out using a Shimadzu GCMS-QP2010 Ultra combination using either the same GC column and conditions as above, or one of the following columns: a fused silica RTX-Wax capillary column (30 m × 0.32 mm id, 0.25 μm film, Restek) or a fused silica SP2380 capillary column (30 m × 0.32 mm id, 0.2 μm film, Varian Inc., Lake Forest, CA, USA). For the RTX-Wax column, the oven was programmed from 50 °C for 2 min, and then at 8 °C min−1 to 220 °C, and for the SP2380 column from 50 °C for 5 min, and then at 6 °C min−1 to 230 °C. Electron impact mass spectra were acquired at 70 eV.
Gas chromatography coupled with Fourier transform infrared spectroscopy (GC/FT-IR) was carried out on a Shimadzu GC2010 equipped with a DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, USA), coupled to a DiscovIR-GC (Spectra Analysis, Marlborough, Massachusetts, USA) infrared detector (4000–750 cm−1, resolution 8 cm−1). Injections of 1 μl were performed in splitless mode, with an injector temperature of 250 °C. The column oven temperature was held at 70 °C for 1 min, and then increased to 250 °C at 7 °C.min−1, and held for 2 min. Helium was used as carrier gas at a column head pressure of 170 kPa.
Derivatization
Dimethyl disulfide (DMDS) (50 μl) and 50 μl of a 5 % solution of iodine in diethyl ether were added to a hexane gland extract containing one female equivalent in ca. 50 μl (Buser et al. 1983). The reaction mixture was kept at 50 °C overnight. After addition of a drop of an aqueous solution of 10 % sodium thiosulfate, 200 μl hexane were added. The hexane solution was carefully separated, concentrated to ca. 20 μl, and either analyzed by GC/MS using the RTX-5 column as described above or submitted to chemical reduction with lithium aluminum hydride (LAH) as follows. The DMDS-derivatized extract was concentrated to dryness and re-dissolved in 1 ml dry diethyl ether. A small amount of LAH was added, and the mixture was left for 2 h at room temperature. Subsequently, a drop of conc. HCl was added, and the mixture was passed through a Pasteur pipette containing silica gel and sodium sulfate. The target compounds were eluted with diethyl ether. The eluate was concentrated to dryness, re-dissolved in hexane, and analyzed by GC/MS using the RTX-5 or RTX-Wax columns as described above.
Chemicals
(7Z,10Z)-7,10-Hexadecadienal (Z7,Z10–16:Ald) was synthesized as described below and in the Supplementary Information. (9Z,12Z)-9,12-Octadecadienal (Z9,Z12–18:Ald) was obtained by reduction (LAH, Et2O) and subsequent oxidation (pyridinium dichromate, CH2Cl2) of commercially available methyl linoleate. Hexadecanal (16:Ald) was purchased from Sigma-Aldrich (St. Louis, MO, USA). (Z)-9-Hexadecenal (Z9–16:Ald) was purchased from Bedoukian (Danbury, CT, USA). Other hexadecenals were available in our laboratory from previous work. For field tests, the compounds were purified by column chromatography on silica gel impregnated with 5 % silver nitrate and had purities >99 % by GC analysis.
Synthesis
General Procedures
Reagents and solvents were purchased from Sigma-Aldrich or Merck and were used without further purification. Syntheses with air- or moisture-sensitive reagents were carried out in dried glassware under nitrogen using standard vacuum line techniques. Silica gel 60 (0.063–0.200 mm, Merck) was used for column chromatography. Nuclear magnetic resonance (NMR) data were acquired on a Bruker Fourier 300 spectrometer (300 MHz for 1H and 75 MHz for 13C), and chemical shifts (δ) are reported in ppm relative to the internal standard tetramethylsilane. Coupling constants J are given in Hz. Mass spectral data were acquired on the Shimadzu GCMS-QP2010 Ultra GC/MS combination described above.
Synthesis of (7Z,10Z)-7,10-Hexadecadienal
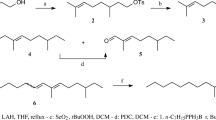
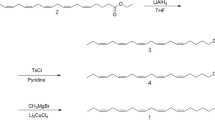
Experimental details of the synthesis of Z7,Z10–16:Ald and its intermediates are described in the Supplementary Information and are outlined in Fig. 1. Briefly, the tetrahydropyranyl derivative (THP ether) of 7-hydroxyheptanal (2) was prepared by reaction of 1,7-heptanediol (1) with 3,4-dihydro-2H-pyran (DHP) (Nishiguchi et al. 2000), followed by oxidation of the hydroxyl group of the resulting monoprotected diol using pyridinium dichromate (PDC) in CH2Cl2 (Corey and Schmidt 1979). In parallel, (Z)-3-nonen-1-ol (3) was converted to (Z)-1-bromo-3-nonene (4) using triphenylphosphine dibromide (PPh3Br2) (Horner et al. 1959; Wiley et al. 1964). The corresponding Wittig salt 5 was obtained by treatment of the bromide 4 with triphenylphosphine (PPh3) in acetonitrile. Wittig reaction of 2 with 5 using sodium bis(trimethylsilyl)amide [NaN(SiMe3)2] for the generation of the ylide (Bestmann et al. 1976), furnished the THP-protected dienol 6, which was deprotected (H+, MeOH) and oxidized (PDC, CH2Cl2) to yield Z7,Z10–16:Ald. 1H-NMR (CDCl3): δ 0.91 (t, 3H, J = 6.9), 1.32–1.47 (m, 10H), 1.58–1.72 (m, 2H), 1.96–2.14 (m, 4H), 2.44 (dt, 2H, J = 7.2, 1.9), 2.75–2.82 (m, 2H), 5.28–5.46 (m, 4H), 9.78 (t, 1H, J = 1.8). 13C-NMR (CDCl3): δ 202.8, 130.3, 129.6, 128.4, 127.8, 43.9, 31.5, 29.4, 29.3, 28.8, 27.2, 27.0, 25.6, 22.6, 22.0, 14.1. EI-MS: m/z (%) = 41 (63), 54 (25), 55 (40), 67 (100), 68 (27), 69 (22), 79 (45), 81(80), 95 (35), 98 (28), 109 (13), 119 (5), 121 (4), 123 (4), 133 (3), 137 (3), 151 (2), 179 (1), 193 (1), 236 (M+, 1).
Field Tests
Four separate trapping experiments were carried out to test the attractiveness of Z7,Z10–16:Ald, and its mixtures with (Z)-7-hexadecenal (Z7–16:Ald), and/or Z9–16:Ald to males. Traps consisted of a 20 L-bucket filled with a solution of ca. 300 g sodium chloride and ca. 5 ml liquid glycerin soap in 1.25 L water. A plastic roof was installed above the bucket with a clearance of ca. 12 cm at the highest point. The lure was suspended with a pin below the roof. Lures containing synthetic compounds were prepared by loading white rubber septa (Sigma-Aldrich, catalog #Z553905) with 100 μl of an appropriate solution of Z7,Z10–16:Ald or its mixture with other aldehydes in hexane. Septa treated with 100 μl of hexane were used as controls. Virgin females used as lures were confined in a cylindrical plastic container (8 cm long, 6.5 cm diam) open at both ends and covered with plastic mesh. Females were used 2–3 d after emergence. Traps were placed at 3.0–3.5 m height on the orchard rows, with a distance of 20–22 m between the traps.
In Experiments 1–3, we tested the attractiveness of the main pheromone candidate Z7,Z10–16:Ald at different locations. The treatments were (1) Z7,Z10–16:Ald (500 μg dose), (2) a virgin female, and (3) hexane control and there were four replicates in each experiment. Traps were checked every 3–4 d, at which time the captured insects were counted and removed from the traps, and live females were replaced with a new individual. All collected adults were preserved in alcohol and their genitalia prepared for taxonomic identification. Experiment 1 was carried out from August 18 until September 8, 2015, in an apple orchard in Colbún Poniente (35° 43′ 12.99″S, 71° 27′ 33.59″W; 6.5 ha planted with varieties ‘Royal Gala’ and ‘Scarlet’ at a density of 1389 trees/ha). Experiment 2 was carried out from August 28 until September 22, 2015, in Colbún Oriente (35° 43′ 10.44″S, 71° 27′ 42.42″W; same characteristics as Colbún Poniente), and Experiment 3 was conducted from August 28 until September 22, 2015, in San Javier (35° 34′ 53.00″S, 71° 44′ 50.39″W; 6.5 ha planted with ‘Royal Gala’ and ‘Galaxy’ at a density of 889 trees/ha). All three orchards had a similar incidence of C. valdiviana (ca. 70 % of the trees had at least one active gallery), but the infestation at the San Javier orchard was of much higher severity (more active galleries per tree).
Experiment 4 was conducted from September 8 until November 10, 2015, at the Colbún Poniente orchard described above and was intended to test the influence of the minor hexadecenals on the attractiveness of the major compound. Treatments were (1) 500 μg Z7,Z10–16:Ald, (2) 500 μg Z7,Z10–16:Ald + 50 μg Z7–16:Ald, (3) 500 μg Z7,Z10–16:Ald + 50 μg Z9–16:Ald, (4) 500 μg Z7,Z10–16:Ald + 50 μg Z7–16:Ald + 50 μg Z9–16:Ald, (5) a virgin female, and (6) hexane control. There were six replicates of each treatment. Traps were checked once a week during the duration of the trial, at which time captured insects were counted and removed from the traps, and live females were replaced with new individuals. All collected adults were preserved in alcohol and their genitalia prepared for taxonomic identification.
Data Analysis
Differences between treatments in Experiments 1 to 3 were analyzed using the mean male captures per trap and per day for all sampling dates. Experiment 4 was analyzed using the males captured per trap and per day for each sampling date. Since the distributions of the data did not fulfill the assumption of homoscedasticity, the non-parametric Kruskal-Wallis multiple comparison test with Bonferroni correction was used (Siegel and Castellan 1988; Sokal and Rohlf 1995).
Results
Analyses of Gland Extracts
GC/MS analyses of pheromone gland extracts revealed the presence of five compounds which were recognized as potential pheromone candidates based on their mass spectra (peaks A-E, in the order of elution from the RTX-Wax column) (Fig. 2). GC-EAD analyses of the extracts showed that male antennae responded reproducibly to the main compound D (data not shown).
Compound A had retention indices of 1819 (RTX-5) and 2132 (RTX-Wax), and its mass spectrum was virtually identical to that of the database spectrum for hexadecanal. Comparison of the retention times and the mass spectrum with those of an authentic reference sample confirmed its assignment as hexadecanal.
Compounds B and C were identified tentatively as hexadecenals, based on their mass spectra (m/z = 220, 238) and retention indices (1799 and 1801 on RTX-5, respectively; and 2157 and 2160 on RTX-Wax, respectively). The diagnostic fragments present in the mass spectra of the DMDS adducts suggested a Δ7- or Δ8-hexadecenal for compound B (m/z = 159, 173, 332) and a Δ6- or Δ9-hexadecenal for compound C (m/z = 145, 187, 332). In the case of aldehydes, the position of the double bond cannot be unambiguously determined from low resolution mass spectra of DMDS adducts, because the formyl group and the terminal ethyl group of the carbon chain are isobaric. In the present case, comparison of retention times and mass spectra of the natural compounds and their DMDS derivatives with those of all eight possible synthetic isomers of the candidate hexadecenals, revealed compound B to be Z7–16:Ald and compound C as Z9–16:Ald (Table S1, Figs. S1, S2).
The mass spectrometric fragmentation pattern and the apparent molecular ion at m/z 236 (Fig. 3a) indicated the major EAD-active compound D to be a hexadecadienal. Its infrared spectrum, obtained by GC/FT-IR, confirmed the presence of a carbonyl group (1708, 2744 cm−1) (Fig. 4) and suggested the double bonds to show Z-configuration, as E-configured double bonds are characterized by an absorption at ca. 970 cm−1, which was not observed. Furthermore, the absence of absorptions above 3007 cm−1 indicated that the double bonds were probably not conjugated and not located at positions 2, 14, or 15 (penultimate and last positions along the chain). Dimethyl disulfide derivatization of the gland extract resulted in the formation of several adducts with similar (but not identical) mass spectra showing apparent molecular ions at m/z 362 (Fig. 5a). The increase of 126 amu indicated the addition of two methylthio groups and a sulfur atom to the proposed hexadecadienal. This is characteristic for the formation of heterocyclic adducts containing sulfur, resulting from dienes with double bonds separated by 1, 2, or 3 methylene groups (Vincenti et al. 1987). It also has been reported that the addition reaction in this case is not stereospecific and yields several diastereomers (Vincenti et al. 1987), which further supported our conclusion. The mass spectra of cyclic DMDS adducts are characterized by cleavage of the C-C bonds adjacent to the ring, and the resulting fragments can subsequently lose CH3S or CH3SH, forming secondary fragments that are of diagnostic value as well. In our case, the mass spectra of the adducts obtained upon derivatization of the pheromone gland extract suggested that the double bonds of the original dienal were located at either the 5,8- or the 7,10-positions (Fig. 5a). In the case of skipped dienes, the formation of tetrahydrothiophene and tetrahydrothiopyran derivatives in addition to the expected thietane has been reported (Carballeira et al. 1994), but this does not affect the possibility of assigning the original double bond locations. Further information was obtained by treatment of the DMDS derivative with LiAlH4, resulting in the reduction of the carbonyl group to a hydroxyl group. Comparison of the mass spectra of the aldehyde and alcohol derivatives showed that the molecular ion and some of the diagnostic fragments were shifted to +2 amu in the case of the alcohol derivative (Fig. 5b), indicating that these fragments contained the terminal hydroxyl group. At the same time, some other diagnostic fragments remained unchanged, which we interpreted as fragments containing the alkyl terminus of the chain. This allowed us to conclude that the double bonds in the original dienal must have been located at the 7,10-positions. Furthermore, both the mass spectrum and the infrared spectrum of synthetic Z7,Z10–16:Ald were identical to those of the natural product. Finally, the gas chromatographic retention times of synthetic Z7,Z10–16:Ald matched those of the natural compound on three different stationary phases (retention indices: 1793 on RTX-5, 2206 on RTX-Wax, and 2462 on SP2380), confirming the assigned structure of the main compound.
The mass spectrum of compound E showed an apparent molecular ion at 264 (+28 amu compared to compound D), and the retention indices on two columns (1994 on RTX-5 and 2410 on RTX-Wax) were both approximately 200 units higher than those of compound D, which suggested it to be a bis-homologue of Z7,Z10–16:Ald. Additionally, the mass spectrometric fragmentation pattern was basically close to that of Z7,Z10–16:Ald (Fig. 3b). Comparison of analytical data of synthetic Z9,Z12–18:Ald with those of compound E confirmed this assignment.
The amount of Z7,Z10–16:Ald present in the gland was determined to be 14.3 ± 13.7 ng (N = 17), and the relative ratios of compounds A-E were estimated to be 3:3:5:88:1 (N = 6).
Field Tests
Field studies showed that the traps captured exclusively C. valdiviana individuals. Table 1 summarizes the mean captures per trap per day and the total number of males captured in Experiments 1–3. At all three locations, traps baited with synthetic Z7,Z10–16:Ald captured significantly more males than control traps. Traps containing virgin females captured fewer males than traps baited with Z7,Z10–16:Ald.
Experiment 4 was run for 9 weeks, and Table 2 reports the mean captures per trap per day after each weekly revision of the traps, as well as the total trap catches. The numbers of captured males in traps baited with synthetic compounds were significantly higher than in unbaited control traps or in traps containing virgin females. The traps containing Z7,Z10–16:Ald + Z9–16:Ald captured significantly more males than traps baited with other treatments, with the exception of week 2.
Discussion
Chemical analyses of female gland extracts and field bioassays with synthetic compounds allowed us to identify a sex pheromone for C. valdiviana. The main compound produced by females is Z7,Z10–16:Ald, and this compound alone is sufficient to attract males in the field.
Addition of the minor compound Z9–16:Ald to the major compound almost always increased trap catches significantly. On the other hand, in most weeks the captures of traps baited with a binary mixture of Z7,Z10–16:Ald and Z7–16:Ald, or a ternary mixture of Z7,Z10–16:Ald with both monoenes did not differ from the captures in traps containing Z7,Z10–16:Ald alone. These results suggest a possible synergistic action of Z9–16:Ald, but do not allow clear conclusions concerning the biological significance of Z7–16:Ald. The minor compounds identified in this investigation may, however, play a role in interspecific communication similar to those of certain polyenes in winter moths (Szöcs et al. 2004).
The temporal dynamics of trap captures during the experiments indicated that the flight period of C. valdiviana starts in early September. This is in accord with previously reported first appearances of adults (Artigas 1994; Ureta 1957). Thus, the low number of captures during the first week of Experiment 4 may be due to small numbers of adults emerging at that time and/or unfavorable weather conditions, such as low temperatures and rainfall. The flight period extends to at least two months, as the traps kept capturing adults until the end of Experiment 4 in mid-November with a peak by mid-late October (Table 2). The decline in captures towards the end of this bioassay may be due to a lower number of adults emerging and/or a lower attractiveness of the lures after prolonged exposure to field conditions.
The main compound Z7,Z10–16:Ald has not been reported before as a sex pheromone of Lepidoptera. However, it is typical of Type I-structures (Ando et al. 2004), which have a straight chain with 10–18 carbon atoms, a terminal oxygenated functional group, and one or more double bonds along the chain. Examples of dienes from this group showing homo-conjugation of double bonds are relatively scarce and include 9,12-tetradecadienyl compounds identified from several species of Pyralidae and Noctuidae (El-Sayed 2014), E4,Z7–13:OAc from the Gelechiidae Phthorimaea operculella (Persoons et al. 1976), and E11,E14–18:Ald from the Endromidae Andraca bipunctata (Ho et al. 1996). The positions and the geometry of the double bonds in Z7,Z10–16:Ald and Z9,Z12–18:Ald identified from C. valdiviana suggest these compounds to be derived from a linoleic acid precursor. Biosynthesis of Z7,Z10–16:Ald would include a chain-shortening step via β-oxidation, followed by transformation of the carboxyl moiety into the aldehyde group. Examples of compounds from Lepidoptera with a presumably similar biosynthesis are Z9,Z12–18:Ald and Z9,Z12,Z15–18:Ald from the Noctuidae Achaea janata (Persoons et al. 1993), as well as the Arctiidae Estigmene acrea (Hill and Roelofs 1981), and Hyphantria cunea (Hill et al. 1982), which may be derived from linoleic and linolenic acid precursors, respectively, and which represent structural intermediates between type I and type II pheromones (Ando et al. 2004). Structurally similar compounds with Z,Z-configured homo-conjugated double bonds also have been identified from midges (Diptera: Cecidomyiidae), and the biosynthesis of these compounds also was suggested to include chain-shortening steps starting with a linoleate precursor (Hall et al. 2012).
This is the first time that an aldehyde is reported as the sex pheromone of a species of Cossidae. Chilecomadia is one of the two genera of the subfamily Chilecomadiinae (Angulo and Olivares 2008) at a basal position in the phylogeny of this family (Schoorl 1990). The sex pheromones known from other Cossidae moths of the subfamilies Cossinae and Zeuzerinae generally are structurally limited to a few acetates and the occasional occurrence of primary alcohols. Females of Cossus cossus produce and males are attracted to (Z)-5-dodecenyl acetate (Z5–12:OAc) and (Z)-5-tetradecenyl acetate (Z5–14:OAc) (Capizzi et al. 1983), while the related species C. insularis uses (E)-3-tetradecenyl acetate (E3–14:OAc) as the sex attractant (Chen et al. 2006). Interestingly, the (Z)-isomer, (Z)-3-tetradecenyl acetate (Z3–14:OAc), is attractive to males of Holcocerus insularis (Jintong et al. 2001). The pheromone of H. hippopaecolus consists of Z5–12:OAc and (Z)-7-tetradecenyl acetate (Z7–14:OAc) (Fang et al. 2005), and H. artemisiae uses a mixture of Z5–12:OAc and Z5–14:OAc (Zhang et al. 2009). The pheromone of H. arenicola has been reported to consist of Z5–14:OAc and Z7–14:OAc (Jing et al. 2010), and a 4-component mixture including Z7–14:OAc, E3–14:OAc, (3Z,5E)-3,5-tetradecadienyl acetate (Z3,E5–14:OAc), and (Z)-7-tetradecenol (Z7–14:OH) is highly attractive to males of H. vicarius (Yang et al. 2012, 2015). The pheromone of Isoceras sibirica contains Z7–14:OAc, (Z)-9-tetradecenyl acetate (Z9–14:OAc), and (Z)-9-hexadecenyl acetate (Z9–16:OAc) (Zhang et al. 2011). Biologically active compounds produced by Zeuzera pyrina include (2E,13Z)-2,13-octadecadienyl acetate (2E,13Z-18:OAc), (3E,13Z)-3,13-octadecadienyl acetate (3E,13Z-18:OAc), and (Z)-13-octadecenyl acetate (Z13–18:OAc) (Malosse et al. 1993; Tonini et al. 1986), which also are known from clearwing moths (El-Sayed 2014). Most recently, the pheromone of Coryphodema tristis was reported to consist of Z9–14:OAc and (Z)-9-tetradecenol (Z9–14:OH) (Bouwer et al. 2015).
The identification of the female-produced sex pheromone of C. valdiviana will enable the development of pheromone-based methods for monitoring and control of this pest insect. The high attractiveness of traps baited with Z7,Z10–16:Ald alone makes these traps an attractive tool for the detection of early-arriving and monitoring of low-density populations. Traps were attractive for at least 2 months under field conditions, thus minimizing the need to replace the baits at shorter intervals. Furthermore, several studies have examined the potential of pheromones of other cossid moths in control programs (Pasqualini et al. 1992, 1993, 1999). For example, a combined UV light-pheromone trap has been developed for the mass trapping of leopard moths, Zeuzera pyrina, in olive orchards, which captured significantly more insects (males and females) than either light or pheromone traps, with a concurrent decrease of the percentage of infested trees and number of active galleries and an increase of fruit yield within the three-year period of the study (Hegazi et al. 2009). Populations of the leopard moth also were suppressed in olive orchards through mating disruption (Hezagi et al. 2010). Promising results for mating disruption were recently reported using the pheromone of Cossus insularis (Nakanishi et al. 2013). Considering that species within the family Cossidae show a similar biology and habits, these examples indicate that pheromone-based methods may provide an effective alternative in the control of Chilecomadia spp.
References
Ando T, Inomata S-J, Yamamoto M (2004) Lepidopteran sex pheromones. Top Curr Chem 239:51–96
Angulo AO, Olivares TS (1991) Chilecomadia valdiviana (Philippi) (Lepidoptera: Cossidae) asociado a Ulmus glabra Hudson forma pendula (Laud.) Rehder (“Olmo pendula”) en la VIII Región (Concepción, Chile). Bosque 12:67–68
Angulo AO, Olivares TS (2008) Catálogo crítico e ilustrado de los Cossidae de Chile (Lepidoptera: Cossidae). Lepidoptera Novae 1:119–133
Artigas JN (1994) Entomología Económica. Insectos de interés agrícola, forestal, médico y veterinario. Vol. 2. Ediciones Universidad de Concepción, Chile, pp. 479–486
Bestmann HJ, Stransky W, Vostrowsky O (1976) Darstellung lithiumsalzfreier Ylidlösungen mit Natrium-bis(trimethylsilyl)amid als base. Chem Ber 109:1694–1700
Bouwer MC, Slippers B, Degefu D, Wingfield MJ, Lawson S, Rohwer ER (2015) Identification of the sex pheromone of the tree infesting cossid moth Coryphodema tristis (Lepidoptera: Cossidae). PLoS One 10:e0118575
Buser H-R, Arn H, Guerin P, Rauscher S (1983) Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal Chem 55:818–822
Capizzi A, Tonini C, Arsura E, Guglielmetti G, Massardo P, Piccardi P (1983) Sex pheromone components of the European goat moth, Cossus cossus. J Chem Ecol 9:191–200
Carballeira NM, Shalabi F, Cruz C (1994) Thietane, tetrahydrothiophene and tetrahydrothiopyran formation in reaction of methylene-interrupted dienoates with dimethyl disulfide. Tetrahedron Lett 35:5575–5578
Cerda LA, Lewis PD (1993) Estudio y seguimiento del taladrador de la Madera Chilecomadia valdiviana (Lep.: Cossidae) en plantaciones de Eucalyptus nitens y E. delegatensis en el predio Santa Luisa de Forestal Rio Vergara S.A., in: Barros Asenjo S, Prado JD, Alvear CS (eds.), Actas Simposio Los Eucaliptos en el Desarrollo Forestal de Chile Instituto Forestal, Santiago pp. 339–353
Cerda L, Angulo A, Durán A, Olivares T (2000) Insectos asociados a bosques del centro sur de Chile. In: Baldini A, Pancel L (eds) . Agentes de daño en el bosque nativo Editorial Universitaria, Santiago de Chile, pp. 201–281
Chen X, Nakamuta K, Nakanishi T, Nakashima T, Tokoro M, Mochizuki F, Fukumoto T (2006) Female sex pheromone of a carpenter moth, Cossus insularis (Lepidoptera: Cossidae). J Chem Ecol 32:669–679
Corey EJ, Schmidt G (1979) Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett 5:399–402
Degefu DT, Hurley BP, Garnas J, Wingfield MJ, Ahumada R, Slippers B (2013) Parallel host range expansion in two unrelated cossid moths infesting Eucalyptus nitens on two continents. Ecol Entomol 38:112–116
El-Sayed AM (2014) The Pherobase: Database of pheromones and semiochemicals <http://www.pherobase.com>
Fang Y-L, Sun J-H, Zhao C-H, Zhang Z-N (2005) Sex pheromone components of the sandthorn carpenterworm Holcocerus hippophaecolus. J Chem Ecol 31:39–48
Gebeyehu S, Hurley BP, Wingfield MJ (2005) A new lepidopteran insect pest discovered on commercially grown Eucalyptus nitens in South Africa. S Afr J Sci 101:26–28
Gentili P (1988) Análisis de la distribución geográfica de Cossidae (Lepidoptera: Ditrysia) de la Patagonia andina. Rev Chil Hist Nat 61:191–198
Gentili P (1989) Revisión sistemática de los Cossidae (Lep.) de la Patagonia Andina. Revista de la Sociedad Entomológica Argentina 45:1–75
Hall DR, Amarawardana L, Cross JV, Francke W, Boddum T, Hillbur Y (2012) The chemical ecology of cecidomyiid midges (Diptera: Cecidomyiidae). J Chem Ecol 38:2–22
Hegazi E, Khafagi WE, Konstantopoulou M, Raptopoulos D, Tawfik H, Abd El-Aziz GM, Abd El-Rahman SM, Atwa A, Aggamy E, Showeil S (2009) Efficient mass-trapping method as an alternative tactic for suppressing populations of leopard moth (Lepidoptera: Cossidae). Ann Entomol Soc Am 102:809–818
Hezagi EM, Khafagi WE, Konstantopoulou M, Schlyter F, Raptopoulos D, Shweil S, Abd El-Rahman S, Atwa A, Ali SE, Tawfik H (2010) Suppression of leopard moth (Lepidoptera: Cossidae) populations in olive trees in Egypt through mating disruption. J Econ Entomol 103:1621–1627
Hill AS, Roelofs WL (1981) Sex pheromone of the saltmarsh caterpillar moth, Estigmene acrea. J Chem Ecol 7:655–668
Hill AS, Kovalev BG, Nikolaeva LN, Roelofs WL (1982) Sex pheromone of the fall webworm moth, Hyphantria cunea. J Chem Ecol 8:383–396
Ho HY, Tao YT, Tsai RS, Wu YL, Tseng HK, Chow YS (1996) Isolation, identification, and synthesis of sex pheromone components of female tea cluster caterpillar, Andraca bipunctata Walker (Lepidoptera: Bombycidae) in Taiwan. J Chem Ecol 22:271–285
Horner L, Oediger H, Hoffmann H (1959) Reaktionen mit Triphenylphosphin-Dihalogeniden. Liebigs Ann Chem 626:26–34
Jing X, Zhang J, Luo Y, Zong S, Liu P, Jia J (2010) Identification and field evaluation of the female sex pheromone of the sand Salix carpenterworm Holcocerus arenicola Staudinger (Lepidoptera: Cossidae). Z Naturforsch 65c:403–411
Jintong Z, Yan H, Xianzuo M (2001) Sex pheromone of the carpenterworm, Holcocerus insularis (Lepidoptera, Cossidae). Z Naturforsch 56c:423–429
Kliejunas JT, Tkacz BM, Burdsall HH Jr, Denitto GA, Eglitis A, Haugen DA, Wallner WE (2001) Pest risk assessment of the importation into the United States of unprocessed eucalyptus logs and chips from South America. Gen. TeC. Rep. FPL-GTR-124. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest products Laboratory 134 p
Malosse C, Descoins C, Frerot B (1993) Identification of a minor component of the sex pheromone of the leopard moth (Zeuzera pyrina L.; Lepidoptera, Cossidae) by capillary gas chromatography. J High Resolut Chromatogr 16:123–125
Nakanishi T, Nakamuta K, Mochizuki F, Fukumoto T (2013) Mating disruption of the carpenter moth, Cossus insularis (Staudinger) (Lepidoptera: Cossidae) with synthetic sex pheromone in Japanese pear orchards. J Asia Pac Entomol 16:251–255
Nishiguchi T, Hayakawa S, Hirasaka Y, Saitoh M (2000) Selective monotetrahydropyranylation of 1,n-diols catalyzed by aqueous acids. Tetrahedron Lett 9843–9846
Olivares TS, Rodríguez MA, Angulo AO (2010) Un nuevo género, nuevas especies y nuevos registros de nóctuidos alto andinos IV (Lepidoptera: Noctuidae). SHILAP-Rev Lepidopt 38:139–151
Pasqualini E, Antropoli A, Faccioli B (1992) Attractant performance of a synthetic sex pheromone for Zeuzera pyrina L. (Lepidoptera; Cossidae). Bollettino dell’Istituto di Entomologia “Guido Grandi” della Universita degli Studi di Bologna 46:101–108
Pasqualini E, Antropoli A, Faccioli G, Molfese M (1993) Zeuzera pyrina L. (Lepidoptera; Cossidae): Results of five year researches on sex attractant. Bulletin IOBC-WPRS 16:189–194
Pasqualini E, Natale D, Witzgall P, El-Sayed A (1999) Zeuzera pyrina and Cossus cossus (Lepidoptera; Cossidae) control by pheromones: four years advances in Italy. Bulletin IOBC-WPRS 22:115–124
Persoons CJ, Voerman S, Verwiel PEJ, Ritter FJ, Nooyen WJ, Minks AK (1976) Sex pheromone of the potato tuberworm moth, Phthorimaea operculella: isolation, identification and field evaluation. Entomol Exp Appl 20:289–300
Persoons CJ, Vos JD, Yadav JS, Prasad AR, Sighomony S, Jyothi KN, Prasuna AL (1993) Indo-Dutch cooperation on pheromones of Indian agricultural pest insects: Sex pheromone components of Diacrisia obliqua (Arctiidae), Achaea janata (Noctuidae) and Amsacta albistriga (Arctiidae). Bulletin IOBC-WPRS 16:136–140
Petersen JG (1988) Chilecomadia valdiviana (Philippi) (Lepidoptera: cossidae), asociado a Nothofagus pumilio (Poepp. et Endl) Krasser (Lenga) en la Región de Magallanes. Anales del Instituto de la Patagonia 18:51–55
Prado E (1991) Artrópodos y sus enemigos naturales asociados a plantas cultivadas en Chile. Boletín Técnico N° 169, Instituto de Investigaciones Agropecuarias, La Platina, 203 p
Ripa R, Larral P (eds) (2008) Manejo de plagas en paltos y cítricos, Colección Libros INIA N°, vol 23. La Cruz, Chile
Schoorl JW (1990) A phylogenetic study of Cossidae based on external adult morphology. Zoologische Verhandelingen 263:1–295
Siegel S, Castellan NJ (1988) Nonparametric statistics for behavioral sciences. McGraw-Hill, New York, 399 pp
Sokal RR, Rohlf FJ (1995) Biometry: The principles and practice of statistics in biological research. W.H. Freeman, New York, 937 p
Szöcs G, Tóth M, Kárpáti Z, Zhu J, Löfstedt C, Plass E, Francke W (2004) Identification of polyene hydrocarbons from the northern winter moth Operophtera fagata, and development of a specific lure for pheromone traps. Chemoecology 14:985–100
Tonini C, Cassani G, Massardo P, Guglielmetti G, Castellari PL (1986) Study of female sex pheromone of leopard moth, Zeuzera pyrina L Isolation and identification of three components. J Chem Ecol 12:1545–1558
Ureta RE (1957) Revisión de la Familia Cossidae (Lep. Het.) en Chile. Bol Mus Nac Hist Nat 27:127–153
Vincenti M, Guglielmetti G, Cassani G, Tonini C (1987) Determination of double bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal Chem 59:694–699
Wiley GA, Hershkowitz RL, Rein BM, Chung BC (1964) Studies in organophosphorus chemistry. I Conversion of alcohols and phenols to halides by tertiary phosphine dihalides. J Am Chem Soc 86:964–965
Yang M, Zhang J, Zong S, Luo Y, Cao C, Fan L, Liu H, Xin H (2012) Synthesis and field evaluation of sex attractants of Holcocerus vicarius (Lepidoptera: Cossidae). Sci Silvae Sin 48:61–66
Yang M-H, Liu H-X, Liu J-L, Jing X-Y, Zhang J-T, Fan L-H, Wang S-F (2015) Sex pheromone components of the carpenterworm moth, Holcocerus vicarius. Entomol Exp Appl 154:199–205
Zhang J, Jing X, Luo Y, Li Z, Zong S, Yang M (2009) The sex pheromone of the sand sagebrush carpenterworm, Holcocerus artemisiae (Lepidoptera, Cossidae). Z Naturforsch 64c:590–596
Zhang J, Liu H, Zhao W, Liu J, Zhong S (2011) Identification of the sex pheromone of Isoceras sibirica Alpheraky (Lepidoptera, Cossidae). Z Naturforsch 66c:527–533
Acknowledgments
Financial support from Fondo Nacional de Desarrollo Científico y Tecnológico (Fondecyt grant 1140779 to JB), Fondo de Equipamiento Científico y Tecnológico (Fondequip grant EQM130154), and from Deutscher Akademischer Austauschdienst (via material resources program to JB) is gratefully acknowledged. HH likes to thank the Comisión Nacional de Investigación Científica y Tecnológica (Conicyt) for a doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Herrera, H., Barros-Parada, W., Flores, M.F. et al. Identification of a Novel Moth Sex Pheromone Component from Chilecomadia valdiviana . J Chem Ecol 42, 908–918 (2016). https://doi.org/10.1007/s10886-016-0761-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0761-4