Abstract
Gas chromatography–electroantennographic detection analysis of sex pheromone gland extracts of the common forest looper Pseudocoremia suavis (Lepidoptera: Geometridae), a polyphagous defoliator of introduced Pinaceae and many New Zealand trees, revealed four compounds that elicited antennal responses. The two major active compounds (6Z)-cis-9,10-epoxynonadec-6-ene and (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene were identified by comparison with known standards. Of the two minor active compounds, one was tentatively identified as (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene, whereas the other could not be identified because of insufficient amounts in extracts. (6Z)-cis-9,10-Epoxynonadec-6-ene, (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene, and (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene were present in P. suavis gland extracts from Eyrewell Forest, a Pinus radiata plantation in the South Island of New Zealand, in a ratio of 35:65:5, respectively. Trapping trials in Eyrewell Forest established that (6Z)-cis-9,10-epoxynonadec-6-ene attracted male P. suavis. However, addition of (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene to the lure at <10% of (6Z)-cis-9,10-epoxynonadec-6-ene reduced capture of male moths, suggesting that one of its enantiomers was acting as a behavioral antagonist. During January–March of 2005, a blend trial involving single, binary, and ternary mixtures of the three components at Eyrewell Forest and at three other sites (two in the South Island and one in the North Island) revealed the existence of a second taxon of P. suavis at the three additional sites that was attracted to lures containing (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene, either singly or in binary and ternary mixtures with (6Z)-cis-9,10-epoxynonadec-6-ene and (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene. This second taxon was not attracted to lures loaded solely with (6Z)-cis-9,10-epoxynonadec-6-ene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common forest looper Pseudocoremia suavis (Butler) (Lepidoptera: Geometridae) is a polyphagous moth endemic to New Zealand, the larvae of which eat the leaves of a wide variety of native and exotic trees and shrubs, including southern beech (Nothofagus spp., Nothofagaceae), Podocarpaceae, and introduced Pinaceae (Dugdale, 1958; Stephens, 2001). In the 1950s and 1960s, P. suavis was responsible for major defoliation in Pinus radiata (D. Don) plantations at Eyrewell Forest (North Canterbury, South Island, NZ; White, 1974), and in the 1970s, it also caused considerable damage in Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] stands in Kaingaroa Forest (Bay of Plenty, North Island, NZ; Alma, 1977).
There are over 30 species of Pseudocoremia in New Zealand (Stephens, 2001). The genus is endemic to New Zealand and Norfolk Island, but has close affinities to geometrids from the Western Pacific (Dugdale, 1989). Both males and females of P. suavis are known to demonstrate developmental polymorphism, and the species appears not to have clearly synchronized generations (Berndt et al., 2004).
Our objective was to identify the sex pheromone of P. suavis as a tool for further study of its ecology and phenology, and for detection and delimitation surveys. A sex pheromone may also be useful for controlling future outbreaks by using mating disruption, lure and kill, or mass trapping technologies (e.g., Suckling and Karg, 2000).
To date, sex pheromones or attractants have been identified for more than 120 geometrids but none from the genus Pseudocoremia (El-Sayed, 2006). Typically, geometrids have sex pheromones consisting of single or multicomponent blends of unsaturated hydrocarbons and/or epoxides, with enantiomeric specificity often serving as a species-isolating mechanism (Mori, 1998; Millar, 2000; Ando et al., 2004). These types of compounds have also been identified from the pheromone glands of lymantriids, arctiids, noctuids, pyralids, and crambids (Ando et al., 2004; El-Sayed, 2006).
In this study, we describe the identification of two compounds in pheromone gland extracts of P. suavis from Eyrewell Forest, by gas chromatography–electroantennographic detection (GC-EAD) and gas chromatography–mass spectrometry (GC-MS) analysis, and confirm their biological activity with field bioassays. We also establish that P. suavis is likely to be a species complex with two distinct pheromone taxa that so far are only distinguishable by differential attraction to pheromone components and by geographic location. This is also the first identification of a sex pheromone of a New Zealand geometrid.
Methods and Materials
Insects
A colony of P. suavis was established at Forest Research (now Ensis), Christchurch, with material gathered from Eyrewell Forest, a pine plantation in North Canterbury (43.4170°S, 172.4235°E; see Berndt et al., 2004). Pupae were placed individually in plastic containers (Lily® 35-ml portion cups with lids, Huhtamaki Henderson Ltd., Auckland, NZ) at HortResearch, Lincoln, under a 12-hr light/12-hr dark reverse photophase lighting regime, at 22°C and 50% RH. Newly emerged female P. suavis were transferred to a humidified, 350-mm3 clear polycarbonate plastic box and provided with 10% sugar solution.
Pheromone Gland Extracts
The pheromone glands of 24- to 48-hr-old female moths were excised and placed into 20–30 μl of n-hexane (BDH Laboratory Supplies, Poole, UK), contained within a liquid-nitrogen-cooled 0.5-ml conical vial (Wheaton, Millville, NJ, USA). Gland extracts were taken from females 2–3 hr into the scotophase, when moths were actively calling. After all glands had been excised, the vial and its contents were brought to room temperature, and the liquid phase was transferred to a 1.1-ml conical glass vial (Alltech, Deerfield, IL, USA). The volume of extract was then reduced to 10 μl by using a stream of argon and was then stored at −18°C.
Gas Chromatography–Electroantennographic Detection
Pheromone gland extracts of P. suavis females were analyzed by GC-EAD with a Varian 3800 GC equipped with two columns of differing polarity, coupled to an EAD Recording Unit (Syntech, Hilversum, The Netherlands). Extracts were run on DB-5 (30 m × 0.25 mm ID × 0.5 μm film; Agilent Technologies, Palo Alto, CA, USA) and DB-Wax columns (30 m × 0.25 mm ID × 0.5 μm film; Agilent) with 1:1 split outlets. Helium was used as carrier gas (1 ml/min), and injections were in splitless mode. Injector and detector temperatures were 220 and 300°C, respectively, and the GC oven was programmed from 80°C/min to 240°C at 10°C/min, hold 35 min. An excised male P. suavis antenna was positioned between two glass electrodes containing BE Ringer's solution with 10% polyvinylpyrrolidone (molecular weight 360,000; Sigma Chemical Co., North Parramatta, NSW, Australia). Each glass electrode held a length of 1-mm silver wire to connect the preparation to the recording unit. The EAD exit port temperature was maintained at 200°C, and the antennal preparation was placed in a charcoal-filtered and humidified airstream (400 ml/min). Kováts retention indexes (KIs; Kováts, 1965; Marques et al., 2000) were calculated for the compounds that elicited strong antennal responses to compare relative retention times.
Gas Chromatography–Mass Spectrometry
The retention times and mass spectra of the active compounds in a P. suavis pheromone gland extract [1 μl = 5.8 FE (female equivalents/aliquot)] were compared with those of known standards on BPX-70 (30 m × 0.25 mm ID × 0.25 μm film; SGE, Victoria, Australia) and VF-5ms (30 m × 0.25 mm ID × 0.25 μm film; Varian Inc., Walnut Creek, CA, USA) columns, by using a Varian 3800 GC in splitless mode, coupled to a Varian 2200 MS. Helium was used as carrier gas (1 ml/min) with the transfer line at 250°C and the ion trap temperature at 200°C. The GC injector temperature was set at 220°C, and the oven was programmed from 80°C/min to 140°C at 10°C/min, hold 5 min, then to 200°C at 5°C/min, hold 25 min (BPX-70 column), or 80°C/min to 240°C at 10°C/min, hold 35 min (VF-5ms column).
Chemicals
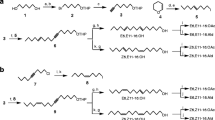
(6Z)-cis-9,10-Epoxynonadec-6-ene (6Z-cis-9,10-epo-19Hy) and (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene (3Z,6Z-cis-9,10-epo-19Hy) were synthesized at HortResearch, Palmerston North (see below). Samples of (3Z,6Z)-cis-9, 10-epoxyhenicosa-3,6-diene (3Z,6Z-cis-9,10-epo-21Hy), (3Z,6Z)-9S,10R-epoxyhenicosa-3,6-diene, and (3Z,6Z)-9R,10S-epoxyhenicosa-3,6-diene were supplied by T. Ando, Tokyo University of Agriculture and Technology. (6Z)-9S,10R-Epoxyhenicos-6-ene (6Z-9S,10R-epo-21Hy) was available from previous work (El-Sayed et al., 2005).
Synthesis of Mono- and Diunsaturated Racemic Epoxides
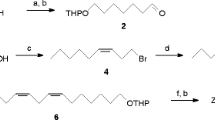
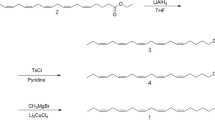
6Z-cis-9,10-Epo-19Hy. 6Z-cis-9,10-Epo-19Hy was synthesized by the methods of Zhang et al. (1999) and Soulie and Lallemand (1995). The final step (reduction of the triple bond with Lindlar's catalyst) was carried out at 0°C, and the final product contained less than 2% of the E isomer. A small quantity of E-isomer-enriched (25%) final product was also produced for GC analysis by carrying out the same reaction at room temperature. 6Z-cis-9,10-epo-19Hy: 1H NMR (CDCl3, 400 MHz): δ (ppm) 5.50 (1H, m), 5.38 (1H, m), 2.90 (2H, m), 2.35 (1H, dt, J = 13.4 Hz, 6.4 Hz), 2.15 (1H, dt, J = 13.4 Hz, 6.7 Hz), 2.01 (2H, q, J = 6.9 Hz), 1.50 (4H, m), 1.24 (18H, m), 0.86 (3H, t, J = 6.9 Hz), 0.85 (3H, t, J = 6.9 Hz). 13C NMR (CDCl3, 100 MHz): δ (ppm) 132.71, 123.81, 57.24, 56.58, 31.90, 31.50, 29.58, 29.58, 29.53, 29.31, 29.25, 27.79, 27.42, 26.61, 26.22, 22.68, 22.57, 14.21, 14.06.
3Z,6Z-cis-9,10-Epo-19Hy
3Z,6Z-cis-9,10-Epo-19Hy and its regioisomers (3Z,9Z)-cis-6,7-epoxynonadeca-3,9-diene (3Z,9Z-cis-6,7-epo-19Hy) and (6Z,9Z)-cis-3,4-epoxynonadeca-6,9-diene (6Z,9Z-cis-3,4-epo-19Hy) were synthesized by the method of Ando et al. (1993). 3Z,6Z-cis-9,10-epo-19Hy: 1H NMR (CDCl3, 400 MHz): 5.43 (3H, m), 5.27 (1H, m), 2.91 (2H, m), 2.77 (2H, t, J = 7.0 Hz), 2.37 (1H, m), 2.19 (1H, m), 2.04 (2H, pent, J = 7.3 Hz), 1.5 (4H, m), 1.24 (12H, m), 0.94 (3H, t, J = 7.5 Hz), 0.85 (3H, t, J = 6.7 Hz). 13C NMR (CDCl3, 100 MHz): 132.65, 131.18, 127.08, 124.63, 57.65, 56.86, 32.30, 29.98, 29.98, 29.94, 29.72, 28.19, 27.02, 26.65, 26.13, 23.10, 20.99, 14.67, 14.54.
Field Bioassays—Eyrewell Forest Trials 1–5
An initial series of bioassays (Trials 1–5) were carried out in mature stands of P. radiata in Eyrewell Forest, during December 2004 and January 2005. In all trials, either green delta traps (Clare et al., 2000) or green Unitrap (International Pheromone Systems Ltd., Cheshire, UK) bucket traps were suspended from rope strung between two pine trees at a height of 2 m, with >20-m spacing between treatments and >100-m spacing between replicates. Trap positions within each replicate were rerandomized, and sticky inserts were replaced at each check for capture of male P. suavis. Test compounds were loaded onto red rubber septa (Thomas Scientific Inc., Philadelphia, PA, USA).
-
Trial 1.
Trial 1 (December 7–14, 2004) investigated the attraction of P. suavis males to various combinations of the three candidate racemic epoxides, based on ratios established from analysis of P. suavis gland extracts by GC. Delta traps were checked four times at intervals of 1–2 d. Treatments were (1) blank control, (2) 100:0:0, (3) 0:100:0, (4) 35:65:0, and (5) 35:65:5 μg loadings of 6Z-cis-9,10-epo-19Hy, 3Z,6Z-cis-9,10-epo-19Hy, and 3Z,6Z-cis-9,10-epo-21Hy (N = 5).
-
Trial 2.
A dose response trial based on treatment 2 (6Z-cis-9,10-epo-19Hy) from trial 1 was set up from December 14 to 20, 2004. Delta traps were checked four times at intervals of 1–3 d. Treatments were (1) blank control, (2) 10, (3) 100, and (4) 1000 μg doses of 6Z-cis-9,10-epo-19Hy (N = 4).
-
Trial 3.
A third trial was undertaken from January 26 to February 14, 2005 to investigate whether the addition of 3Z,6Z-cis-9,10-epo-19Hy to lures inhibited capture of male P. suavis. Delta traps were checked six times at intervals of 3–4 d. Treatments were (1) blank control, (2) 100:0, (3) 99.35:0.65, (4) 93.5:6.5, (5) 35:65, and (6) 35:0 μg loadings of 6Z-cis-9,10-epo-19Hy and 3Z,6Z-cis-9,10-epo-19Hy (N = 5).
-
Trial 4.
During trials 2 and 3, on several occasions, traps captured >20 male moths in a 3- to 4-d period. Because of the relatively large size of P. suavis (wingspan of c. 30 mm), trap saturation was likely to be a problem. Therefore, in trial 4, we investigated nonsaturating bucket traps for capture of P. suavis males. We were also interested in establishing an alternative to the use of dichlorvos strips (Agrisense-BCS Ltd., Pontypridd, UK) as the insecticide “knockdown” component of this system because of availability and toxicity concerns. We compared trap catch of P. suavis males in delta traps with trap catch in bucket traps containing either (1) dichlorvos strips, (2) diazinon strips [20-mm strips of dog flea collar (Vitapet Corp. Ltd., Gracefield, NZ) with 15% diazinon as the active ingredient], or (3) 200 ml of water plus 5 ml of 200 Fluid 350 cs paraffin oil (Ajax Chemicals, Auckland, NZ).
Trial 4 ran from December 20 to 24, 2004 with traps checked daily between 0800 and 1000 hr (N = 5). Septa were loaded with 100 μg of 6Z-cis-9,10-epo-19Hy. Bucket traps were opened inside a polyethylene bag to avoid the loss of live males. Numbers of live and dead moths in each bucket trap treatment and the delta trap treatment were recorded.
-
Trial 5.
Trial 5 (December 24, 2004 to January 19, 2005) compared trap catch in delta traps and the bucket trap oil-and-water-system over a 4-wk period using lures loaded with 100 μg of 6Z-cis-9,10-epo-19Hy. Traps were checked every 7–10 d, rotating the positions of each trap type at each count date.
P. suavis Taxa Trial
To discover how widespread P. suavis was in exotic forestry blocks in the Canterbury region, we placed a replicate of trial 1 in a small south-facing, hillside forestry block at Cass Bay in Lyttelton Harbor, Banks Peninsula (43.5984°S, 172.6902°E), from December 26, 2004 to January 6, 2005.
A last trapping trial was set up in early 2005 (same treatments as trial 1) to investigate the possibility of P. suavis comprising more than one taxon, e.g., cryptic species. It was undertaken at three sites on the South Island in Canterbury: Eyrewell Forest (February 15–March 9, N = 5, traps checked five times at intervals of 3–5 d); Burnham Plantation, Kerrs Road, Burnham (43.5793°S, 172.3399°E; February 8–March 8, N = 5, traps checked five times at intervals of 3–4 d, except for the last occasion when traps were left for 15 d because of low catches during that period); Cass Bay (January 21–March 7, N = 3, traps checked six times at intervals of 5–7 d, except for the last occasion when traps were left for 12 d), and one site in the North Island [Forest Research/Ensis campus, Rotorua (38.1615°S, 176.2669°E); February 17–March 7, N = 3, traps checked five times at intervals of 3–4 d]. These trials were run concurrently to avoid temporal differences in response by P. suavis males to the candidate pheromone components (e.g., Steck et al., 1982; Szöcs et al., 1993). Green delta traps were used at all sites.
Statistical Analyses
Only treatments that trapped moths were included in the statistical analyses. For each trial, the sum of moths captured per trap was analyzed for treatment effects using analysis of variance (ANOVA; SAS Institute Inc., 1998). Residual plots were used to check the validity of the ANOVA assumptions, and log10(x + 1) transformations of the summed captures were undertaken when necessary to remove variance heterogeneity. Means were compared by Fisher's protected least significant differences test (P = 0.05; SAS Institute Inc., 1998).
Results
Identification of Gland Components Eliciting Antennal Responses
Antennal responses were elicited by four compounds in gland extracts of P. suavis from Eyrewell Forest, only two of which (peaks 1 and 2, Fig. 1) gave peaks detectable by GC (KIs of 2052 and 2058, respectively, on the DB-5 column and 2341 and 2405, respectively, on the DB-Wax column). Male antennae were also screened with a range of synthetic standards of unsaturated hydrocarbons, epoxides, and ketones using GC-EAD analysis. From the standards tested, the diene monoepoxide 3Z,6Z-cis-9,10-epo-21Hy had retention times indistinguishable from those of compound 4 on both columns.
A search of the literature indicated that C19 trienes and the corresponding diunsaturated monoepoxides occur most frequently as sex attractants in the family Geometridae (El-Sayed, 2006), and comparison of the KIs for peaks 1 and 2 suggested that they might have differing degrees of unsaturation because their retention indices were relatively closer on the DB-5 column than the DB-Wax column. In addition, the relative retention times of compounds 2, 3, and 4 (Fig. 1) suggested that they could be a homologous series. Therefore, we hypothesized that compounds 1–4 were C19, C20, or C21 diene or triene alkenes, or the corresponding mono- and diunsaturated monoepoxides.
GC-MS analysis of 5.8 FE of P. suavis gland extract on the VF-5ms column revealed two closely eluting peaks, the mass spectra of which were consistent with those of a mono- and a diunsaturated monoepoxide, respectively (Ando et al., 1993, 1995; Millar, 2000). The spectrum of compound 1 had a base peak of m/z 67 [C5H7]+ with diagnostic ions at m/z (relative intensity) 71 (18) [C5H11]+, 81 (80) [C6H9]+, 97 (31) [C7H13]+, 110 (12) [C8H14]+, 124 (5) [C9H16]+, 153 (7) [C10H17O]+, 155 (5) [M-C9H17]+, 169 (2) [M-C8H15]+, 209 (7) [M-C5H11]+, 262 (1) [M-H2O]+, and 280 (1) [M]+, and matched the spectrum of a 6Z-cis-9,10-epo-19Hy standard. The retention times of 1 and the 6Z-cis-9,10-epo-19Hy standard were the same on the VF-5ms and BPX-70 columns (KIs of 2054 and 2413, respectively), and 6Z-cis-9,10-epo-19Hy and 6E-cis-9,10-epo-19Hy were easily separated on the VF-5ms column (KIs of 2054 and 2067, respectively), confirming compound 1 as 6Z-cis-9,10-epo-19Hy.
The spectrum of compound 2 had a base peak of m/z 79 [C6H7]+ with diagnostic ions at m/z 108 (21) [C8H12]+, 122 (9) [C9H14]+, 209 (2) [M-C5H9]+, 249 (4) [M-C2H5]+, 260 (3) [M-H2O]+, and 278 (4) [M]+, and matched the spectrum of authentic 3Z,6Z-cis-9,10-epo-19Hy. The retention times of compound 2 and the 3Z,6Z-cis-9,10-epo-19Hy standard were the same on the VF-5ms and BPX-70 columns (KIs of 2059 and 2486, respectively). 3Z,6Z-cis-9,10-epo-19Hy eluted later than its two regioisomers, 3Z,9Z-cis-6,7-epo-19Hy (KI 2050) and 6Z,9Z-cis-3,4-epo-19Hy (KI 2056) on the VF-5ms column, confirming compound 2 as 3Z,6Z-cis-9,10-epo-19Hy.
Whereas it was not possible to identify minor compound 3 from gland extracts because of the small amount present, we were able to tentatively identify compound 4 as 3Z,6Z-cis-9,10-epo-21Hy. The spectrum and retention time (KI 2261) of compound 4 were the same as those of 3Z,6Z-cis-9,10-epo-21Hy on the VF-5ms column, with a base peak of m/z 79 [C6H7]+, and diagnostic ions at m/z 108 (45) [C8H12]+, 122 (8) [C9H14]+, 237 (5) [M-C5H9]+, 277 (2) [M-C2H5]+, 288 (1) [M-H2O]+, and 306 (1) [M]+. In addition, 6Z-9S,10R-epo-21Hy eluted well before this peak (KI 2255). However, we were not able to categorically confirm the structure of compound 4 by GC-MS analysis because it coeluted with another compound in gland extracts on the BPX-70 column.
Field Bioassays
A total of 127 male P. suavis were captured in trial 1 at Eyrewell Forest over seven nights, and all of these moths, with the exception of 1 in treatment 4, were attracted by the single-component lure loaded with 100 μg of 6Z-cis-9,10-epo-19Hy (Fig. 2A).
Attraction of P. suavis males to lures consisting of (A) the three GC-EAD active components in P. suavis pheromone gland extracts, (B) various doses of 6Z-cis-9,10-epo-19Hy, and (C) addition of 3Z,6Z-cis-9,10-epo-19Hy to a lure containing 6Z-cis-9,10-epo-19Hy. All trials were undertaken at Eyrewell Forest, between December 2004 and January 2005. Amounts on the x-axis are micrograms per lure. Treatments labeled with the same letter are not significantly different (P > 0.05).
In a dose response trial (Fig. 2B), more male P. suavis moths were attracted to lures loaded with 1000 μg of 6Z-cis-9,10-epo-19Hy (P < 0.05) than to those loaded with either 100 or 10 μg. One hundred sixty-two moths were captured over six nights, with a strong positive correlation between dose and number of moths captured (r 2 = 0.84, df = 14, P < 0.001).
In the third trial (Fig. 2C), we tested the effect of the addition of 3Z,6Z-cis-9,10-epo-19Hy to a lure containing 6Z-cis-9,10-epo-19Hy. A total of 297 moths were trapped during the 19 nights, and the results indicated that 3Z,6Z-cis-9,10-epo-19Hy was inhibitory. Addition of <10% of 3Z,6Z-cis-9,10-epo-19Hy significantly reduced trap capture of male moths (P < 0.05).
In the fourth and fifth trials at Eyrewell Forest, we tested an alternative trapping system for capturing male P. suavis because of the likelihood of saturation of delta traps. Forty-eight moths were captured during the four nights of trial 4, and the results (Fig. 3) indicated that bucket traps, with either a diazinon strip or with water and oil, were as effective as the delta trap (P > 0.05). Bucket traps with a dichlorvos pesticide strip trapped fewer male moths (P < 0.05) than did the delta traps, suggesting a repellent effect from the dichlorvos strip.
Mean catches (±SEM) of P. suavis males in a trapping trial to test an alternative trapping system to delta traps for capturing male P. suavis. All treatments used a 100-μg lure, loaded with 6Z-cis-9,10-epo-19Hy. The trial was carried out at Eyrewell Forest in December 2004. Treatments labeled with the same letter are not significantly different (P > 0.05).
In the bucket-trap diazinon strip trapping system, all moths were alive (13 moths trapped in total) when the traps were cleared the following morning, whereas only 1 moth was alive in the bucket-trap dichlorvos strip system (6 moths trapped), indicative of the relative vapor pressures of these two insecticides (1.4×10−4 and 1.2×10−2 mm Hg at 20°C, respectively; Windholz, 1983). In the bucket-trap water–oil system, 12 moths were trapped, and all of these were immobilized in the water–oil solution. In trial 5, we trapped 193 moths over a 4-wk period, and there was no difference (P > 0.05, data not shown) in mean catches of P. suavis males, using either delta traps or the bucket trap water–oil system.
P. suavis Taxa Trial
Trap catches indicated the presence of P. suavis in a forestry block in Cass Bay during late December 2004 and early January 2005. However, male moths were not attracted to treatment 2 (100 μg of 6Z-cis-9,10-epo-19Hy), but instead to treatments 3–5 (a total of 12 moths trapped over 11 nights). A repeat of trial 1 at four forestry sites (Fig. 4) confirmed the existence of two distinct taxa of P. suavis, based on attraction of male moths to the racemates of the two major pheromone components identified from gland extracts of female moths from Eyrewell Forest. A preliminary morphological investigation of the male genitalia of P. suavis has revealed no consistent differences between moths from Eyrewell Forest and moths from Rotorua, Burnham, or Cass Bay (Stephens, personal communication).
Mean catches (±SEM) of P. suavis males in a blend trial of the three components in P. suavis pheromone gland extracts that elicited EAD responses, at four locations in New Zealand. Trials were undertaken between January 2005 and March 2005. Amounts on the x-axis are micrograms per lure, and treatments labeled with the same letter are not significantly different (P > 0.05).
At the Eyrewell site, only 2 of the moths captured (115 moths trapped in total) were attracted to lures other than 6Z-cis-9,10-epo-19Hy (treatment 2), consistent with trial 1. However, at Rotorua (85 moths trapped), Burnham (170 moths trapped), and Cass Bay (66 moths trapped), the majority of P. suavis males were attracted to treatments 3–5, with only 3 being attracted to other treatments (1 moth in treatment 2 at Burnham and Cass Bay, and 1 moth in the blank control at Burnham).
At the Burnham site, the three-component lure of 6Z-cis-9,10-epo-19Hy, 3Z,6Z-cis-9,10-epo-19Hy, and 3Z,6Z-cis-9,10-epo-21Hy trapped more moths (P < 0.05) than the single component 3Z,6Z-cis-9,10-epo-19Hy lure, suggesting that 3Z,6Z-cis-9,10-epo-21Hy might be a component of the sex pheromone of this population of P. suavis. However, in an earlier trial at Eyrewell Forest in March of 2003, 100-μg doses of 3Z,6Z-cis-9,10-epo-21Hy or its enantiomers in single-component lures failed to attract any moths (Brockerhoff, unpublished data), indicating that this diunsaturated monoepoxide is not active as a single component.
We also attracted small numbers of two other closely related Pseudocoremia spp. (Stephens, 2001) to 6Z-cis-9,10-epo-19Hy lures over the course of the trial at the Rotorua site [16 Pseudocoremia fenerata (Felder and Rogenhofer) males during February and March], the Burnham site [1 Pseudocoremia leucelaea (Meyrick) male in March], and at Cass Bay (3 P. leucelaea males in February). Similarly, a trap baited with 6Z-cis-9,10-epo-19Hy in a P. radiata block at Whataroa (43.1167°S, 170.3085°E), South-Westland, in early January 2005 captured 2 P. leucelaea males but no P. suavis. At Cass Bay, P. productata (Walker) adults were observed in the P. radiata plantation on numerous occasions during January and February 2005, but no males of this species were ever captured in baited traps.
Discussion
We have identified 6Z-cis-9,10-epo-19Hy and 3Z,6Z-cis-9,10-epo-19Hy from pheromone gland extracts of the common forest looper P. suavis. We have also tentatively identified the homologous diene monoepoxide 3Z,6Z-cis-9,10-epo-21Hy in gland extracts. All of these compounds have previously been found as either sex pheromone components or attractants in other geometrids of the subfamily Ennominae (El-Sayed, 2006).
At Eyrewell Forest, male P. suavis moths were attracted to 6Z-cis-9,10-epo-19Hy as a single component. Because we used the racemate in our trapping trials, the active attractant may be one of the enantiomers or some mixture of the two enantiomers. For example, Millar et al. (1991) found that racemic 6Z-cis-9,10-epo-19Hy was attractive to the geometrid Euchlaena madusaria, and the individual enantiomers failed to attract moths of this species, whereas Xanthotype sospeta (a sympatric species) was selectively attracted by 6Z-9S,10R-epo-19Hy and 6Z-9R,10S-epo-19Hy antagonized attraction. Therefore, removal of one of the enantiomers from the blend may enhance, reduce, or eliminate attraction of male P. suavis at the Eyrewell site.
Addition of 3Z,6Z-cis-9,10-epo-19Hy to the lure inhibited capture of moths at Eyrewell Forest. Because 3Z,6Z-cis-9,10-epo-19Hy was identified in gland extracts from female moths from Eyrewell Forest, this observed inhibitory effect was possibly due either to one of the enantiomers in the racemic mix or to an incorrect blend of the two enantiomers. Thus, determination of the natural enantiomer of 3Z,6Z-cis-9,10-epo-19Hy or blend from gland extracts may aid in developing a better lure for P. suavis males at Eyrewell Forest.
Both 6Z-cis-9,10-epo-19Hy and 3Z,6Z-cis-9,10-epo-19Hy attracted male moths but not at the same location. Therefore, P. suavis is likely to be a species complex comprising two distinct pheromone taxa. This finding will be verified by further trapping trials using the enantiomers of the monoepoxides identified in gland extracts of P. suavis from Eyrewell Forest. In addition, an investigation to determine subtle differences in morphology of genitalia for this species complex and DNA analysis of P. suavis from the various locations is underway. There are other examples in the New Zealand Lepidoptera where identification of sex pheromone components has enabled cryptic species to be recognized. For example, Foster et al. (1991) investigated the sex pheromones of distinct geographic populations of various tortricid species in the genera Planotortrix and Ctenopseustis, and Frérot and Foster (1991) elucidated two distinct pheromone taxa within the noctuid Graphania mutans.
A cladistic analysis by Stephens (2001) indicated that P. suavis belongs to the clade containing P. leucelaea, P. fenerata, and P. monacha. Our study of the sex pheromone of P. suavis supports this finding. We captured both P. fenerata and P. leucelaea in our field trials, in addition to P. suavis. Both P. suavis and P. fenerata have been recorded as exotic forestry pests (Alma, 1977; Nuttall, 1983), and P. leucelaea also feeds on exotic pines (Dugdale, personal communication). Whereas P. monacha, like its host genus Phyllocladus spp., is restricted in its distribution, being characteristic of upland or montane forest sites (Dugdale, personal communication), the other three species are widespread throughout New Zealand.
At the Rotorua site, P. fenerata males were attracted to 6Z-cis-9,10-epo-19Hy, and whereas P. leucelaea is also present (Alma, unpublished data), we did not trap any of this species during our trial. However, P. leucelaea were attracted in low numbers to 6Z-cis-9,10-epo-19Hy at three sites in the South Island. Further trapping trials, using enantiomers of the two major pheromone components identified from P. suavis gland extracts, could be useful in establishing species-isolating mechanisms among these three sympatric species.
Of the trapping systems tested for capture of P. suavis, bucket traps with water and oil were most appropriate for long-term studies. However, it may be possible to further refine the trapping system because P. suavis males were caught in reasonable numbers in bucket traps with neither an insecticidal strip nor water and oil (Gibb, unpublished data). Such traps would be useful for collection of live specimens, for example, for GC-EAD or DNA analyses.
References
Alma, P. J. 1977. Selidosema [= Pseudocoremia] suavis (Butler) (Lepidoptera: Geometridae). Forest and Timber Insects in New Zealand, No. 11. Forest Research Institute, Rotorua, pp. 4.
Ando, T., Ohsawa, H., Ueno, T., Kishi, H., Okamura, Y., and Hashimoto, S. 1993. Hydrocarbons with a homoconjugated polyene system and their monoepoxy derivatives: Sex attractants of geometrid and noctuid moths distributed in Japan. J. Chem. Ecol. 19:787–798.
Ando, T., Kishi, H., Akashio, N., Qin, X., Saito, N., Abe, H., and Hashimoto, S. 1995. Sex attractants of geometrid and noctuid moths: Chemical characterization and field test of monoepoxides of 6,9-dienes and related compounds. J. Chem. Ecol. 21:299–311.
Ando, T., Inomata, S., and Yamamoto, M. 2004. Lepidopteran sex pheromones. Top. Curr. Chem. 239:51–96.
Berndt, L., Brockerhoff, E. G., Jactel, H., Weis, T., and Beaton, J. 2004. Biology and rearing of Pseudocoremia suavis, an endemic looper (Lepidoptera: Geometridae) with a history of outbreaks on exotic conifers. N. Z. Entomol. 27:73–82.
Clare, G., Suckling, D. M., Bradley, S. J., Walker, J. T. S., Shaw, P. W., Daly, J. M., McLaren, G. F., and Wearing, C. H. 2000. Pheromone trap colour determines catch of non target insects. N. Z. Plant Prot. 53:216–220
Dugdale, J. S. 1958. Structural characters of the larva of Selidosema [= Pseudocoremia] suavis (Butler) (Lepidoptera: Geometridae, Subfamily Ennominae). N. Z. Entomol. 2:24–33.
Dugdale, J. S. 1989. New Zealand Lepidoptera; basic biogeography. N.Z. J. Zool. 16:679–687.
El-Sayed, A. M. 2006. The Pherobase: Database of Insect Pheromones and Semiochemicals. http://www.pherobase.com.
El-Sayed, A. M., Gibb, A. R., Suckling, D. M., Bunn, B., Fielder, S., Comeskey, D., Manning, L. A., Foster, S. P., Morris, B. D., Ando, T., and Mori, K. 2005. Identification of sex pheromone components of the painted apple moth: a tussock moth with a thermally labile pheromone component. J. Chem. Ecol. 31:633–657.
Foster, S. P., Dugdale, J. S., and White, C. S. 1991. Sex pheromones and the status of the greenheaded and the brownheaded leafroller moths in New Zealand. N.Z. J. Zool. 18:63–74.
Frérot, B. and Foster, S. P. 1991. Sex pheromone evidence for two distinct taxa within Graphania mutans (Walker). J. Chem. Ecol. 17:2077–2093.
Kováts, E. 1965. Gas chromatographic characterization of organic substances in the retention index system, pp. 229–247, in J. C. Giddings and R. A. Keller (eds.). Advances in Chromatography, Vol. 1. Edward Arnold Ltd., London.
Marques, F. A., McElfresh, J. S., and Millar, J. G. 2000. Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 11:592–599.
Millar, J. G. 2000. Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 45:575–604.
Millar, J. G., Giblin, M., Barton, D., and Underhill, E. W. 1991. Synthesis and field screening of chiral monounsaturated epoxides as lepidopteran sex attractants and sex pheromone components. J. Chem. Ecol. 17:911–929.
Mori, K. 1998. Chirality and insect pheromones. Chirality 10:578–586.
Nuttall, M. 1983. Pseudocoremia fenerata (Felder) (Lepidoptera, Geometridae): A native looper. Forest and Timber Insects in New Zealand, No. 56. Forest Research Institute, Rotorua, pp. 4.
SAS Institute Inc. 1998. Statview. SAS Institute Inc., Cary, NC.
Soulie, J. B. and Lallemand, Y. 1995. Access to unsaturated chiral epoxides. Part II. Synthesis of a component of the sex pheromone of Phragmatobia fuliginosa. Tetrahedron: Asymmetry 6:625–636.
Steck, W. F., Underhill, E. W., Bailey, B. K., and Chisholm, M. D. 1982. (Z)-7-Tetradecenal, a seasonally dependent sex pheromone of the W-marked cutworm, Spaelotis clandestina (Harris) (Lepidoptera: Noctuidae). Environ. Entomol. 11:1119–1122.
Stephens, A. E. A. 2001. Pseudocoremia (Lepidoptera: Geometridae: Ennominae) Systematics, biogeography and host plant associations. MSc thesis, Victoria University of Wellington, New Zealand.
Suckling, D. M. and Karg, G. 2000. Pheromones and semiochemicals, pp. 63–99, in J. Rechcigl and N. Rechcigl (eds.). Biological and Biotechnical Control of Insect Pests. CRC Press, Boca Raton, FL.
Szöcs, G., Tóth, M., Francke, W., Schmidt, F., Philipp, P., König, W. A., Mori, K., Hansson, B. S., and Löfstedt, C. 1993. Species discrimination in five species of winter-flying geometrids (Lepidoptera) based on chirality of semiochemicals and flight season. J. Chem. Ecol. 19:2721–2735.
White, T. R. C. 1974. A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema [= Pseudocoremia] suavis in a plantation of Pinus radiata in New Zealand. Oecologia 16:279–301.
Windholz, M. (ed.) 1983. The Merck Index. Tenth Edition. Merck & Co., Inc.
Zhang, Z., Wang, Z., Wang, Y., Liu, H., Lei, G., and Shi, M. 1999. A facile synthetic method for chiral 1,2-epoxides and the total synthesis of chiral pheromone epoxides. Tetrahedron: Asymmetry 10:837–840.
Acknowledgments
We thank John Allen (HortResearch, Palmerston North) and Diane Steward and Robert Franich (Forest Research, Rotorua) for mass spectrometry, and Carter Holt Harvey, Selwyn Plantation Board, and John and Jenny Taylor for access to trapping sites. Tetsu Ando, Tokyo University of Agriculture and Technology, Japan, provided samples of 3Z,6Z-cis-9,10-epo-21Hy, (3Z,6Z)-9S,10R-epoxyhenicosa-3,6-diene, and (3Z,6Z)-9R,10S-epoxyhenicosa-3,6-diene. Technical assistance in the field was provided by Paula Thompson (HortResearch, Lincoln) and Belinda Gresham (Ensis, Rotorua). Thanks are also due to Barry Donovan for placement and checking of a trap at Whataroa and to John Dugdale, Andréa Stephens, and David Logan for commenting on earlier versions of the manuscript. Funding was provided by the New Zealand Foundation for Research, Science, and Technology (under C04X0302 to Forest Research) and the New Zealand Forest Health Collaborative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibb, A.R., Comeskey, D., Berndt, L. et al. Identification of Sex Pheromone Components of a New Zealand Geometrid Moth, the Common Forest Looper Pseudocoremia suavis, Reveals a Possible Species Complex. J Chem Ecol 32, 865–879 (2006). https://doi.org/10.1007/s10886-006-9031-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9031-1