Abstract
The dotted white geometrid moth, Naxa seriaria Motschulsky (Lepidoptera: Geometridae), is a pest of Oleaceae in Korea, Japan, and China. In this study, we identified (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene (C-21 pentaene) as the only compound in extracts of the pheromone glands of female N. seriaria causing a response from receptors on the antennae of males in analyses by gas chromatography with electroantennographic detection. The synthetic sex pheromone elicited dose-dependent electrophysiological responses from antennae of male N. seriaria. In field tests, more male moths were captured in traps baited with synthetic C-21 pentaene than in unbaited traps, and increasing the loading of C-21 pentaene in the lure increased catches of male moths. Significantly more male N. seriaria moths were caught in delta traps than in bucket traps. Based on these results, C-21 pentaene is proposed to be the major, if not the only, component of the sex pheromone of N. seriaria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dotted white geometrid moth, Naxa seriaria Motschulsky (Lepidoptera: Geometridae), is mainly distributed in Russia and East Asia including Korea, Japan, and China. This species is known as oligophagous and feeds predominantly on Oleaceae species (Kim et al. 2013; Zhang et al. 2008). In particular cases, this insect pest causes serious damage to the privets, Ligustrum obtusifolium and Ligustrum japonicum, which are very popular tree species for hedges. The larva of N. seriaria consumes the leaves inside the web and significantly reduces plant growth. The main control method of N. seriaria relies on ground spray of synthetic insecticides, such as fenitrothion and deltamethrin. However, there are objections to ground spraying because of extensive drift of pesticide and safety concerns in urban areas. This highlights the need for development of other effective control methods for N. seriaria. Identification of the sex pheromone could provide alternative approaches to management of this pest: traps baited with the pheromone could give information for timing application of insecticides, and control by mass trapping or mating disruption may be possible.
On the other hand, Chinese privet, Ligustrum sinense, was recently introduced into New Zealand, the east coast of Australia, and United States, and has become one of the most destructive weeds in these countries (Greene and Blossey 2012; Merriam and Feil 2002). Hudson et al. (2014) reported that removing Chinese privet rehabilitated forest diversity and promoted secondary succession. Various control methods have been used to control privets such as mowing, cutting, burning, and spray of herbicides. Classical biological control could be highly effective for control of invasive plants and might offer economic savings and ecological benefits (Baars 2011). Shaw et al. (2018) and Zhang et al. (2008) reported N. seriaria as a potential biological control agent against Chinese privet because of its narrow host range and voracious appetite. To evaluate the effectiveness of an imported natural enemy, continuous monitoring of the population density is essential. Insect pheromones are one of the most effective tools for monitoring of target insects (Howse et al. 2013; Witzgall et al. 2010).
During field tests of pheromone trapping of spruce corn worm, Dioryctria abietella (Denis & Schiffermüller) (Lepidoptera; Pyralidae), we observed strong attraction of male N. seriaria to traps baited with (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene (C-21 pentaene). Based on this observation, we identified the C-21 pentaene in pheromone gland extracts of female N. seriaria and confirmed it as major sex pheromone component by electrophysiological study and field attraction tests.
Methods and Materials
Insects
Larvae of N. seriaria (second and third instar) were collected at Hongcheon County, Gangwon Province (37°46′06.5”N 127°53′52.3″E) in Korea on 22 May 2018. Collected insects were reared in plastic cages (30 × 40 × 20 cm) at room temperature, and fresh L. obtusifolium leaves were supplied for food. Pupae of N. seriaria were transferred to individual containers (9.5 cm dia × 4 cm high). Newly emerged adults were separated by sex, and an aqueous solution containing ascorbic acid (0.005%) and sucrose (3%) was supplied to provide vitamin and nutrients.
Extraction and Identification of Sex Pheromone
Pheromone glands of three- to five-day-old female N. seriaria were removed (N = 107) at 3–4 h into the scotophase. Each pheromone gland was extracted with 10 μL hexane for 3 min. The gland was removed and the extract was concentrated with nitrogen gas and stored at −70 °C before use.
Pheromone gland extracts were analyzed via gas chromatography (Agilent 7890A; Santa Clara, CA, USA) coupled with a mass-selective detector (Agilent 5977B MSD) equipped with an HP-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folsom, CA, USA). The gland extracts were combined in in 30 μL distilled hexane and 1 μL (equivalent to 3.56 glands) of the extract was injected via auto-sampler (G4513A, Agilent) in splitless mode. Carrier gas was helium at 1 mL/min, and the oven temperature was programmed from 40 °C to 190 °C at 30 °C/min, held for 5 min and then programmed to 215 °C at 10 °C/min, held for 5 min and finally programmed to 320 °C at 10 °C/min. The retention index of synthetic C-21 pentaene was calculated in relation to a homologous series of n-alkanes (C8-C28) using a gas chromatograph (Agilent 7890B) with flame ionization detection. Columns coated with DB-5MS (30 m × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folsom, CA, USA) and HP-Innowax (30 m × 0.25 mm i.d., film thickness = 0.25 μm; J&W Scientific) were used. The oven temperature was held at 40 °C for 1 min, raised to 250 °C at 6 °C/min, and held at 250 °C for 4 min. Carrier gas was helium at 1.5 mL/min.
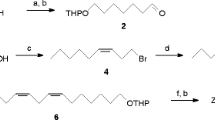
Synthesis (3Z,6Z,9Z,12Z,15Z)-Heneicosapentaene (Fig. 1).
Synthesis of major component of sex pheromone of Naxa seriaria (1) (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene (61.5%); (2) ethyl (5Z,8Z,11Z,14Z,17Z)-eicosapentaenoate; (3) (5Z,8Z,11Z,14Z,17Z)-eicosapentaen-1-ol (83.0%); (4) (5Z,8Z,11Z,14Z,17Z)-eicosapentaen-1-yl p-toluenesulfonate (72.8%). THF = tetrahydrofuran, TsCl = p-toluenesulfonyl chloride
Chemicals and Instrumentation
Lithium aluminum hydride (powder, purity 95%), tetrahydrofuran (purity 99.9%), sodium hydroxide (dry, purity 95%), pyridine (purity 99.8%), dichloromethane (purity 99.8%), p-toluenesulfonyl chloride (purity 98%), ethyl acetate (purity 99.8%), acetone (purity 99.9%), NaI (99.5%), methyl magnesium bromide solution (2.0 M in diethyl ether), dilithium tetrachlorocuprate (II) (0.1 M in THF), and ammonium chloride (purity 99.5%) were purchased from Sigma Aldrich (MI, USA). Eicosapentaenoic acid ethyl ester (purity 96%) was purchased from TCI Chemicals (Tokyo, Japan). Magnesium sulfate anhydrous (purity 99.5%) was purchased from Daejung Chemicals (Siheung, Korea).
1H NMR (at 400 MHz or 600 MHz) and 13C NMR (at 150 MHz) spectroscopic data were recorded on an Advance 400 MHz and 600 MHz spectrometer (Bruker, Germany) in CDCl3.
(5Z,8Z,11Z,14Z,17Z)-Eicosapentaen-1-Ol (3)
A solution of lithium aluminum hydride (4.82 g, 127 mM) in 250 mL of tetrahydrofuran was cooled to −10 °C, and ethyl (5Z,8Z,11Z,14Z,17Z)-eicosapentaenoate (2) (30 g, 99.8 mM) in 250 mL of tetrahydrofuran was added over 1 h. The reaction mixture was stirred overnight at room temperature. After cooling the inner flask temperature to −10 °C, 5 mL of cold distilled water were added to quench the reaction. Tetrahydrofuran and a solution of sodium hydroxide (15%) were added to the reaction mixture and stirred for 30 min at room temperature. The reaction mixture was washed with distilled water and dried with anhydrous magnesium sulfate. After filtration, solvent was removed with a rotary evaporator (N-1300 V-WB, Eyela, Singapore), the residue was purified by flash chromatography, and 25.0 g of compound (3) was obtained (yield: 83.0%). 1H-NMR (400 MHz, CDCl3): δ 5.20–5.40 (m, 10H), 3.60 (m, 2H), 2.87–2.65 (m, 8H), 2.15–1.95 (m, 4H), 1.58 (m, 3H), 1.42 (m, 2H), and 0.94 (t, 3H).
(5Z,8Z,11Z,14Z,17Z)-Eicosapentaen-1-Yl p-Toluenesulfonate (4)
p-Toluenesulfonyl chloride (24.78 g, 130 mM) dissolved in dichloromethane was added using a dropping funnel to a solution of (5Z,8Z,11Z,14Z,17Z)-eicosapentaen-1-ol (3) (25.0 g, 86.7 mM) and pyridine (13.71 g, 173. 3 mM) in 300 mL of dichloromethane. The reaction mixture was stirred for 1 h at room temperature and concentrated with a rotary evaporator. The residue was washed with distilled water and dried with anhydrous magnesium sulfate. After filtration, the solvent was removed with a rotary evaporator. The residue was purified by flash chromatography to give 18.2 g of compound (4) (yield: 72.8%). 1H-NMR (400 MHz, CDCl3): δ 7.76 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 8.1 Hz, 2H), 5.28–5.36 (m, 10H), 4.00 (t, J = 6.4 Hz, 2H), 2.83–2.72 (m, 8H), 2.43 (s, 3H), 2.07–1.97 (m, 4H), 1.63 (m, 2H), 1.37 (m, 2H), and 0.95 (t, J = 7.5 Hz, 3H).
(3Z,6Z,9Z,12Z,15Z)-Heneicosapentaene (1)
Methyl magnesium bromide (2.99 g, 25.1 mM) and dilithium tetrachlorocuprate (II) (4.4 g, 20.1 mM) were added to a solution of (5Z,8Z,11Z,14Z,17Z)-eicosapentaen-1-yl p-toluenesulfonate (4) (2.0 g, 4.5 mM) in 20 mL of tetrahydrofuran and stirred for 3 h at room temperature. Saturated aqueous ammonium chloride solution was added carefully to quench the reaction. After concentration using a rotary evaporator, 200 mL of ethyl acetate and distilled water were added to wash the residue. The organic layer was dried with anhydrous magnesium sulfate, concentrated, and then purified by flash chromatography to yield 1.23 g of compound (1) (yield: 61.5%). 1H-NMR (600 MHz, CDCl3): δ 5.43–5.31 (m, 10H), 2.86–2.80 (m, 8H), 2.10–2.03 (m, 4H), 1.37–1.26 (m, 6H), 0.99–0.96 (t, 3H), 0.90–0.87 (t, 3H). 13C NMR (150 MHz, CDCl3): δ 132.03, 130.49, 128.57, 128.56, 128.21, 128.18, 127.92, 127.90, 127.56, 127.04, 31.58, 31.52, 29.32, 27.22, 25.65, 25.55, 25.29, 22.56, 20.55, 14.24, 14.04. EI-MS m/z: 79 (100) [C6H7]+, 91 (64) [C7H7]+, 67 (54) [C5H7]+, 80 (48) [C6H8]+, 93 (29) [C7H9]+, 77 (34) [C6H5]+, 55 (32) [C4H7]+, 105 (29) [C8H9]+, and 81 (22) [C6H9] + .
Electrophysiology
We evaluated the electrophysiological response of antennae of male N. seriaria to female pheromone gland extract using gas chromatography with electroantennographic detection (GC-EAD), and responses to the synthetic sex pheromone were assessed using electroantennogram (EAG) recording.
Three- to five-day-old male N. seriaria were used for GC-EAD analyses. Each individual was inserted into a plastic pipette tip (standard 50–1000 uL, Eppendorf, Hamburg, Germany) and immobilized with Utility Wax (5 × 300 mm; Atria Co, Seoul, Korea). An electrolytically-sharpened tungsten wire (0.005″, 25 FEET; A-M system, USA) was used as a reference electrode and connected through the left eye. The last segment of the right antenna was removed and connected to a recording glass electrode filled with 0.1 N KCl.
The GC was fitted with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific) and carrier gas was nitrogen at 1 mL/min. The column effluent was split using a Presstight Connector (Universal Y; Restek, USA) at 1:1 ratio between FID and EAD. The effluent capillary to the EAD was passed through a heated transfer line (EC-03; Syntech, Germany) set at 280 °C and connected to glass tube (I.D. = 70 mm) which was supplied with 6 mL/s of filtered,-humidified air. The moth antenna was located 0.5 cm from the end of the glass tube. Female pheromone gland extract was injected in splitless mode with an inlet temperature of 200 °C. The oven temperature was programed from 40 °C to 250 °C at 10 °C/min and then 320 °C at 30 °C/min. FID and EAD signals were digitized with an IDAC-4 (Syntech, Germany) and processed using GC-EAD software (GC-EAD 2014, vers. 1.2.5; Syntech).
EAG responses of male N. seriaria to synthetic C-21 pentaene were evaluated using excised antennae of three- to five-day-old male N. seriaria (N = 7). The basal segment was connected to the reference glass electrode, and the tip segment was connected to the recording glass electrode, both electrodes filled with 0.1 N KCl. Five doses of C-21 pentaene were dissolved in distilled hexane, and applied to a paper disc (8 mm; Advantec MF, Inc., Dublin, CA, USA). After the solvent was allowed to evaporate for 30 s, each treated paper disc was inserted into a Pasteur pipette (L = 150 mm; Witeg ®, Wertheim, Germany). The tip of the Pasteur pipette was inserted at right angles into a glass tube (I.D. = 70 mm) and stimulated for 1.5 s with filtered, humidified air using a Stimulus Controller (CS-55, Syntech, Germany). The continuous flow rate and pulse flow rate were 6 mL/s and 3.5 mL/s, respectively. Stimuli were repeated three times in each antenna and intervals between each stimulus were 50 s. The control paper disc was treated with hexane only. Signals were digitized with an IDAC-4 (Syntech) and processed by Auto Spike software (V3.0, Syntech). EAG amplitude values (mV) were analyzed by one-way ANOVA, and mean treatment values were compared by Duncan’s test (SAS 9.4; SAS Institute Inc., USA).
Field Experiments
Field experiments were carried out at Hongcheon County, Gangwon Province, Korea (37°46′06.5”N 127°53′52.3″E) from 17 June to 2 July 2019, and numbers of male moths caught in traps were counted on 20, 24, 27 June, and 2 July 2019. Various doses (0.1, 1, 2, and 3 mg per lure) of synthetic C-21 pentaene dissolved in hexane were loaded into a sleeve stopper septa (bottom I.D. × O.D. 2.4 mm × 5.3 mm, white; Sigma Aldrich, USA). Control received only hexane. Delta traps (170 × 275 × 195 mm, yellow; Korean Institute of Insect Pheromone, Daejeon, Korea) with lure were hung approximately 1.5 m above the ground. The interval between traps in the same block was approximately 10 m, and blocks were 30–50 m apart. Traps were arranged in a randomized block design with four replicates and catches of male moths were analyzed by ANOVA. Differences between mean catches for each treatment were tested for significance by Duncan’s multiple range test (SAS 9.4, SAS Institute Inc. NC, USA).
Catches of male N. seriaria moths in delta traps were compared with those in bucket traps (17.5 cm dia × 23 cm height; Korean Institute of Insect Pheromone) in Hongcheon County, Gangwon Province, Korea (37°46′06.5”N 127°53′52.3″E) from 17 June to 2 July 2019, and the numbers of male moths caught in traps were counted on 20, 24, 27 June, and 2 July 2019. Each trap baited with synthetic C-21 pentaene (1 mg) was hung approximately 1.5 m above the ground. The numbers of male N. seriaria caught in the two types of trap were compared by t-test (SAS 9.4, SAS Institute Inc. NC, USA).
Results
Identification of N. seriaria Sex Pheromone
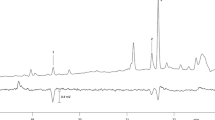
Analyses of pheromone gland extracts from female N. seriaria by GC-EAD revealed that one compound elicited a response from male antennae of N. seriaria (Fig. 2A) with retention time 18.70 min and retention index 2041 on the DB-5MS column used. In GC-MS analyses of the pheromone gland extracts using a similar HP-5MS column, the major electron ionization (EI) ions of the compound that elicited antennal responses were: m/z 79 (100) [C6H7]+, 91 (65) [C7H7]+, 67 (55) [C5H7]+, 55 (41) [C4H7]+, 105 (21) [C8H9]+, 119 (18) [C9H11]+, and 133 (10) [C10H13]+ (Fig. 2C). Other ions at m/z 150 and m/z 190 are characteristic of polyunsaturated fatty acids with ω 6-unsaturation, and the ion at m/z 108 indicated a polyene with a double bond in the 3-position (Ando et al. 2004; Holman and Rahm 1971; Karunen 1974). Synthetic (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene (C-21 pentaene) had identical retention time (Fig. 2B) and mass spectrum (Fig. 2C) to the EAD-active compound. Retention index on the polar HP-INNOWAX column was 2295.
(A) Gas chromatogram of pheromone gland extract from female moth and corresponding electroantennogram trace from male Naxa seriaria; antennal response was shown at retention time 18.71 min (*). (B) Gas chromatogram of synthetic (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene. (C) EI mass spectra of (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene produced by female N. seriaria (top) and the synthetic compound (bottom)
Electrophysiological Responses of Male N. seriaria to C-21 Pentaene
Mean EAG responses of adult male N. seriaria to C-21 pentaene are shown in Fig. 3. Significant differences were observed in the responses to C-21 pentaene at loadings on the filter paper above 30 μg compared to responses to the hexane control (F5,36 = 3.54, P = 0.011). The highest EAG response of male N. seriaria to synthetic C-21 pentaene was observed at 100 μg. The mean EAG response to 1000 μg was lower, but not significantly so.
Field Experiments
The effect of sex pheromone dose on the number of male N. seriaria caught in traps is shown in Fig. 4. There was a positive correlation between the number of male N. seriaria caught in traps and sex pheromone dose (N = 4, F7,12 = 74.51, P < 0.0001, Duncan’s test). Most moths were caught in traps baited with 3 mg of C-21 pentaene, the highest loading tested.
Mean numbers of male N. seriaria caught in the delta trap and bucket trap are shown in Fig. 5. Significantly more males were captured in the delta trap than in the bucket trap (N = 5, t = −6.76, P = 0.0017).
Discussion
In this study, we identified (3Z,6Z,9Z,12Z,15Z)-heneicosapentaene as the major component of the female-produced sex pheromone of N. seriaria and confirmed attractiveness through a field test. It is possible that this is the only component of the pheromone as no other electrophysiologically-active compounds were detected in GC-EAD analyses of pheromone gland extracts. However, the attractiveness of the synthetic compound was not compared with the attractiveness of a virgin female moth, and so the possibility of the presence of additional minor components in the pheromone cannot be excluded.
Lepidopteran sex pheromones can be categorized into several groups according to structure and biosynthetic origin (Ando et al. 2004; Löfstedt et al. 2016). Type I pheromones have functional groups such as alcohols, acetates, and aldehydes at a terminal position (Ando et al. 2004) and are biosynthesized de-novo from acetate (Bjostad and Roelofs 1981; Jones and Berger 1978; Löfstedt et al. 2016). More than 75% of lepidopteran sex pheromones belong to the Type I pheromone family. Type II pheromones, by contrast, consist of straight-chain, polyunsaturated hydrocarbons and the epoxy derivatives (Löfstedt et al. 2016; Millar 2000). Type II pheromones are derived from linoleic and linolenic acids, which were obtained in the larval stage from host plants (Löfstedt et al. 2016; Stanley-Samuelson et al. 1988). Type II pheromones are the second largest group in lepidopteran sex pheromones and mostly occur in Geometroidea and Noctuoidea, which are highly evolved taxa of the lepidopteran group (Ando et al. 2004; Millar 2000). This study revealed that N. seriaria uses C-21 pentaene as the major component of the female-produced sex pheromone. This is a typical Type II pheromone, but has not been reported previously as a sex pheromone component in Lepidoptera (Ando 2019). Furthermore, identification of sex pheromones of Orthostixinae species have not been reported until recently (Ando 2019), and this study could be a starting point to identify other sex pheromones of Orthostixinae species.
Synthetic C-21 pentaene elicited dose-dependent antennal responses in EAG measurements. Dose-dependent relationship between insect pheromones and their electrophysiological responses has been reported in many lepidopteran species (Fescemyer and Hanson 1990; Molnár et al. 2018; Sans et al. 1997; Yan et al. 2018). Typically when the concentration of the pheromone reaches a certain level, the EAG response does not increase further. This study showed that EAG response of male N. seriaria to female sex pheromone did not increase at doses above 100 μg.
In the field attraction test, more adult male N. seriaria were attracted to traps baited with sex pheromone than unbaited traps. A positive relationship between sex pheromone dose and trap capture of male N. seriaria was observed. Pheromone dose is an important factor for practical use of pheromone traps in the field. We did not investigate the saturation level of C-21 pentaene for N. seriaria male adults in this study. Saturation level of N. seriaria sex pheromone could be determined in future study by shortening the time interval of counting the number of male adults caught in a delta trap because of the space limitation of the sticky plate.
In this study, the effectiveness of two pheromone trap types, delta trap and bucket trap, which are most commonly used in control and monitoring of lepidopteran species, on male capture of N. seriaria was investigated. The delta trap was more effective than the bucket trap. Trap design greatly affects the trap capture rate of pheromone traps for many insect species (Kim and Park 2013; Malo et al. 2001; Strong et al. 2008). Factors such as pheromone emission pattern, trap opening, and the design compatibility for flying speed or size of moth might influence trap efficiency (Haynes et al. 2007; Lewis and Macaulay 1976). Lewis and Macaulay (1976) compared the patterns of pheromone plumes spreading from six types of traps. Among these, the triangular trap produced a long thin pheromone plume, but the covered funnel trap produced a much broader pheromone plume. Atmospheric dispersion and molecular properties are also decisive factors that determine the form of the odor plume (Murlis et al. 1992). Regnier and Law (1968) reported that pheromones with molecular weights under 250 Da diffused more rapidly. The sex pheromone of N. seriaria is a long-chain hydrocarbon with a molecular weight of 286 Da and corresponding low volatility that might affect the effectiveness of the bucket trap. Strong et al. (2008) reported that the trapping efficiency of the bucket trap was much lower than those of diamond, delta, and wing traps for monitoring of the fir corn worm, Dioryctria abietivorella, which uses the even less volatile (3Z,6Z,9Z,12Z,15Z)-pentacosapentaene as an essential component of the sex pheromone.
In conclusion, we identified C-21 pentaene as the major, if not the only, component of the sex pheromone produced by female N. seriaria moths, and showed the synthetic compound is highly attractive to male moths in the field.. This sex pheromone could be useful not only for obtaining accurate monitoring data for timing application of insecticides but also for controlling N. seriaria by mass trapping or mating disruption.
References
Ando T, Inomata SI, Yamamoto M (2004) Lepidopteran sex pheromones. Top Curr Chem 239:51–96
Ando T (2019) Internet database: https://lepipheromone.sakura.ne.jp/lepi_phero_list_eng.html
Baars JR (2011) Classical biological control for the management of alien invasive plants in Ireland. Biol Environ Proc R Irish Acad 213–222
Bjostad LB, Roelofs WL (1981) Sex pheromone biosynthesis from radiolabeled fatty acids in the redbanded leafroller moth. J Biol Chem 256:7936–7940
Fescemyer HW, Hanson FE (1990) Male european corn borer, Ostrinia nubilalis (Hübner), antennal responses to analogs of its sex pheromone. J Chem Ecol 16:773–790
Greene BT, Blossey B (2012) Lost in the weeds: Ligustrum sinense reduces native plant growth and survival. Biol Invasions 14:139–150
Haynes KF, Mclaughlin J, Stamper S, Rucker C, Webster FX, Czokajlo D, Kirsch P (2007) Pheromone trap for the eastern tent caterpillar moth. Environ Entomol 36:1199–1205
Holman RT, Rahm JJ (1971) Analysis and characterization of polyunsaturated fatty acids. Prog Chem Fats Other Lipids 9:13–90
Howse P, Stevens JM, Jones OT (2013) Insect pheromones and their use in pest management. Springer Science & Business Media
Hudson JR, Hanula JL, Horn S (2014) Impacts of removing Chinese privet from riparian forests on plant communities and tree growth five years later. For Ecol Manag 324:101–108
Jones IF, Berger RS (1978) Incorporation of (1−14C) acetate into cis-7-dodecen-1-ol acetate, a sex pheromone in the cabbage looper (Trichoplusia ni). Environ Entomol 7:666–669
Karunen P (1974) Polyunsaturated hydrocarbons from Polytrichum commune spores. Phytochemistry 13:2209–2213
Kim J, Park IK (2013) Female sex pheromone components of the box tree pyralid, Glyphodes perspectalis, in Korea: field test and development of film-type lure. J Asia Pac Entomol 16:473–477
Kim Y, Cho Y, Kang YK, Choi M, Nam SH (2013) A study of the major insect pest communities associated with Hibiscus syriacus (Columniferae, Malvaceae). J Ecol Environ 36:125–129
Löfstedt C, Wahlberg N, Millar JG (2016) Evolutionary patterns of pheromone diversity in Lepidoptera, chapter 4. In: Allison JD, Cardé RT (eds) Pheromone communication in moths. University of California Press, Berkeley, pp 43–82
Lewis T, Macaulay EDM (1976) Design and elevation of sex-attractant traps for pea moth, Cydia nigricana (Steph.) and the effect of plume shape on catches. Ecol Entomol 1:175–187
Malo EA, Cruz-Lopez L, Valle-Mora J, Virgen A, Sanchez JA, Rojas JC (2001) Evaluation of commercial pheromone lures and traps for monitoring male fall armyworm (Lepidoptera: Noctuidae) in the coastal region of Chiapas. Mexico Fla Entomol 84:659–664
Merriam RW, Feil E (2002) The potential impact of an introduced shrub on native plant diversity and forest regeneration. Biol Invasions 4:369–373
Millar JG (2000) Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu Rev Entomol 45:575–604
Molnár PB, Bognár C, Erdei AL, Fujii T, Vági P, Jósvai JK, Kárpáti Z (2018) Identification of the female-produced sex pheromone of an invasive greenhouse pest, the European pepper moth (Duponchelia fovealis). J Chem Ecol 44:257–267
Murlis J, Elkinton JS, Carde RT (1992) Odor plumes and how insects use them. Annu Rev Entomol 37:505–532
Regnier FE, Law JH (1968) Insect pheromones. J Lipid Res 9:541–551
Sans A, Riba M, Eizaguirre M, Lopez C (1997) Electroantennogram, wind tunnel and field responses of male Mediterranean corn borer, Sesamia nonagrioides, to several blends of its sex pheromone components. Entomol Exp Appl 82:121–127
Shaw RH, Cock MJ, Evans HC (2018) The natural enemies of privets (Ligustrum: Oleaceae): a literature review, with particular reference to biological control. CAB Rev 13:1–24
Stanley-Samuelson DW, Jurenka RA, Cripps C, Blomquist GJ, de Renobales M (1988) Fatty acids in insects: composition, metabolism, and biological significance. Arch Insect Biochem Physiol 9:1–33
Strong WB, Millar JG, Grant GG, Moreira JA, Michael Chong J, Rudolph C (2008) Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctria abietivorella. Entomol Exp Appl 126:67–77
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecology 36:80–100
Yan Q, Zheng MY, Xu JW, Ma JF, Chen Y, Dong ZP, Liu L, Dong SL, Zhang YN (2018) Female sex pheromone of Athetis lepigone (Lepidoptera: Noctuidae): identification and field evaluation. J Appl Entomol 142:125–130
Zhang YZ, Hanula JL, Sun JH (2008) Survey for potential insect biological control agents of Ligustrum sinense (Scrophulariales: Oleaceae) in China. Fla Entomol 91:372–383
Acknowledgements
This study was carried out with the support of ‘R&D Program for Forest Science Technology (Project No. “2019146B10-2021-AB02)’ provided by Korea Forest Service (Korea Forestry Promotion Institute).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SC., Koo, EC., Lee, DH. et al. Identification of the Female-Produced Sex Pheromone of the Dotted White Geometrid Naxa seriaria (Lepidoptera: Geometridae). J Chem Ecol 46, 927–934 (2020). https://doi.org/10.1007/s10886-020-01214-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01214-1