Abstract
Background
T-helper (Th) 22 and Th17 cells are implicated in the pathogenesis of autoimmune diseases. The roles of Th22 cells in the pathophysiology of rheumatoid arthritis (RA) remain unsettled.
Materials and Methods
CD4+IFNγ−IL17−IL-22+ T cells (Th22 cells), CD4+IFNγ−IL-22−IL17+ T cells (pure Th17 cells), CD4+IL17+ T cells (Th17 cells), and CD4+IFNγ+ T cells (Th1 cells) in RA, osteoarthritis patients, and healthy controls were examined by flow cytometry. Plasma IL-22 and IL-17 levels were examined by enzyme-linked immunosorbent assay.
Results
Th22 cells, pure Th17 cells, Th17 cells, and interleukin-22 were significantly elevated in RA patients compared with osteoarthritis and healthy controls, but there were no significant differences regarding Th1 cells and interleukin-17. Th22 cells showed a positive correlation with interleukin-22 as well as pure Th17 cells or Th17 cells in RA patients. Additionally, the percentages of Th22 cells, pure Th17 cells as well as Th17 cells correlated positively with both C-reactive protein levels and 28-joints disease activity score.
Conclusion
Together, our results indicated a possible role of Th22 pure Th17 cells and Th17 cells in RA, and blockade of the interleukin-22 may be a reasonable therapeutic strategy for RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) represents an example of autoimmune diseases. With a prevalence of 1% worldwide, the pathogenesis of RA is not clear yet [1]. Bone and cartilage destruction in the course of persistent inflammation is a serious clinical problem in the pathophysiology of RA. The activation of T cells recognizing autoantigens is implicated in the pathogenesis of RA. It has been well established for many years that Th1 cells have been implicated to play an important pathogenetic role in RA [2]. Th1 cells are predominant in synovial fluid of RA [3]. However, the situation of Th1 in the peripheral blood is more controversial: with either decreased or unchanged Th1 profile have been reported in RA patients [4, 5]. In recent years, additional effector T-cell subsets have been described, including interleukin-17 (IL-17)-producing cells (Th17 cells) [6–11]. T-helper (Th) 17 cells are inflammatory CD4+ T cells that produce IL-17A but not IFN-γ [12]. These cells and their secreted cytokines are found to be elevated in the peripheral blood of RA patients. In synovial fluid of RA patients, levels of Th17 cells were demonstrated to be much higher than that in peripheral blood, suggesting a pathogenetic role of Th17 in RA [13]. The exact mechanism of Th17 cells in pathogenesis of RA remains to be elucidated.
Th22 subset is a more recently identified CD4+ T-helper subset, which is characterized by secretion of IL-22 but not IL-17 or IFN-γ [14–16]. Th22 cells showed distinct differences in the profile of altered genes compared to other T cells such as Th1, Th2, and Th17 cells, confirming an individual signature for the Th22 subset [15]. The clonal stability, the selective expression of transcription factors, PDGF receptor and CCR-10 [14], and the fact that naive T cells differentiate toward the Th22 phenotype in the presence of TNF-α and IL-6 [14] provide strong evidence that Th22 cells represent a terminally differentiated and independent T-cell subtype. It has been established that Th22 cells are increased within psoriasis lesions and peripheral blood in psoriasis patients [17]. Stefanie et al. have identified Th22 cells that infiltrate the epidermis in individuals with inflammatory skin disorders [15]. The proinflammatory Th22 responses were synergistically dependent on IL-22 and TNF-α [15]. These mean that Th22 cells are probably implicated in the pathophysiology of some autoimmune diseases.
The effector cytokine of Th22 cells is IL-22, which belongs to the IL-10 cytokine family [18]. IL-22 signals through a heterodimeric receptor complex consisting of the IL10 receptor (IL-10R) β chain and the IL-22R [19, 20]. The precise function of IL-22 remains unclear. Recent studies implicated that IL-22 was involved in the pathogenesis of some autoimmune diseases such as systemic lupus erythematosus (SLE) [21], systemic sclerosis (SS) [22], and inflammatory bowel disease (IBD) [23] in humans. However, the situation of IL-22 was different in different diseases. Decreased plasma IL-22 level was found in patients with SLE [21]. Otherwise, there was increased IL-22 in the serum samples from psoriasis patients [24]. Consistently, increased IL-22 has also been reported in serum samples from Crohn disease patients [23].
IL-17, the main effector cytokine of Th17 cells, plays an important role in the acute mechanisms in host defense. It was involved in the pathogenesis of many autoimmune diseases such as ankylosing spondylitis, multiple sclerosis, and Crohn’s disease [25]. Although the amount of IL-17 in serum of RA patients is hard to detect, increasing IL-17 levels have been demonstrated in synovial fluid of patients with RA [26, 27].
Up to date, there is no data about Th22 cells (CD4+IFNγ−IL17−IL-22+ T cells) and pure Th17 cells (CD4+IFNγ−IL-22−IL17+ T cells) in patients with RA. To investigate their roles in the pathogenesis of RA, we measured the frequencies of Th22, pure Th17, Th17, Th1, and plasma IL-22 or IL-17, and examined their correlations with disease activity.
Materials and Methods
Patients and Controls
A total of 30 patients with active RA according to the criteria of the American College of Rheumatology were included in this study [28]. Each patient with active RA was defined by a DAS28 score ≥2.6 [29]. This group consisted of 23 women and seven men, with mean ± SD disease duration of 10.7 ± 8.6 years. The mean age of the patients was 53.9 ± 10.9 years. The demographic and key clinical information of the RA patients are summarized in Table I. All of the patients did not receive immunosuppressive or immunomodulatory drugs for at least 2 months when sampling. Eleven osteoarthritis (OA) patients (six females and five males; mean age 55 ± 5.3 years) as disease controls and 23 healthy controls (18 females and five males; mean age 54 ± 10.8 years) were also recruited in the study. Enrollment took place between March 2010 and January 2011 in two centers: the Department of Orthopedics, Qilu Hospital, Shandong University and the Department of Rheumatology, Shandong Provincial Hospital, Shandong University, China. The study was approved by the Institutional Review Boards of each participating center. Informed consent was obtained from each patient before being included in the study.
Flow Cytometric Analysis
Intracellular cytokines were studied by flow cytometry to reflex the cytokine-producing cells. Briefly, heparinized peripheral whole blood (400 μl) with an equal volume of Roswell Park Memorial Institute 1640 medium were incubated for 4 h at 37°C, 5% CO2 in the presence of 25 ng/ml of phorbol myristate acetate (PMA), 1 μg/ml of ionomycin, and 1.7 μg/ml Golgiplug (monensin; all from Alexis Biochemicals, San Diego, CA, USA). PMA and ionomycin are pharmacological T-cell-activating agents that mimic signals generated by the T-cell receptor (TCR) complex and have the advantage of stimulating T cells of any antigen specificity. Monensin was used to block intracellular transport mechanisms, thereby leading to an accumulation of cytokines in the cells. After incubation, the cells were stained with PE-Cy5-conjugated anti-CD4 monoclonal antibodies at room temperature in the dark for 20 min. The cells were next stained with FITC-conjugated anti-interferon (IFN)-γ monoclonal antibodies, PE-conjugated anti-IL-17 monoclonal antibodies, and APC-conjugated anti-IL22 monoclonal antibodies after fixation and permeabilization. All the antibodies were from eBioscience (San Diego, CA, USA). Isotype controls were given to enable correct compensation and confirm antibody specificity. Stained cells were analyzed by flow cytometric analysis using a FACScan cytometer equipped with CellQuest software (BD Bioscience PharMingen).
IL-22 and IL-17 Enzyme-Linked Immunosorbent Assay (ELISA)
Peripheral blood was collected into heparin-anticoagulant vacutainer tubes. Plasma was obtained from all subjects by centrifugation and stored at −80°C for determination of cytokines. IL-22 and IL-17 levels were determined with a quantitative sandwich enzyme immunoassay technique in accordance with the manufacturer's recommendations (lower detection limit 9 pg/ml; eBioscience).
Clinical Assessment
Disease activity score in 28 joints (DAS28) [26] was calculated in our study. Each patient with active RA was defined by a DAS28 score ≥2.6. At the time of clinical assessment for disease activity, blood samples were collected for the measurement of levels of C-reactive protein (CRP).
Statistical Analysis
Results were expressed as mean ± SD or median (range). Statistical significance was determined by ANOVA, and difference between two groups was determined by Newman–Keuls multiple comparison test (q test) unless the data were not normally distributed, in which case Kruskal–Wallis test (H test) and Nemenyi test were used. The Pearson or Spearman correlation test was used for correlation analysis depending on data distribution. All tests were performed by SPSS 17.0 system. P value less than 0.05 was considered statistically significant.
Results
Elevated Th22 Cells Correlated with Increased Plasma Level of IL-22 in RA Patients
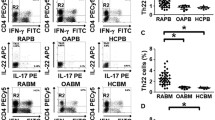
We analyzed the frequency of Th22 based on cytokine patterns after in vitro activation by PMA/ionomycin in short-term cultures. Th22 was defined as CD4+IFNγ−IL17−IL-22+ T cells to exclude Th1 or Th17 cells. The expression of a typical dot-plot of Th22 cells in representative RA, OA patients, and healthy controls is shown in Fig. 1c, e, g. The percentage of Th22 cells was significantly elevated in RA patients (1.56 ± 0.86%) compared to OA patients (0.76 ± 0.26%, P < 0.05) or healthy controls (0.74 ± 0.31%, P < 0.05) (Fig. 2a).
The percentages of circulating Th1 cells, pure Th17 cells, and Th22 cells in representative rheumatoid arthritis (RA), osteoarthritis (OA) patients, and healthy controls. Heparinized peripheral whole blood from all subjects were stimulated with phorbol myristate acetate, ionomycin, and monensin for 4 h, and then stained with labeled antibodies as described in “Materials and Methods”. a Lymphocytes were gated by flow cytometry. b, d, f The percentages of circulating Th1(CD4+IFNγ+) cells from RA, OA patients, and healthy controls. c, e, g The percentages of circulating pure Th17 cells (CD4+IFNγ−IL-22−IL17+ T cells) and Th22 (CD4+IFNγ−IL17−IL-22+ T cells) from RA, OA patients, and healthy controls
The percentages of circulating Th1 cells, pure Th17 cells, Th17 cells, and Th22 cells in rheumatoid arthritis (RA), osteoarthritis (OA) patients, and healthy controls (HC). a The percentages of circulating Th22 (CD4+IFNγ−IL17−IL-22+) cells from RA, OA patients, and healthy controls. Significantly increased percentage of Th22 cells was found in RA patients (1.56 ± 0.86%) compared to OA (0.76 ± 0.26%) (*P < 0.05) and healthy controls (0.74 ± 0.31%) (*P < 0.05). b The percentages of circulating pure Th17 (CD4+IFNγ−IL-22−IL17+) cells from RA,OA patients, and healthy controls. Significantly increased percentage of pure Th17 cells was found in RA patients (2.14 ± 0.86%) compared to OA (1.21 ± 0.43%) (*P < 0.05) and healthy controls (1.18 ± 0.49%) (*P < 0.05). c The percentages of circulating Th1(CD4+IFNγ+) cells from RA, OA patients, and healthy controls. For Th1 cells, there was no significant difference between RA (10.86 ± 3.51%) patients and OA (10.39 ± 2.38%) or healthy controls (10.48 ± 2.12%). d The percentages of circulating Th17 (CD4+IL17+) cells from RA, OA patients, and healthy controls. Significantly increased percentage of Th17 cells was found in RA patients (2.96 ± 1.10%) compared to OA (1.23 ± 0.36%) (*P < 0.05) and healthy controls (1.28 ± 0.26%) (*P < 0.05)
Plasma IL-22 was investigated by ELISA. The level of IL-22 was significantly increased in RA patients [median, 36.62 pg/ml (range, 21.89–171.85)] compared with OA [median, 23.68 pg/ml (range, 17.61–29.96 pg/ml), P < 0.05] and healthy controls [median, 22.72 pg/ml (range, 13.61–32.90), P < 0.05] (Fig. 3a).
Concentration of IL-22 and IL-17 in plasma from RA, OA patients, and healthy controls. a Concentration of IL-22 in plasma from RA, OA patients, and healthy controls. The level of IL-22 was significantly increased in RA patients [median, 36.62 pg/ml (range, 21.89–171.85)] compared with OA [median, 23.68 pg/ml (range, 17.61–29.96 pg/ml)] (*P < 0.05) or healthy controls [median, 22.72 pg/ml (range, 13.61–32.90)] (*P < 0.05). b Concentration of IL-17 in plasma from RA, OA patients, and healthy controls. As to IL-17, there was no significant difference between RA (15.56 ± 3.77 pg/ml, P > 0.05) patients and OA (14.55 ± 2.89 pg/ml, P > 0.05) or healthy controls (14.80 ± 2.92 pg/ml, P > 0.05)
A positive correlation was found between Th22 cells and plasma level of IL-22 (r = 0.67, P = 0.034; Fig. 4) in RA patients. However, Th1, pure Th17, or Th17 cells failed to show a statistical correlation with plasma level of IL-22 (P = 0.323, P = 0.709, or P = 0.747).
Elevated Th17 Cells in RA Patients
Pure Th17 was defined as CD4+IFNγ−IL17+IL-22− T cells to exclude Th1 or Th22 cells. The expression of a typical dot-plot of pure Th17 cells in representative RA, OA patients, and healthy controls is shown in Fig. 1c, e, g. Similarly, we found significantly increased percentage of pure Th17 cells in RA patients (2.14 ± 0.86%) compared with OA patients (1.21 ± 0.43%, P < 0.05) and healthy controls (1.18 ± 0.49%, P < 0.05) (Fig. 2b). In addition, the frequency of Th17 cells (CD4+IL17+) increased significantly in RA patients (2.96 ± 1.10%) compared to OA patients (1.23 ± 0.36%, P < 0.05) or healthy controls (1.28 ± 0.26%, P < 0.05) (Fig. 2d). However, there was no significant difference regarding plasma IL-17 between RA patients (15.56 ± 3.77 pg/ml, P > 0.05) and OA patients (14.55 ± 2.89 pg/ml, P > 0.05) or healthy controls (14.80 ± 2.92 pg/ml, P > 0.05) (Fig. 3b).
For Th1 cells, there was no significant difference between RA (10.86 ± 3.51%) patients and OA patients (10.39 ± 2.38%) or healthy controls (10.48 ± 2.12%) in this study (Figs. 1b, d, f and 2c).
Correlation between Th22, Pure Th17 Cells, Th17, and Th1 Cells in RA Patients
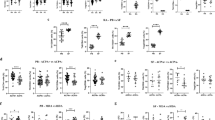
In RA patients, a significant positive correlation was found between Th22 cells and pure Th17 cells (r = 0.52, P = 0.003) as well as Th17 cells(r = 0.51, P = 0.004) (Fig. 5). However, Th1 cells failed to show a significant correlation with Th22 cells (P = 0.751), pure Th17 cells (P = 0.940), and Th17 cells (P = 0.884).
Correlation between Th22 cells and pure Th17 cells as well as Th17cells in RA patients. a Correlation between the percentages of Th22 cells and the percentages of pure Th17 cells in RA patients. Positive correlation was found between Th22 cells and pure Th17 cells (r = 0.52, P = 0.003). b Correlation between the percentages of Th22 cells and the percentages of Th17 cells in RA patients. A positive correlation was found between Th22 cells and Th17 cells (r = 0.51, P = 0.004)
Th22 Cells, Pure Th17 Cells, and Th17 Cells Positively Correlated with the Disease Activity in RA Patients
In patients with RA, there were positive correlations between the percentage of Th22 cells and CRP level or DAS28 (r = 0.619, P < 0.01 or r = 0.518, P = 0.003, respectively) (Fig. 6a, b). Consistently, positive correlations were also found between the percentage of pure Th17 cells (r = 0.879, P < 0.001 or r = 0.690, P < 0.001, respectively) (Fig. 6c, d) or Th17 cells (r = 0.897, P < 0.001 or r = 0.697, P < 0.001, respectively) (Fig. 6e, f) and CRP level as well as DAS28. However, the percentage of Th1 cells was not correlated with either CRP level or DAS28 (P = 0.856 or P = 0.634), and plasma level of IL-22 or IL-17 failed to show a statistical correlation with CRP level or DAS28 (P = 0.660 and P = 0.544 or P = 0.859 and P = 0.690).
Correlation between the percentages of both Th22 cells (r = 0.619, P < 0.01 and r = 0.518, P = 0.003, respectively), pure Th17 cells (r = 0.879, P < 0.001 and r = 0.690, P < 0.001, respectively) as well as Th17 cells (r = 0.897, P < 0.001 or r = 0.697, P < 0.001, respectively) and CRP levels as well as DAS28
Discussion
RA is one of the autoimmune diseases in which multiple joints are damaged by inflammation. Abnormality of cellular immunity has been widely demonstrated in RA, and many proinflammatory cytokines as well as chemokines contribute to the pathogenesis of inflammatory arthritis. In recent years, the idea that arthritis is mainly driven by the Th1 subset has been questioned. A new T-cell subset, Th17 cells, the main cells producing IL-17, are increased in many models of autoimmunity and are often considered to be the principal driver of inflammation. There are considerable data showing that Th17 cells are increased in RA [5, 13]. More recently, a unique IL-22-producing CD4+ T-helper subset, which expresses neither IL-17 nor IFN-γ, has been identified in human blood [14–16]. In analogy to the Th17 subset, cells with this cytokine profile have been named as Th22 subset that represents a distinct human T cell compared with Th1, Th2, and Th17 cells subset [14]. Although previous studies suggested that Th22 cells may play important roles in certain autoimmune diseases such as psoriasis [17], it is not clear whether they are involved in RA yet.
To study whether Th22 is involved in the development of RA, the frequencies of Th22 cells were examined in the peripheral blood of patients with RA, OA, and healthy controls. Our results demonstrated that the numbers of Th22 cells (defined as CD4+IL-22+IL-17−IFNγ−) were significantly increased in the peripheral blood of patients with RA compared with OA and healthy controls, implicating that Th22 may be involved in the pathogenesis of RA. It was reported that Th22 cells also co-express the chemokine receptor CCR6 and CCR4 [16], which are necessary for T-cell migration to the lesion. Significantly higher levels of CCL22 [30] and CCL20 [31], the ligand of CCR4 and CCR6, respectively, were found in synovial fluid from RA patients compared with OA and healthy controls. This will enable migration of CCR4 and CCR6 expressing Th22 cells to the joints, which support that CCL22 and CCL20 could play a role in attracting Th22 cells to the joints. Therefore, we speculate that the Th22 cells of peripheral blood may reach the joints and play some pathophysiologic roles in this way. However, the clinical association between Th22 cells and RA remains to be elucidated.
Considerable evidence suggests Th17 cells and Th1 cells have been linked to the development of autoimmune diseases [13, 32]. To further investigate the role of Th17 cells and Th1 cells in the pathogenesis of RA, we also examined the percentages of pure Th17 (CD4+IFNγ−IL-22−IL17+) cells, Th17 cells (CD4+IL17+) as well as Th1 (CD4+IFNγ+) cells in RA, OA patients, and healthy controls. The percentage of pure Th17 cells and Th17 cells in the peripheral blood of patients with RA was much higher than that in OA and healthy controls. In consistence with the study of Hui et al., the percentage of Th17 cells in the peripheral blood of patients with RA was demonstrated to be much higher than that in healthy controls [5]. In contrast to the results of Th22 and Th17 cells, there was no statistical difference in Th1 frequencies between patients with RA and OA or healthy controls.
In this study, there was a positive correlation between Th22 cells and pure Th17 cells as well as Th17 cells in patients with RA, suggesting that differentiation of Th22 and Th17 cells may be driven in an isotropic manner in RA. IL-6 is not only required for IL-17 induction from naïve T cells [8] but also can promote the expression of IL-22 [33]. In addition, IL-23 was essential for human Th17 differentiation [34], and IL-23 treatment can induce IL-22 production [33]. These might contribute to the positive correlation between Th22 cells and Th17 cells in our study, and the potential correlations between Th22 cells and Th17 cells need further research. However, statistical correlations between Th1 cells and Th22 cells or Th17 cells were not found in our study.
IL-22 is a member of the IL-10 family of cytokines and represents an important effector molecule of activated Th22 cells [14, 35]. IL-22 has been implicated in the pathogenesis of many autoimmune inflammatory diseases, such as psoriatic skin [36] and Crohn’s disease [23, 37]. As to RA, elevated IL-22 levels have been detected in synovial tissues from patients with RA, and high levels of IL-22R1 expression were observed in both the lining and sublining layers of rheumatoid synovium [38]. IL-22 can activate important kinases such as ERK1/2 and p38 MAKP which play important roles in leading to proliferation of synovial fibroblast and MCP-1 production, respectively [38, 39]. In accordance with increasing percentage of Th22 cells, elevated concentration of plasma IL-22 was observed in RA patients. Th22 cells, Th17 cells, and Th1 cells are the major T cells subsets producing IL-22 [14, 16, 31]. In Duhen’s study, the frequency of Th22 cells among total IL-22-producing T cells ranged from 37% to 63%, whereas Th17 cells ranged from 10% to 18% and the average frequency of Th1 cells was approximately 35% [14]. That might account for the positive correlation between plasma IL-22 and the frequency of Th22 cells in our study. Otherwise, there was no correlation between Th1 cells, pure Th17 cells as well as Th17 cells and plasma levels of IL-22 in RA patients in our study. It will be especially important to investigate how IL-22 work in RA and their potent correlation with other cells and cytokines. Another main cytokine secreted by Th22 cells is TNF-α, which Th22 cells proinflammatory responses depend on and induce a characteristic Th22 signature [15]. TNF-α is a main pathogenic cytokine in RA and is also critical for the induction of inflammatory chemokines and adhesion molecules. In addition, TNF-α is demonstrated to induce proliferation of synovial fibroblast, which leads to pannus formation [40]. Blockage of TNF-α has been proven to be the standard treatment of patients with rheumatoid arthritis for many years.
IL-17 has been shown to be an important cytokine involving in RA, and increasing IL-17 was examined in the synovial fluid of patients with RA. However, the situation of IL-17 in peripheral blood of RA patients is inconsistent in several studies [41, 42], with either increased or unchanged levels being found. In the present study, plasma IL-17 was determined. In accordance with Jan’s reports, we did not find a significant difference in plasma IL-17 levels among RA patients, OA patients, and healthy controls. Therefore, further studies are needed to elucidate the precise function of IL-17 in the development of RA
To investigate the potential roles of Th profile and related cytokines in RA, the relationships between RA disease activity (assessed by CRP and the DAS28) and Th22, pure Th17, Th17, or Th1 cells as well as IL-22 or IL-17 were also examined in the present study. Results showed that the frequency of Th22 cells positively correlated with CRP as well as DAS28 in patients with RA. Consistent with previous reports by Leipe et al., a positive correlation between the frequency of Th17 cells and the disease activity of RA was also observed [43]. However, there was no correlation between Th1 cells, IL-22 or IL-17, and CRP as well as DAS28. As mentioned above, IL-22 is not only secreted by Th22 cells. The proportion of Th1 cells among total IL-22-producing T cells is also relatively large. Moreover, Th1 cells were not significantly increased in our present study. These might explain the discrepancy on the correlation between Th22 or IL-22 and CRP as well as DAS28 in our experiment.
In conclusion, our data demonstrated that the percentages of Th22 cells, pureTh17 cells as well as Th17 cells were significantly increased in RA patients compared to OA and healthy controls. Plasma levels of IL-22, which correlated positively with Th22 cells, were also found to be elevated in RA patients. These support the hypothesis that Th22 cells, pure Th17 cells, and Th17 cells contribute to the pathogenesis of RA. Blockade of Th22 cells might be of clinical profit in RA patients. Further studies are necessary to clarify the situation of Th22 cells in RA joints and the pathophysiologic role of Th22 in RA.
Abbreviations
- RA:
-
Rheumatoid arthritis
- OA:
-
Osteoarthritis
- CRP:
-
C-reactive protein
- DAS28:
-
28-joints disease activity score
- PB:
-
Peripheral blood
- SF:
-
Synovial fluid
- SLE:
-
Systemic lupus erythematosus
- SS:
-
Systemic sclerosis
- IBD:
-
Inflammatory bowel disease
- PDGF:
-
Platelet-derived growth factor
- CCR:
-
Chemokine receptor
- ERK1/2:
-
Extracellular regulated kinase1/2
- MAPK:
-
Mitogen-activated protein kinases
References
Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10.
Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9.
Berner B, Akca D, Jung T, Muller GA, Reuss-Borst MA. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–35.
Kawashima M, Miossec P. mRNA quantification of T-bet, GATA-3, IFN-gamma, and IL-4 shows a defective Th1 immune response in the peripheral blood from rheumatoid arthritis patients: link with disease activity. J Clin Immunol. 2005;25:209–14.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56.
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40.
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–4.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41.
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. New Engl J Med. 2009;361:888–98.
Shahrara S, Huang Q, Mandelin AM, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93.
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63.
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85.
Trifari S, Kaplan C, Tran E, Crellin N, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71.
Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83.
Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9.
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9.
Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–32.
Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604–6.
Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–4.
Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–38.
Lo YH, Torii K, Saito C, Furuhashi T, Maeda A, Morita A. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J Dermatol Sci. 2010;58:225–7.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61.
Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7(4):R784–95.
Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164(5):2832–8.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, Jørgensen A, et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–9.
Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–12.
Bettelli E, Oukka M, Kuchroo VK. Th-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50.
Zheng Y, Danilenko D, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51.
Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7.
Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71.
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23.
Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–84.
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–46.
Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line: pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–82.
Gitter BD, Labus JM, Lees SL, Scheetz ME. Characteristics of human synovial fibroblast activation by IL-1 beta and TNF alpha. Immunology. 1989;66:196–200.
Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62(10):2876–85.
Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol. 2010;71(4):334–41.
Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–85.
Acknowledgments
This study was partially supported by research funding from the National Natural Science Foundation (30600680, 81070407, and 30973018), the Shandong Technological Development Project (2005BS03022, Q2008C07, and BS2009SW014), and the SRF for ROCS, SEM, GIIFSDU (yzc10147), and IIFSDU (2009TS063, 2009TS071).
Disclosures
The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lei Zhang, Jian-min Li, and Xin-guang Liu contributed equally to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Frequencies of T-helper cells and the related ratio in RA, OA patients, and healthy controls (DOC 30 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Li, Jm., Liu, Xg. et al. Elevated Th22 Cells Correlated with Th17 Cells in Patients with Rheumatoid Arthritis. J Clin Immunol 31, 606–614 (2011). https://doi.org/10.1007/s10875-011-9540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9540-8