Abstract
Psoriasis is a common chronic skin disease with a largely unknown pathogenesis. We demonstrate here that transgenic over-expression of interleukin (IL)-22 in mice resulted in neonatal mortality and psoriasis-like skin alterations including acanthosis and hypogranularity. This cutaneous phenotype may be caused by the direct influence of IL-22 on keratinocytes, since this cytokine did not affect skin fibroblasts, endothelial cells, melanocytes, or adipocytes. The comparison of cytokines with hypothesized roles in psoriasis pathogenesis determined that neither interferon (IFN)-γ nor IL-17, but only IL-22 and, with lower potency, IL-20 caused psoriasis-like morphological changes in a three-dimensional human epidermis model. These changes were associated with inhibited keratinocyte terminal differentiation and with STAT3 upregulation. The IL-22 effect on differentiation-regulating genes was STAT3-dependent. In contrast to IL-22 and IL-20, IFN-γ and IL-17 strongly induced T-cell and neutrophilic granulocyte-attracting chemokines, respectively. Tumor necrosis factor-α potently induced diverse chemokines and additionally enhanced the expression of IL-22 receptor pathway elements and amplified some IL-22 effects. This study suggests that different cytokines are players in the psoriasis pathogenesis although only the IL-10 family members IL-22 and IL-20 directly cause the characteristic epidermal alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a recurrent, chronic skin disease affecting about ten million people in the USA and the European Union alone [1, 2]. Affected people show sharply demarcated, red, and slightly elevated skin lesions with marked silver-white scales. Histologically, alterations include a massively thickened epidermis with diminished keratinocyte differentiation and a vast infiltration of immune cells including T cells, dendritic cells (DCs), macrophages, and granulocytes in the dermis and epidermis [1, 2]. Despite the great medical need, current psoriasis treatment options are limited, which is at least in part due to our restricted knowledge of its pathogenesis. Since the early 1990s, it has been considered a fact that via their mediators, particularly interferon (IFN)-γ, T cells play an important role in the initiation and maintenance of psoriasis [2–4]. However, the exact course of the pathogenic cascade and the precise contributions of each mediator to the pathogenesis of psoriasis are currently unknown.
Data recently published by others and ourselves on interleukin (IL)-22, a novel member of the IL-10–IFN family [5], led us to speculate that this mediator could play an outstanding role in psoriasis pathogenesis. IL-22 is produced by some lymphocyte subsets including activated Th17, Th1, and NK cells [6–10], and its expression is massively increased in psoriatic lesions [11, 12]. Moreover, elevated IL-22 levels were found in the blood from patients with psoriasis and they correlate strongly with the disease severity [13]. IL-22 has no influence on immune cells [11]. However, it regulates functions of a few tissue cell populations [11]. The IL-22 treatment of keratinocytes in vitro inhibits their differentiation and increases their mobility [13–15]. Moreover, it strongly increases the expression of antimicrobial proteins [7, 11, 13–15]. Alterations very similar to those observed in vitro in IL-22-treated keratinocytes are also found in psoriatic lesions [16].
In our current study, we clarify the significance of IL-22 for the psoriatic epidermis alterations by utilizing transgenic (TG) mice, a three-dimensional epidermis model, and patient samples and compare the effects of this cytokine on keratinocytes with the effects of IFN-γ, IL-17, tumor necrosis factor (TNF)-α, and IL-20.
Materials and methods
Generation of IL-22 TG mice
A DNA fragment containing a consensus Kozak sequence and coding region of mouse or human IL-22 was cloned into an expression plasmid under either the EμLCK (mouse IL-22) or the rat insulin II (human IL-22) promoter. The expression cassette with the IL-22 complementary DNA (cDNA) plus human growth hormone poly-A signal was isolated and used for microinjection into fertilized B6C3f1 (Taconic) murine oocytes. Equivalence in the potency of mouse and human IL-22 action on mouse cells had been confirmed by assessing STAT3 phosphorylation in Hepa1-6 cells (data not shown). Microinjection and production of TG mice was performed as previously described [17]. Animal experimentation conformed to AAALAC guidelines and has been approved by the ZymoGenetic's Institutional Animal Care and Use Committee.
Cell culture
Primary human epidermal keratinocytes and dermal fibroblasts were obtained from Lonza and cultured in KGM and FGM-2 medium, respectively, from the same supplier. Adipocytes were generated from primary human subcutaneous preadipocytes (Lonza) by culturing them for 10 days with PGM-2 differentiation medium (Lonza). Primary human dermal microvascular endothelial cells and melanocytes were purchased from Invitrogen and cultured in the respective medium (Invitrogen) according to the supplier’s instructions. Cells were used for experiments after a 24- to 48-h preculture period. For analysis of constitutive IL-22 receptor expression, cells were directly prepared for quantitative RT-PCR (qPCR) analysis after preculture. For signal transduction analysis, keratinocytes were stimulated with 10 ng/ml IL-22 for 0, 10, 20, and 40 min, and fibroblasts, microvascular endothelial cells, melanocytes, and adipocytes were each stimulated or not (control) with 10 ng/ml IL-22 and IFN-γ for 20 min. To investigate chemokine expression, keratinocytes were exposed to either IL-22, IFN-γ, IL-1β, or combinations of IL-22 with either IFN-γ, or IL-1β (all cytokines at 10 ng/ml), or left without additives (control) for 24 h. To investigate the effects of TNF-α, keratinocytes were exposed or not (control) to 10 ng/ml TNF-α for 42 h. EpiDerm-201™ under-developed tissue equivalents of human epidermis (under-developed human epidermis models, HEM) that are composed of stratified human keratinocytes derived from neonatal foreskins were obtained from MatTek in 2006 and 2007 and were cultured in inserts at the air–liquid interface using hydrocortisone-free Epi-201-DM differentiation medium from the same supplier. For stimulation, culture medium was supplemented or not (control) with 20 ng/ml IL-22, 20 ng/ml IL-20, 10 ng/ml IL-17, 1 to 20 ng/ml TNF-α, or a combination of IL-22 and TNF-α (1 and 2 ng/ml). After 72 h, the culture medium was recovered for enzyme-linked immunosorbent assay and biopsies were taken from the HEM tissues and either snap-frozen for histology or lysed for messenger RNA (mRNA) analysis using Invisorb® lysing solution (Invitek). All cytokines mentioned above were purchased from R&D Systems.
Small interfering RNA knockdown
Keratinocytes at a confluence of 30–50% were transfected using 100 pmol STAT3 Stealth RNAi and respective control oligo-ribonucleotide (Invitrogen) and 3 μl Lipofectamine 2000 (Invitrogen; 12-well culture format) according to the supplier’s instructions. Six hours later, the transfection solution was replaced by KGM and, after a further 24 h, cells were stimulated with 10 ng/ml IL-22 (R&D Systems) or left unstimulated (control) for another 24 h.
Patients
For STAT3 mRNA expression analyses, punch biopsies were obtained from diseased and uninvolved skin from adult patients with chronic plaque psoriasis. All skin and blood samples were approved by the clinical institutional review board of the Charité University Hospital, Berlin.
Histological and immunohistochemical analysis
For histological analyses of skin from neonatal IL-22 TG and wild-type (WT) control mice, biopsies were fixed in 10% neutral buffered formalin, embedded in paraffin, routinely processed, sectioned at 5 μm, and stained with hematoxylin and eosin. For immunohistochemical analyses of skin from neonatal IL-22 TG and WT mice, biopsies were frozen in blocks of Tissue-Tek® O.C.T. compound (Tissue Tek) and sectioned at 7 μm on a cryostat. Sections adhered to glass slides were allowed to dry overnight at room temperature, fixed in −20°C acetone, and stained with rat anti-mouse CD3 monoclonal antibody (Ab; clone 17A2, Pharmingen) and with rat anti-mouse F4/80 monoclonal Ab (clone CI:A3-1, Serotec) diluted 1:50 and 1:200, respectively, in phosphate-buffered saline with 10 mg/ml bovine serum albumin for 1 h at room temperature. After extensive washing, detection of primary antibody binding was performed using goat anti-rat immunoglobulin (Ig)G conjugated with horseradish peroxidase (Zymed). Nuclear counter-staining of sections was performed using methyl green (DAKO) before sections were dehydrated and cover-slipped. Frozen biopsies taken from HEM were embedded in tissue-freezing medium (Leica Microsystems) and cryo-cut to 7 μm sections, fixed in −20°C acetone and stained with either Mayer's hemalaun (Dr. K. Hollborn & Söhne GmbH & Co.) or with the following primary Abs: mouse anti-human K10 (clone LHP1, Serotec) diluted 1:50, mouse anti-human Ki67 (clone MIB-1, DAKO) diluted 1:75, or the corresponding isotype control mouse anti-human IgG1 (clone DAK-G01, DAKO) diluted 1:50. Detection of primary Ab binding was performed using the LSAB+ System-AP kit (DAKO). Sections were then counter-stained with Mayer's hemalaun, and covered with Kaiser's glycerin gelatin (Merck). The thickness of living cell layers in HEM and mouse skin sections was measured by means of AxioVision Release 4.6.3 image software (Zeiss, Jena, Germany).
Quantitative RT-PCR
Isolation of total cellular RNA was performed using the Invisorb® RNA kit II (Invitek). Messenger RNA was reverse-transcribed as described previously [12] and analyzed by TaqMan™ PCR using the ABI Prism™ 7700 Sequence Detection System (Applied Biosystems). The expression of human IL-22R1, IL-10R2, IL-20R1, and IL-20R2 was detected using fluorescent probes as described previously [8, 11]. All other detection systems used were purchased from Applied Biosystems. The expression levels were calculated relative to those of hypoxanthine phosphoribosyl-transferase 1 (HPRT).
Enzyme-linked immunosorbent assay
Human TNF-α and CXCL8 was quantified by Immulite™ (DPC Biermann).
Western blot analyses
Cell lysis, protein electrophoresis, and Western blot were performed as described previously [18]. Blotted samples were incubated with polyclonal Abs against phospho-STAT1 (Tyr701), phospho-STAT3 (Tyr705), phospho-STAT5 (Tyr694), and total STAT3 (all from New England Biolabs) followed by incubation with peroxidase-conjugated AffiniPure F(ab′)2 goat anti-rabbit IgG (H and L) secondary Ab fragment (Dianova) and ECL detection (Amersham Pharmacia Biotec).
Statistical analyses
Significance of differences between treatment groups of in vitro cultures and between non-lesional and lesional skin was tested by the Wilcoxon matched-pairs signed-rank test (two-tailed). Significance of differences between WT versus TG mice was tested by the Mann–Whitney U test (two-tailed). In all cases, the SPSS software (SPSS) was used.
Results
IL-22 TG mice show psoriasis-like skin alterations
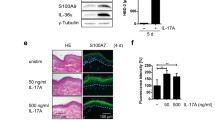
To further explore the biological significance of IL-22, we investigated its role in vivo in the first part of our study using IL-22 over-expressing mice. Two approaches were used: First, mouse IL-22 was over-expressed in mice using the EμLCK promoter, which drives expression in cells of the lymphoid lineage. Second, human IL-22 was over-expressed using the rat insulin II promoter, which leads to a pancreatic islet-specific expression. Interestingly, very similar results were obtained in both cases. The IL-22 TG pups were smaller (Fig. 1a) and had 33% less body weight compared to their WT littermate controls. They had a shiny and stiff skin (Fig. 1a) and had difficulty in moving. Most of the IL-22 TG neonates died within the first few days after birth, although milk was found in their stomachs indicating that they were able to suckle. However, we were able to establish one line from an insulin II promoter-driven IL-22 TG founder that delivers shiny offspring. Histologically, the epidermis of the IL-22 TG mice appeared to be thicker than the epidermis of WT littermate controls (Fig. 1b–e). The granular layer of the TG mice contained fewer keratohyalin granules and the cornified layer was more compact than that of the WT. Importantly, the skin alterations of IL-22 TG mice recapitulate the main features of psoriatic skin (acanthosis: increased number of keratinocytes and thickening of the spinous layer, loss of the granular layer, and hyperkeratosis: thickening of the cornified layer) [2]. As assessed by immunohistochemistry analysis, IL-22 TG mice showed some dermal infiltration of macrophages (Fig. 1f). However, cutaneous T-cell infiltrates commonly found in psoriatic skin lesions were not prominent in IL-22 TG mice as assessed by analysis of CD3, CD4, and CD8 markers (Fig. 1g and data not shown).
IL-22 TG mice exhibit an aberrant skin phenotype. IL-22 TG mice were developed by microinjection into fertilized B6C3f1 murine oocytes of an expression cassette containing either the mouse IL-22 or the human IL-22 cDNA sequence and the human growth hormone poly-A signal under the EμLCK (mouse IL-22) or the rat insulin II (human IL-22) gene promoter. Both constructs gave similar results. a Newborn (day 1) insulin II promoter-driven TG mice compared to WT control mice. b Skin from day 1 EμLCK promoter-driven TG mice compared to WT control mice was analyzed by histology (H&E). Microscopic magnification, 40-fold. c Skin from day 1 rat insulin II promoter-driven TG mice compared to WT control mice was analyzed by histology (H&E). Microscopic magnification, 40-fold. d ,e Thickness of living epidermal cell layers measured on histologic skin sections described in (b) and (c) is given of at least five (mean ± SEM) and one mice, respectively, per genotype. Statistical significance of deviations was tested using the Mann–Whitney U test (d; **p < 0.01). f Immunohistochemistry analysis of F4/80 in skin sections from day 1 insulin II promoter-driven TG mice compared to WT control mice. Microscopic magnification, 40-fold. g Immunohistochemistry analysis of CD3 in skin sections from day 1 insulin II promoter-driven TG mice compared to WT control mice. Microscopic magnification, 40-fold. All images, with the exception of (c) and (e), represent the result from at least five different mice. sc stratum corneum
Among skin cell populations, only keratinocytes are the major target of IL-22
Our first question after analyzing the phenotype of IL-22 TG mice was which cells could be involved in the observed skin alterations induced by IL-22. Already in 2004, we found that IL-22 does not have any effects on immune cells in vitro and in vivo and identified tissues cells, such as keratinocytes, as IL-22 targets [11]. We now investigated other resident skin cells such as primary dermal fibroblasts, dermal microvascular endothelial cells, melanocytes as well as subcutaneous adipocytes and compared their expression of the IL-22 receptor components with that of keratinocytes. The receptor complex that confers the cellular sensitivity to IL-22 is composed of IL-22R1 and IL-10R2 [19–21]. As assessed by qPCR, only fibroblasts and keratinocytes but not endothelial cells, melanocytes, or adipocytes showed coexpression of IL-22R1 and IL-10R2, although IL-22R1 expression in fibroblasts was 14 times lower than in keratinocytes (Fig. 2a). We then analyzed the sensitivity of the different cell populations to IL-22 by assaying the induction of signal transduction. As shown in Fig. 2b, only keratinocytes clearly responded to IL-22 stimulation. This data demonstrated that in the skin only the keratinocytes are a major target of IL-22. Our further investigations therefore focused on these cells.
Among skin cell populations, only keratinocytes are major targets of IL-22. a Skin fibroblasts (FB), microvascular endothelial cells (EC), melanocytes (MC), adipocytes (AC), and keratinocytes (KC) were analyzed for their expression of IL-22 receptor subunits by qPCR. Data from two (mean ± range) independent experiments are given as relative to HPRT expression. b Skin fibroblasts, microvascular endothelial cells, melanocytes, and adipocytes were stimulated with IL-22 or IFN-γ or left without stimulation (control) for 20 min. Keratinocytes were stimulated for 0, 10, 20, and 40 min with IL-22. Cellular content of P-STAT1 (Tyr701), P-STAT3 (Tyr705), P-STAT5 (Tyr694), and total STAT3 was assessed by Western blot analysis
In contrast to IL-22, IFN-γ and IL-17 neither inhibit the differentiation of keratinocytes nor induce psoriasis-like morphological alterations of the epidermis
IL-22 is produced, among others, by Th17 and Th1 cells [6–9]. In the past, two other mediators of these cell populations, IFN-γ (from Th1) and IL-17 (from Th17 cells) were suggested to be responsible for the initiation and maintenance of the psoriasis skin alterations [3, 4, 22, 23]. To compare the IL-22 effects on keratinocytes with those of IFN-γ and IL-17, we applied these cytokines to human epidermis models obtained 3 days before full maturation (here designated as HEM). These cultures were kept in medium containing IL-22, IFN-γ, or IL-17, or in control medium for 3 days and were analyzed afterwards by histological/immunohistochemical, qPCR, and Western blot analyses. Importantly, IL-22 but not IL-17 or IFN-γ induced acanthosis of cultures and decreased granularity in the upper living layer (Fig. 3a–d). To gain insight into the molecular causes of these morphological alterations, we investigated the regulation of more than 110 genes involved in different pathways essential for the physiology of keratinocytes. In doing so, we noticed that only IL-22, but not IL-17 or IFN-γ, decreased the expression of terminal differentiation-regulating genes including late cornified envelope 1B (LCE1B), desmocollin 1 (DSC1), calmodulin like 5 (CALML5), and keratin (K) 10 (Fig. 3e). The terminal differentiation of keratinocytes comprises the dissolution of the cell nucleus and the formation of insoluble protein agglomeration (so-called cornified envelope), resulting in the development of the mechanically resistant cornified layer of the epidermis. Our data suggest that of the tested T-cell mediators only IL-22 inhibits the terminal differentiation of keratinocytes and increases the epidermal thickness in psoriasis. Interestingly, the IL-22 effects on keratinocytes were not associated with any modulation of the expression of several pro- or anti-apoptotic molecules in these cells (Fig. 3f). We further found that IL-22 but not IFN-γ or IL-17 enhanced the keratinocyte mRNA and protein expression of STAT3 (Figs. 3e and 7a), a major signaling molecule implicated in the development of a psoriasis-like skin phenotype [24, 25].
IFN-γ and IL-17 do not share the IL-22-induced effect on keratinocyte differentiation. HEM were stimulated or not (control) in different independent experimental series as indicated with IL-22, IFN-γ, and IL-17 for 72 h. a, b Tissues were analyzed by histology (hemalaun). Images represent the result of one out of three independent experiments for each experimental series. Microscopic magnification, 100-fold. c ,d Mean (±SEM) thickness data of living cell layers from three independent experiments described in (a) and (b) are given as percent of control. e, f Gene expression was analyzed by qPCR. Data from three (mean ± SEM) independent experiments are given as percent of control (e) or relative to HPRT expression (f)
IFN-γ and IL-17, but not IL-22, are major inducers of chemokines in keratinocytes
Another characteristic of the psoriatic skin is the infiltration of immune cells including mixed mononuclear cells in the dermis and epidermis and epidermal islets-like inclusions of neutrophilic granulocytes (Munro’s abscesses). Since a major prerequisite for such infiltration is chemokine production, we systematically analyzed the expression levels of different chemokine classes in IL-22, IFN-γ, and IL-17-treated HEM and conventional keratinocyte cultures. As shown in Fig. 4a, b, IFN-γ induced the expression of Th1-cell (CXCL9, CXCL10) but not DC (CCL20) or neutrophilic granulocyte (CXCL1, CXCL8)-attracting chemokines in keratinocytes. IL-17 induced neither Th1 (CXCL9, CXCL10) nor other T-cell subset (CCL17, CCL22, CCL2, CCL27)-specific chemokines but was a strong inducer of DC (CCL20) and granulocyte-attracting (CXCL1, CXCL2, CXCL5, CXCL8) chemokines (Fig. 4c, d). Interestingly, IL-22 showed IL-17-like effects. In fact, IL-22 did not induce Th1 or Th17-specific chemokines (it even reduced the constitutive CCL22 expression) but had an enhancing effect on the DC and granulocyte-specific chemokines (Fig. 4a, c, d). This effect was smaller than that observed with IL-17. Furthermore, IL-22 did not modify the inducing effect of IFN-γ or IL-1β on T-cell and DC-specific chemokines (Fig. 4b).
IFN-γ, IL-17, and IL-22 have different capacities to induce chemokines in keratinocytes. a HEM were stimulated with IL-22 or IFN-γ, or left without stimulation (control) for 72 h. Gene expression was analyzed by qPCR. Data from three (mean ± SEM) independent experiments are given as relative to HPRT expression. b Keratinocytes were exposed to either IL-22, IFN-γ, IL-1β, or combinations of IL-22 with either IFN-γ, or IL-1β, or left without additives (control) for 24 h. Gene expression was analyzed by qPCR. Data from three (mean ± SEM) independent experiments are given as relative to HPRT expression. c HEM were stimulated with IL-22 or IL-17, or left without stimulation (control) for 72 h. Gene expression was analyzed by qPCR. Data from three (mean ± SEM) independent experiments are given as relative to HPRT expression. d HEM were stimulated with IL-22 or IL-17, or left without stimulation (control) for 72 h. CXCL8 concentration in the culture supernatant was analyzed by Immulite™. Data from five (mean ± SEM) independent experiments are given. Statistical significance of deviations was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05)
TNF-α enhances some effects of IL-22
In recent years, targeting of TNF-α has shown an outstanding therapeutic success in psoriasis patients [26, 27] and therewith has revealed the importance of this mediator in psoriasis pathogenesis. For this reason, we investigated whether TNF-α also induces psoriasis-like alterations in keratinocytes and whether there would be any interaction between IL-22 and TNF-α. TNF-α alone was not a major modulator of the expression of differentiation-regulating genes as shown using conventional keratinocyte cultures (Fig. 5a). It minimally upregulated K16 expression but had no influence on LCE1B, DSC1, CALML5, or kallikrein (KLK)7 expression levels in keratinocytes. This was in contrast to IL-22, which clearly regulated the expression of all these genes both in conventional keratinocyte cultures [13] and in the HEM (Fig. 3e). It should be noted that TNF-α is a major inducer of chemokines in keratinocytes. As show in Fig. 5b, TNF-α induced chemokines attracting Th1 cells, Th17 cells, DCs, and neutrophilic granulocytes. In the HEM, we observed complete tissue fragility when a concentration of TNF-α greater than 2 ng/ml was used (data not shown). When 2 ng/ml TNF-α was applied, this alone led to an only very weak if any increase in epidermal thickness with some spongiosis, but, in combination with IL-22, provoked a more pronounced epidermal thickness than did IL-22 alone (Fig. 5c). Notably, we did not observe any amplification of IL-22-caused morphological changes at 1 ng/ml TNF-α (data not shown), although both 1 and 2 ng/ml TNF-α enhanced the IL-22-driven upregulation of K16 expression, CXCL8 secretion (Fig. 5d), and S100A7 expression (data not shown). Further on, we investigated the mechanism of the TNF-α-induced amplification of IL-22 effects by accessing the possible influence of TNF-α on elements of the IL-22 receptor and signal transduction pathway. As demonstrated in Fig. 5f, a very small but reproducible increase of IL-22R1, IL-10R2, and STAT3 expression was observed in TNF-α-treated versus control keratinocytes. In contrast, the expression of STAT1 and components of the type I IL-20 receptor complex, IL-20R1 and IL-20R2, was either slightly decreased (IL-20R1) or not influenced (STAT1, IL-20R2) by TNF-α treatment. In line with these in vitro observations, we found nearly doubled IL-22R1 expression in the skin of psoriasis patients compared to samples from healthy participants (1.371 ± 0.279 versus 0.696 ± 0.151; p = 0.06). Of note, TNF-α did not induce IL-22 production in lymphocytes (data not shown). Conversely, IL-22 did not induce TNF-α production by keratinocytes although it very slightly upregulated the expression of TNFR1, the only TNF-α receptor on these cells (Fig. 5e). No influence of IL-22 was detected with respect to the expression of p50 or p65 of NF-κB, a major TNF-α-activated transcription factor (Fig. 5e).
TNF-α enhances the effect of IL-22 on HEM. a, b, f Keratinocytes were stimulated or not (control) with TNF-α for 42 h. Gene expression was analyzed by qPCR. Data from six (mean ± SEM) independent experiments are given as relative to HPRT expression. Statistical significance of deviations was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05). c HEM were stimulated with IL-22, TNF-α, a combination of IL-22 and TNF-α, or left without stimulation (control) for 72 h. Tissues were analyzed by histology (hemalaun). Images represent the result from two experiments. Microscopic magnification, 100-fold. d HEM were stimulated with IL-22, TNF-α, a combination of IL-22 and TNF-α, or left without stimulation (control) for 72 h. K16 gene expression was analyzed by qPCR. CXCL8 concentration in the culture supernatant was analyzed by Immulite™. Data from four (mean ± SEM) independent experiments are given as percent of culture without additives. e HEM were stimulated with IL-22 or left without stimulation (control) for 72 h. TNF-α concentration in culture medium of three independent experiments was quantified by Immulite™. Gene expression was analyzed by qPCR. Data from five (mean ± SEM) independent experiments are given as relative to HPRT expression. Statistical significance of deviations was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05)
IL-22 and IL-20 show very similar effects on keratinocytes
Interestingly, the skin phenotype observed in our IL-22 TG mice (Fig. 1) was very similar to that of IL-20 TG mice [17]. IL-20 is also a member of the IL-10–IFN family, which, however, acts via two distinct receptor complexes, IL-20R1/IL-20R2 and IL-22R1/IL-20R2 [28]. Studies from others [29, 30] and ourselves [31] have shown elevated IL-20 levels in psoriatic lesions. To compare the IL-20 effects on keratinocytes with those of IL-22, we again used HEM and stimulated them with IL-20 and IL-22 for 3 days. As demonstrated in Fig. 6a, b, IL-20, like IL-22, was able to induce acanthosis of cultures, although this effect was less pronounced as compared to that induced by IL-22 and was not accompanied by a clear reduction of the granular layer. Moreover, IL-20, like IL-22, reduced the expression of LCE1B, DSC1, CALML5, K1, and K10, and discretely enhanced K16 expression (Fig. 6c). In line with the different degrees of acanthosis, in almost all cases, the effects of IL-20 were less pronounced than those of IL-22 (Fig. 6a–c). As formerly discussed for IL-22 [11], the IL-20-induced acanthosis was not due to an increased proliferation of keratinocytes either, since no enhanced Ki67 staining was observed in these cultures (Fig. 6a).
IL-20 exerts IL-22-like effects on HEM. HEM were stimulated with IL-20, IL-22, or left without stimulation (control) for 72 h. a Tissues were analyzed by histology (hemalaun) and immunohistochemistry (K10, Ki67). Images represent the result from five experiments. Microscopic magnification, 100-fold. b Mean (±SEM) thickness data of living cell layers from five independent experiments described in (a) are given. Statistical significance of deviations was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05). c, d Gene expression was analyzed by qPCR. Data from five (mean ± SEM) independent experiments are given as relative to HPRT expression. Statistical significance of deviations was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05). hem hemalaun
We then compared the influence of IL-20 and IL-22 on transcription factor and chemokine expression. As shown in Fig. 6c, IL-20 like IL-22 treatment led to an increased STAT3 expression, whereby IL-20 was less potent than IL-22. Interestingly, neither IL-20 nor IL-22 regulated the expression of STAT1 in keratinocytes (Fig. 6c). Regarding the chemokines, IL-20 induced an IL-22-like expression pattern either, again with a lower potency (Fig. 6d). The only qualitative difference found between both cytokines was a very slight induction of CCL27 expression by IL-20.
STAT3 is essential for the IL-22-induced inhibition of differentiation-regulating genes in keratinocytes
As described above, among the tested cytokines, those that had more or less clear inhibiting influence on the terminal differentiation of keratinocytes (IL-22, IL-20, and TNF-α) had also a significant inducing effect on the keratinocyte STAT3 expression. Potent STAT3 induction was especially obvious for IL-22. In IL-22-treated HEM, the upregulation of STAT3 could also be observed at the protein level (Fig. 7a). Since STAT3 is known to be strongly activated by IL-22 and IL-20 in keratinocytes, the upregulation of STAT3 expression may confer an elevated cellular sensitivity to IL-22 and IL-20 action. Interestingly, lesional skin from psoriasis patients also showed increased STAT3 mRNA expression compared to non-lesional skin from the same patients (Fig. 7b). To further go into detail, we investigated whether STAT3 is necessary for the ability of IL-22 to modulate the expression of differentiation-regulated genes. By means of the small interfering RNA (siRNA) technique, we reduced the STAT3 expression in primary keratinocytes to approximately 20% (Fig. 7c). In those keratinocytes, IL-22 only minimally downregulated the expression of DSC1, CALML5, and K10 if at all, demonstrating a clear STAT3 dependency of these regulations.
Elevated keratinocyte STAT3 expression may reconstitute a positive feedback loop for IL-22 action on the keratinocyte terminal differentiation. a HEM were stimulated with IL-22 or left without stimulation (control) for 72 h. Cellular content of STAT3 was analyzed by Western blot analysis. b Paired (lesional versus non-lesional) skin biopsies from psoriasis patients were analyzed by qPCR. Data from seven (mean ± SEM) patients are given as relative to HPRT expression. Statistical significance of differences was tested using the Wilcoxon matched-pairs signed-rank test (*p < 0.05). c Keratinocytes cultured under conventional conditions were transfected with STAT3-specific siRNA or control oligo-ribonucleotide or were left untransfected (control). After 24 h, cells were stimulated or not with IL-22 for another 24 h. Gene expression was analyzed by qPCR and presented as relative to HPRT expression (left) or to every matching transfection group without IL-22 exposure (right)
Discussion
Cytokines play a decisive role in the communication between cells. In recent years, this fact has been increasingly utilized in the development of new powerful medications to treat chronic inflammatory diseases. For example, anti-TNF-α therapy constitutes an enormous improvement in the treatment possibilities for psoriasis [26, 27]. However, TNF-α is a pluripotent cytokine with numerous effects on tissue cells as well as immune cells and, therefore, neutralization of this cytokine appears to have side effects [27]. There continues to be a great interest in identifying mediators that are important for the psoriasis pathogenesis, but that do not have broad effects especially on the immune system.
A large body of data demonstrates that IL-22, a cytokine that can be produced by special T- and NK-cell subsets, may be such a mediator. As we have shown in early studies, IL-22 mRNA is massively elevated in psoriatic skin lesions [11]. Interestingly and in contrast to other disease-related cytokines, this cytokine is also present in the blood of such patients, and the IL-22 plasma levels strongly correlate with the disease severity [11, 13]. In keratinocytes, IL-22 induces molecular changes that are very similar to that in keratinocytes from psoriatic lesions. Most importantly, it impairs the normal terminal differentiation of these cells, as deduced from studies on conventional keratinocyte cultures and HEM ([13–15] and Figs. 3 and 6). Here, we show for the first time that TG over-expression of IL-22 in mice caused profound epidermal alterations, which are characteristics of psoriasis: acanthosis, loss of granular layer, and a compact cornified layer. These changes appeared to be the result of direct IL-22 action on the epidermis since, (a) among skin cells, only keratinocytes are major targets of this cytokine (Fig. 2), and (b) these changes can be reproduced in IL-22-treated HEM ([14, 15] and Figs. 3 and 6). Furthermore, we have previously demonstrated that IL-22 does not act on immune cells [11], further supporting the exclusive, direct induction of psoriatic keratinocyte alterations by this cytokine. An important role of IL-22 in Th17 mouse models of psoriasis-like skin inflammation was very recently demonstrated by the groups of Ouyang and Fouser [9, 32].
In our present study, we also compared the influence of IL-22 on keratinocytes with that of cytokines hypothesized to play a major role in the pathogenesis of psoriasis and other chronic inflammatory disorders: IFN-γ, IL-17, IL-20, and TNF-α [2, 4, 26, 27, 33–38]. We observed that IFN-γ and IL-17 strongly induced Th1 cell and neutrophilic granulocyte-attracting chemokines, respectively (Fig. 4). Furthermore, IL-17 enhanced the expression of CCL20, a chemokine that recruits DCs and, in combination with CCL17 and CCL22, recruits Th17 cells in epithelial tissues [39, 40]. However, neither IFN-γ nor IL-17 caused psoriasis typical morphological changes in the HEM or modulated the expression of differentiation-associated genes in these cultures (Fig. 3). In addition to the induction of diverse chemokines, TNF-α at least showed some amplifying action on the acanthosis-inducing effect of IL-22, which may be due to its enhancing effect on the expression of the IL-22 receptor and signal transduction elements (Fig. 5). IL-20 did provoke changes in the HEM that were very similar to those of IL-22. This is in line with the previously demonstrated skin phenotype of IL-20 TG mice [17], which is very similar to that of the IL-22 TG mice described here. In our study on HEM, the IL-20 effects on the culture morphology and the expression of differentiation-associated genes were clearly less pronounced than those by IL-22 (Fig. 6).
All these data clearly show that different cytokines exert very different effects on the epidermis and, therefore, should play different roles in the pathogenesis of psoriasis. We hypothesize that the development of psoriatic lesions occurs in at least two different stages: a proximal stage in which immune cells infiltrate the skin (Fig. 8a) and a distal stage in which the psoriasis typical epidermal (keratinocyte) alterations form (Fig. 8b). In the proximal stage, by inducing Th1, Th17, macrophage, DC, and neutrophilic granulocyte-attracting chemokines, TNF-α may initiate the infiltration process. The T-cell mediators IFN-γ and IL-17 may also be important in the proximal stage of psoriasis pathogenesis. In fact, IFN-γ causes Th1-cell infiltration and activation of antigen-presenting cells, and IL-17 is a strong inducer of neutrophilic granulocyte-attracting chemokines and may therefore play a major role in the development of the psoriasis typical Munro’s micro-abscesses. However, our data imply that IL-22 and IL-20, but not IFN-γ or IL-17, are the mediators responsible for the distal stage of the pathogenic cascade by directly causing the keratinocyte alterations. Whereas in the psoriatic lesion IL-22 is produced by infiltrated Th1 and Th17 cells, IL-20 is derived from activated keratinocytes and monocytic immune cells.
Suggested roles of the different cytokines in the pathogenesis of psoriasis. a Regulation of chemokine production. b Regulation of keratinocyte terminal differentiation. For details, refer to the “Discussion” section. Th1 Th1 cells, Th17 Th17 cells, Mo monocytes, DCs dendritic cells, N neutrophilic granulocytes
The massive thickness of the psoriatic epidermis is probably not only the result of the impaired keratinocyte terminal differentiation in the upper epidermis layers but may also be due to the hyperproliferation of basal layer keratinocytes [16]. We hypothesize that the IL-22 and IL-20-induced acanthosis is basically only the result of the observed impairment of the terminal differentiation of the keratinocytes. The keratinocyte terminal differentiation is the prerequisite for the generation of corneocytes from living keratinocytes and is therefore the basis of the formation of the desquaming cornified layer [41]. This process takes place in the granular layer of the epidermis. It includes the dissolution of the cell organelles, the formation of insoluble agglomerations that replace the cytoplasmic membrane and that are generated from K1 and K10 filaments and other proteins such as filaggrin, involucrin, loricrin, small proline-rich proteins, and LCE proteins by transglutaminase-based cross-linking, as well as the formation of corneodesmosomes between formed corneocytes. This process is strongly disrupted in psoriatic lesions. IL-22 and IL-20 inhibited the expression of several differentiation-associated genes in keratinocytes (K1, K10, profilaggrin, involucrin, loricrin, LCE1B, the corneodesmosomal protein DSC1, the corneodesmosomal protein cleaving enzyme KLK7, and the transglutaminase cofactor CALML5) [13, 14], and Figs. 3 and 6). These effects were not associated with changes in pro- or anti-apoptotic protein expression (Fig. 3f). Interestingly, IL-22 and IL-20 simultaneously had a slight enhancing effect on the K16 expression (Fig. 6c), a marker of the activated or regenerative state of keratinocytes and known to be upregulated in psoriatic lesions. As shown by the absence of increased Ki67 expression in HEM (Fig. 6) and the absence of increased DNA synthesis in conventional keratinocyte culture [11], IL-22/IL-20 do not appear to have a major effect on the keratinocyte proliferation. Further studies are necessary to identify the mediator responsible for the keratinocyte hyperproliferation in psoriatic lesions.
Very recently, another study about IL-22, IFN-γ, and IL-17 effects on human keratinocytes was published [42]. Nograles et al. performed microarray analyses of correspondingly treated conventional (two-dimensional) cultures of keratinocytes. This study confirmed our results regarding IL-22 and IFN-γ from 2006 using a similar experimental system and showing that IL-22 regulated the expression of anti-bacterial proteins and a few genes involved in keratinocyte mobility and terminal differentiation, whereas IFN-γ favored the expression of MHC pathway molecules, adhesion molecules, cytokines, some chemokines, and their receptors [13]. Similar to our results presented in Fig. 4, Nograles et al. additionally found that IL-17 induced neutrophilic granulocyte-attracting chemokines and CCL20 and demonstrated enhanced expression of these mediators in lesional skin compared to non-lesional skin from psoriasis patients. Interestingly, the authors did not observe any IL-22-induced chemokines in conventional cultures of keratinocytes. This is in contrast to our current results from HEM (Fig. 4), but in line with our previous study using conventional cultures of keratinocytes [13]. This difference seems to be related to the lacking stratification of the cells in the conventional cultures rather than to the use of neonatal (contained in HEM) versus adult (used in the conventional cultures) sources of keratinocytes.
It should be noted that there actually seem to be some differences between human adult and neonatal keratinocytes, as presented by a recent study [43]. Tjabringa et al. compared full-thickness human skin equivalents generated with either adult (abdominal) or neonatal (foreskin) keratinocytes. The authors found that the cultures derived from adult keratinocytes displayed the correct morphological and molecular characteristics of normal skin and proposed that these cultures may be an accurate model to study normal skin. Due to the presence of psoriasis-associated features like SKALP/elafin expression in skin equivalents derived from neonatal keratinocytes, this model, however, may be mimicking psoriatic skin to some extent. In fact, three-dimensional epidermis composed of stratified human keratinocytes derived from neonatal foreskins is a system used very often to study cytokine-induced changes in keratinocytes to better understand the pathogenesis of psoriasis [14, 15]. No matter what the source of the keratinocytes is, in our opinion, the use of stratified (HEM or full thickness skin models) and conventional keratinocyte cultures each have advantages: Whereas the number of IL-22-regulated genes and the extent of regulation were higher in stratified keratinocytes compared to conventional keratinocyte cultures, the expressional responses to TNF-α were more observable in the less fragile, conventional cultures.
A further interesting finding demonstrated in our current study is the ability of IL-22 and IL-20, and to a small extent also of TNF-α, to enhance the keratinocyte expression of the signal transduction element STAT3 (Figs. 3, 4, 5, 6, and 7). STAT3 is strongly activated by IL-22 and IL-20 in keratinocytes [13, 17, 44] and the loss of STAT3 expression inhibited the IL-22 effect on some differentiation-regulating genes in these cells (Fig. 7c). The enhancement of STAT3 expression may increase the sensibility of the cells towards a repeated IL-22 or IL-20 exposure and may also constitute a positive feedback regulation of the IL-22 and IL-20 action. Interestingly, lesional skin from psoriasis patients also showed increased STAT3 mRNA expression (Fig. 7b). The high significance of STAT3 activation for the IL-22 effects is also supported by TG mice with keratinocyte-specific expression of a constitutively active STAT3 variant, which also developed a psoriasis-like skin phenotype [24].
Whereas IL-22 uses a receptor complex composed of IL-22R1 and IL-10R2, IL-20 has two receptor complexes, IL-20R1/IL-20R2 and IL-22R1/IL-20R2 [28]. The similar effects of IL-22 and IL-20 in vitro and in specific TG mice (Fig. 6 as well as Fig. 1 and [17]) led us to assume that IL-20 induced the psoriasis-like alterations via the IL-22R1-containing complex. Moreover, IL-20 TG mice lost their psoriasis-like phenotype when they were bred with IL-22R1-deficient mice, but not when they were bred with IL-20R1-deficient mice (H. S. Haugen, personal communication).
On the basis of all currently available data, we suggest that the therapeutic targeting of IL-22R1 would be a promising therapeutic option for psoriasis for the following reasons. First of all, although the psoriasis pathogenesis is a complex process in which many cytokines including IFN-γ and IL-17 play a major role, patients normally visit the physician during the distal stage of the pathogenesis, in which skin alterations are macroscopically visible. At this stage, therapeutic targeting of IL-22R1 should prevent the pathogenic effects of both IL-22 and IL-20. It should be emphasized that healthy human keratinocytes express IL-22R1 and IL-20R1 at an approximately 3:1 ratio, that is further elevated by TNF-α (Fig. 5) and IFN-γ [31], mimicking the situation in psoriasis. In fact, we found a twofold enhanced IL-22R1 mRNA expression in the skin of psoriasis patients compared to samples from control participants. An IL-22R1-targeting approach is also favored by the limited number of tissue cells expressing IL-22R1 [11]. In the skin, keratinocytes appear to be the only cells that clearly express IL-22R1. Most importantly, immune cells do not express IL-22R1; therefore, upon IL-22R1 blocking no major immunosuppression is expected.
In conclusion, we demonstrate here that IL-22 and IL-20, but not IFN-γ, IL-17, or TNF-α, directly caused epidermal alterations typical for psoriasis and that each of these mediators exerts different effects on the epidermis.
References
Griffiths CE, Barker JN (2007) Pathogenesis and clinical features of psoriasis. Lancet 370:263–271
Schon MP, Boehncke WH (2005) Psoriasis. N Engl J Med 352:1899–1912
Kupper TS, Fuhlbrigge RC (2004) Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 4:211–222
Lew W, Bowcock AM, Krueger JG (2004) Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol 25:295–305
Wolk K, Sabat R (2006) Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 17:367–380
Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C (2006) Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16:902–907
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168:5397–5402
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648–651
Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29:958–970
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21:241–254
Wolk K, Witte E, Reineke U, Witte K, Friedrich M, Sterry W, Asadullah K, Volk HD, Sabat R (2005) Is there an interaction between interleukin-10 and interleukin-22. Genes Immun 6:8–18
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36:1309–1323
Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F (2005) IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 174:3695–3702
Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178:2229–2240
Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K (2007) Immunopathogenesis of psoriasis. Exp Dermatol 16:779–798
Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, Haugen H, Jelinek L, Kelly JD, Madden K, Maurer MF, Parrish-Novak J, Prunkard D, Sexson S, Sprecher C, Waggie K, West J, Whitmore TE, Yao L, Kuechle MK, Dale BA, Chandrasekher YA (2001) Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104:9–19
Wolk K, Kunz S, Crompton NE, Volk HD, Sabat R (2003) Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem 278:18030–18036
Dumoutier L, Van Roost E, Colau D, Renauld JC (2000) Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA 97:10144–10149
Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S (2001) Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem 276:2725–2732
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275:31335–31339
Asarch A, Barak O, Loo DS, Gottlieb AB (2008) Th17 cells: a new paradigm for cutaneous inflammation. J Dermatolog Treat 19(5):259–266
Haider AS, Lowes MA, Suarez-Farinas M, Zaba LC, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG (2008) Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol 180:1913–1920
Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J (2005) Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 11:43–49
Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J (1999) Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J 18:4657–4668
Kleyn CE, Griffiths CE (2006) Infliximab for the treatment of psoriasis. Expert Opin Biol Ther 6:797–805
Philipp S, Wolk K, Kreutzer S, Wallace E, Ludwig N, Roewert J, Hoflich C, Volk HD, Sterry W, Sabat R (2006) The evaluation of psoriasis therapy with biologics leads to a revision of the current view of the pathogenesis of this disorder. Expert Opin Ther Targets 10:817–831
Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC (2001) Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol 167:3545–3549
Otkjaer K, Kragballe K, Funding AT, Clausen JT, Noerby PL, Steiniche T, Iversen L (2005) The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol 153:911–918
Romer J, Hasselager E, Norby PL, Steiniche T, Thorn Clausen J, Kragballe K (2003) Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J Invest Dermatol 121:1306–1311
Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R (2006) Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 15:991–1004
Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA (2008) IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 118:597–607
Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183:2593–2603
Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S (2007) T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med 204:41–47
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177:566–573
Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK (2006) Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med 203:2785–2791
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682
Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK (2006) Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis 12:382–388
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646
Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, Dubois B (2006) Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24:191–201
Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6:328–340
Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, Pensabene C, Coats I, Novitskaya I, Lowes MA, Krueger JG (2008) Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 159:1092–1102
Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J (2008) Development and validation of human psoriatic skin equivalents. Am J Pathol 173:815–823
Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, Thilo J, Asadullah K, Sterry W, Volk HD, Sabat R (2008) Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol 83:1181–1193
Acknowledgments
The authors would like to acknowledge B. Ketel, A. Buss, and Wendy Curtis for excellent technical assistance and E. Wallace for accurately proofreading the manuscript. Prof. J. Lademann is acknowledged for placing the Olympus microscope at our disposal. We also thank the German Ministry of Education and Research for the generous support.
Disclosure
H. S. Haugen, W. Xu, K. Waggie, and M. Anderson are stockholders of ZymoGenetics. Inc. K. Wolk was consultant for Merck Serono S. A. until July 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerstin Wolk and Harald S. Haugen equally contributed to this work.
Rights and permissions
About this article
Cite this article
Wolk, K., Haugen, H.S., Xu, W. et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-γ are not. J Mol Med 87, 523–536 (2009). https://doi.org/10.1007/s00109-009-0457-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-009-0457-0