Abstract
A thermo-sensitive biocompatible amphiphilic material, N-phthaloylchitosan-g-poly(N-vinylcaprolactam) which could self-assemble into spherical micelles with good stability in aqueous medium, was synthesized. Meloxicam (MLX), a strongly hydrophobic pain killer for ankylosing spondylitis, was encapsulated into the hydrophobic cores of the micelles successfully as a model drug. The micelles showed excellent stability after MLX loading. Furthermore, the prepared micelles presented obvious thermo-sensitivity with lower critical solution temperature around 32 °C, where the MLX release rate reduced obviously when the temperature increased above it. This drug delivery system could achieve long time drug release under body temperature. The obtained biosurfactant should be an ideal candidate for applications in the development of long-lasting drug delivery systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease which affects both sexes, usually starting at the 20–30 years old patients [1]. The patients suffering from AS always carry a heavy burden of disease, such as inflammatory pain, poor posture, and ankyloses [2]. Nevertheless, there are few studies on AS up to now, no anti-rheumatic drugs are proved clearly to be effective drugs for reducing the pains generated by AS. As a result, patients with AS have few treatment options, their life qualities are reduced significantly [3]. A lot of studies demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs), such as meloxicam (MLX), presented positive effects on AS [2, 4]. However, most NSAIDs are strongly hydrophobic drugs and have gastrointestinal and cardiovascular toxicity [5, 6]. Long-lasting drug delivery system will decrease the excess drug intake more or less which is conducive to reduce the side effects generated by the drugs. Therefore, the development of an advanced drug carrier to achieve the prolonged release of NSAIDs in human body would be a better choice for treating AS in the present situation.
Chitosan, a natural alkaline polysaccharide, is among the most abundant biopolymers in nature. It is composed of randomly distributed β-(1,4)-linked d-glucosamine and N-acetyl-d-glucosamine units and is predominantly extracted from crustaceans, such as crabs and shrimps [7]. Chitosan is currently receiving a great deal of interests for medical and pharmaceutical applications, such as tissue engineering [8–10], wound healing [11–13], bioimaging [14, 15], and drug delivery [16–19]. The main reason for this increasing attention is contributed to its interesting intrinsic properties, such as non-toxic, biocompatible, and biodegradable. Furthermore, positively charged chitosan at the physiological pH is considered to electronically interact with negatively charged cellular membrane, which can increase retention in cells [20–22].
During the past several decades, stimuli-responsive polymers based on chitosan were synthesized to establish an effective delivery system for drugs which could be triggered by a small change from environmental conditions, such as pH value, ionic strength, and temperature [23]. Recently, poly(N-vinylcaprolactam) (PNVCL) received extensive concerns as a kind of thermo-sensitive polymer with a LCST close to physiological temperature [24–30]. Compared to the other typical thermo-sensitive polymer poly(N-isopropylacrylamide) (PNIPAAm), PNVCL has many attractive characteristics such as biocompatible, non-toxic, and outstanding complexation ability with organic compounds [31, 32]. In this study, a novel tailored biocompatible surfactant N-phthaloylchitosan-g-poly(N-vinylcaprolactam) (PHCS-g-PNVCL) was synthesized. The purpose was to develop a biocompatible carrier to achieve the prolonged release of NSAIDs, such as MLX, under body temperature. MLX-loaded PHCS-g-PNVCL micelles were prepared by the precipitation method (Fig. 1). The stability and temperature stimuli release performance of the MLX-loaded micelles were also investigated.
Experimental section
Materials
Chitosan (CS, degree of N-deacetylation = 95 %, Mw = 50 kDa) was purchased from Aoxing Biotechnology Co. Ltd., China, and used as received. Phthalic anhydride was supplied by the First Reagent Factory of Shanghai (China). N-Vinylcaprolactam (NVCL), N,N-azoisobutyronitrile (AIBN), 3-mercaptopropionic acid (MPA), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were obtained from J&K Scientific Ltd., China, and NVCL was recrystallized from hexane for three times before being used. 1-Hydroxybenzotriazole (HOBt) and MLX were provided by TCI, Japan, and used as received. 1,4-Dioxane and N,N-dimethylformamide (DMF) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), and distilled under reduced pressure from calcium hydride. The water used in all experiments was purified by reverse osmosis (Shanghai RO Micro Q). All other reagents and solvents were of analytical grade and used without further purification.
Synthesis of N-phthaloylchitosan (PHCS)
PHCS was prepared by previously reported method [33]. Briefly, CS (5.00 g, 31.01 mmol pyranose units) was reacted with phthalic anhydride (13.80 g, 93.03 mmol) in DMF (150 mL) at 125 °C under nitrogen atmosphere. After 5 h of reaction, the product was precipitated in cold water. The precipitation was collected, washed with methanol at room temperature, and dried under vacuum to obtain 8.16 g pale yellow PHCS. The degree of substitution (DS) of phthaloyl groups within PHCS was determined to be 1.12 by elemental analysis (EA), and the yield of PHCS was calculated to be 80.5 % based on EA.
Synthesis of monocarboxyl-terminated poly(N-vinylcaprolactam) (PNVCL–COOH)
The PNVCL–COOH polymers were synthesized by free radical polymerization in 1,4-dioxane. In brief, NVCL (2.50 g, 17.96 mmol), MPA (0.24 g, 2.26 mmol), and AIBN (0.05 g, 0.30 mmol) were dissolved in 20 mL purified 1,4-dioxane. The solution was degassed by flushing with nitrogen for 30 min. The polymerization was subsequently conducted under constant stirring at 68 °C for 24 h. After the reaction, the product was precipitated with excess amount of diethyl ether and dried under vacuum at 45 °C overnight to obtain 2.03 g (yield 81.2 %) white powder for the subsequent studies.
Synthesis of PHCS-g-PNVCL copolymer
PHCS (0.15 g, about 0.51 mmol phthaloylated pyranose units) was stirred with PNVCL–COOH (0.60 g, 0.54 mmol) in purified DMF solution. HOBt (0.24 g, 1.78 mmol) was added as a catalyst and stirred until clear solution. Then, EDC (0.35 g, 1.78 mmol) was added as a condensing agent, and then reacted at room temperature for 48 h. The obtained mixture was dialyzed against distilled water and washed with excess ethanol to obtain 0.21 g white powder, PHCS-g-PNVCL. The grafting content (GC) was calculated to be 4.0 % by the following method:
where m g and m o were the weight of grafted copolymer PHCS-g-PNVCL and PHCS, respectively.
Preparation of PHCS-g-PNVCL micelles
Polymeric PHCS-g-PNVCL micelles were prepared by the precipitation method. PHCS-g-PNVCL (5 mg) was dissolved in 2 mL DMF and then drop-wised into distilled water under vigorous stirring. The obtained colloid solution was transferred into a dialysis bag (MWCO = 8–12 kDa) and dialyzed against distilled water for 24 h with four changes. The obtained micelles solution was purified by filtration with a 0.45-μm pore-sized microfiltration membrane.
Drug loading
The entrapment of MLX into PHCS-g-PNVCL micelles was carried out by the precipitation method. Briefly, 5 mg copolymer and 2 mg MLX were dissolved into 2 ml DMF under stirring at room temperature until completely dissolved. Then, the mixture DMF solution was drop-wised into water to prepare MLX-loaded micelles.
Determination of MLX encapsulation efficiency and loading content
The drug encapsulation efficiency and loading content of MLX were determined by the previous reported method [34]. Basically, MLX-loaded polymeric micelles were dissolved in a mixture of DMF:H2O (v:v = 9:1). The amount of MLX in polymeric micelles was determined by UV–vis absorption at 361 nm. The MLX encapsulation efficiency (EE) and loading content (LC) were calculated, respectively, to be 14.66 and 51.3 wt% according to the following equations:
where M o is the amount of encapsulated MLX, M i is the total amount of the added MLX, and M m is the amount of the blank micelles.
In vitro drug-release investigation
The temperature-triggered in vitro drug-release property was investigated by the dynamic dialysis method in phosphate buffer solutions (PBS, pH = 7.4) at 25, 30, 35, and 40 °C. Briefly, a certain amount of the prepared MLX-loaded micelle solutions were placed into a dialysis bag (MWCO = 8–12 kDa) and dialyzed against the PBS at the designed temperature intervals. Samples (5 mL) were periodically removed at the designed time intervals, and the same volume of PBS was added simultaneously. The amount of released MLX was analyzed by a UV–vis spectrophotometer at 361 nm which was determined by the calibration curve. The drug-release studies were performed in triplicate for each of the samples.
Characterizations
All infrared spectra were obtained from samples in KBr pellets using a Nicolet 6700 FT-IR spectrophotometer. 1H NMR spectra were measured on a 500 MHz Bruker DRX500 spectrometer at 25 °C. Elemental analysis was performed using a VARIO EL III elemental analyzer. The LCST of the PNVCL–COOH was analyzed by turbidity starting from a temperature range of 25–40 °C. Transmittance (%T) was monitored with a Brinkmann PC 950 colorimeter (420 nm filter), connected to a 2-cm path length optical probe. The dependence of solution turbidity on temperature was obtained by observing the change of turbidity upon the variation of temperature. Critical micelle concentration (CMC) was determined by using a RF-540 spectrophotofluorometer of SHIMADZU with pyrene as the probe (excited at 335 nm). The diameters of the copolymer aggregations were determined by using a Particle Sizer NICOMP 380 ZLS instrument at a scattering angle of 90°. The amount of released MLX was determined spectroscopically at 361 nm (UV-2550, UV–vis spectrophotometer, SHIMADZU). The TEM was performed by using a JEM-2100 electron microscope at 200 kV.
Results and discussion
Synthesis and characterization of the PHCS-g-PNVCL
The PHCS-g-PNVCL copolymer was synthesized by grafting PNVCL–COOH chains onto the backbone of PHCS. PHCS was firstly synthesized by the reaction of phthalic anhydride and Chitosan, and then PNVCL–COOH was given by radical polymerization method using MPA as chain transfer agents. Subsequently, PHCS-g-PNVCL was obtained by grafting PNVCL–COOH chains onto the backbones of PHCS (Scheme 1).
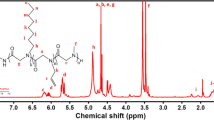
The molecular structures of the synthesized products were characterized by 1H NMR and FT-IR. As shown in Fig. 2a, the 1H NMR spectrum for PNVCL–COOH showed peaks at 4.2 ppm (1H, –NCH–), 3.2 ppm (2H, –NCH2–), 2.3 ppm (2H, –COCH2–), and 1.1–1.9 ppm (6H, –CH2– of the caprolactam ring, and 2H, –CH2– of the PNVCL–COOH main chain). Additionally, two peaks that appeared at 2.8 and 2.95 ppm were attributed to MPA. In the FT-IR spectrum of PNVCL–COOH, the strong peaks at 1631 and 1480 cm−1 were contributed to the amide I band and C–N stretching vibration, while the peaks of monomer NVCL at 1658 cm−1 (C=C) and 3000–3100 cm−1 (C=C and CH=) disappeared. The analysis above indicated that the PNVCL–COOH was synthesized successfully. The molecular weight of PNVCL–COOH (M PNVCL–COOH ) was determined to be 1100 Da by 1H NMR according to the following equation:
where I 4.2ppm and I 2.8ppm were the integration ratios for –NCH– of PNVCL–COOH and the –CH2– of the terminal MPA group, respectively, and M NVCL was the molecular weight of NVCL monomer.
In the 1H NMR spectrum of PHCS (Fig. 2a), two broad peaks at 2.8–5.0 and 7.8–8.0 ppm, corresponding to the hydrogen of chitosan backbone and phthaloyl groups, were observed. As shown in the FT-IR spectrum of PHCS (Fig. 2b), the characteristic peaks at 1776.1 and 1712.5 cm−1 were attributed to the carbonyl anhydride, and the peak at 721.3 cm−1 was referred to the aromatic ring. These results confirmed the molecular structure of PHCS.
New proton peaks at 1.1–1.9 and 3.1–3.2 ppm were appeared in the 1H NMR spectrum of PHCS-g-PNVCL as shown in Fig. 2a, which were attributed to the caprolactam ring and –SCH2CH2– of the grafted PNVCL–COOH chain. Compared to PHCS, the FT-IR spectrum of PHCS-g-PNVCL (Fig. 2b) showed new absorption peaks at 1186.2 and 1089.5 cm−1, which belonged to the dissymmetrical and symmetry stretching vibration peaks of the ester linker, respectively. The absorbance at 1634.5 and 1643.5 cm−1 was attributed to the carbonyl and –SCH2CH2– group, respectively. The 1H NMR and FT-IR analysis of PHCS-g-PNVCL indicated that the PNVCL–COOH chains were grafted onto the PHCS backbone successfully.
LCST determination
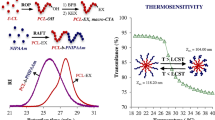
The synthesized PNVCL–COOH is a thermo-sensitive polymer with a LCST. The PNVCL–COOH chains can disperse in water as a dissolved state when the solution temperature is below LCST. However, the chains will change from hydrophilic state into hydrophobic state rapidly as the temperature increases above LCST. The turbidity of the polymer aqueous solution will change correspondingly as the temperature varied. As shown in Fig. 3, when the temperature was below 32 °C, the solution was kept in transparent state. However, once the temperature reached above 32 °C, plenty of white precipitation appeared and the transmittance of the solution decreased dramatically. The LCST of the synthesized PNVCL–COOH in water was determined to be around 32 °C by the turbidity analysis.
Amphiphilic properties of PHCS-g-PNVCL
Due to the existence of both hydrophilic and hydrophobic fragments in the copolymers, the PHCS-g-PNVCL showed amphiphilic properties which could be investigated by fluorescence method using pyrene as the fluorescence probe. Pyrene is poorly soluble and tends to aggregate in the hydrophobic domains of self-assembled structure such as micelles. As a result, the aggregated pyrene would emit strong fluorescence. Among the five peaks in the emission spectrum of pyrene, the intensity ratio of the first peak at 372 nm and the third peak at 385 nm (I 372/I 385) is very sensitive to the polarity of the micro-aqueous media [35, 36]. As shown in Fig. 4, when the concentration of PHCS-g-PNVCL was lower than the CMC, the copolymer dispersed in the aqueous media as single chains, and the intensity ratio (I 372/I 385) remained constant. The ratio decreased dramatically when the concentration increased above CMC. This result was attributed to the encapsulation of pyrene by the formed micelles. The CMC of PHCS-g-PNVCL was determined to be 0.51 mg/L. With such a low CMC, the prepared copolymer micelles could exist stably under highly diluted conditions especially after being intravenously administration [37, 38].
Preparation and characterization of PHCS-g-PNVCL micelles
The hydrophobic phthalic anhydride groups provided the chitosan with a rigid skeleton which rendered the copolymers to self-assemble into micelles in aqueous media and provide better in vitro stability [38]. PHCS-g-PNVCL micelles were prepared by the typical precipitation method. TEM images (Fig. 5a) showed that the self-assembled blank PHCS-g-PNVCL micelles presented in spherical shape, and the diameter of these particles was about 180 nm. The hydrodynamic diameter (D H) and the size distribution (PDI), as shown in Fig. 5c, were determined by DLS to be 210 and 0.282 nm, respectively. The hydrodynamic size measured by DLS was slightly larger than the size determined by TEM at dried condition.
Incorporation of MLX into micelles
MLX was incorporated into the hydrophobic cores of the PHCS-g-PNVCL micelles as a model drug. TEM and DLS were employed to investigate the morphology and size distribution of the drug-loaded micelles (Fig. 5b, d). The TEM images showed that the drug-loaded micelles maintained the spherical shape, and became larger (around 220 nm) than the blank micelles (around 180 nm). Figure 5d shows that the hydrodynamic diameter of micelles (about 280 nm) increased significantly and the PDI (about 0.331) became slightly larger after drug loading as determined by DLS. The particle size determined by TEM was also smaller than that determined by DLS, as discussed above. The increased size of the micelles demonstrated that MLX was loaded into the prepared copolymer micelles successfully.
Evaluation of the thermo-sensitivity of the micelles
Figure 6a shows that the size of the copolymer micelles increased slightly from 210 to 215 nm as the temperature increased from 25 to 32 °C determined by DLS. However, the diameter increased dramatically when the temperature increased above 32 °C. The temperature had a significant impact on the size of the prepared copolymer micelles, which could be attributed to the existence of the thermo-sensitive PNVCL grafts. When the temperature increased above the LCST of PNVCL, the PNVCL transferred from hydrophilic into hydrophobic state. The increased hydrophobicity of PHCS-g-PNVCL leads to the reorganization of micelles. To balance the reduction of hydrophilicity, more polymer surfactant molecules had to join into the micelles, which resulted in the dramatic increase of the micelle size as the temperature increased. Interestingly, the PDI presented no significant change (Fig. 6b), which implied that the size of the copolymer micelles increased uniformly when the temperature increased.
Stability of micelles
The stability was an important parameter for micelles, especially for drug-loaded micelles. As shown in Fig. 6c and d, the size and size distribution were barely changed during 5 weeks, indicative of excellent storage stability of both the prepared blank micelles and drug-loaded micelles.
Temperature-triggered in vitro release of MLX from micelles
In vitro release of MLX from drug-loaded micelles was studied in PBS (pH = 7.4, 0.1 M) medium at different temperatures. As shown in Fig. 7a, the accumulated release pattern of MLX from the PHCS-g-PNVCL micelles was influenced significantly by the environmental temperature. At 25 and 30 °C (<LCST), the drug-release rate and amount were obviously greater than those at 35 and 40 °C (>LCST). About 90 % drug was gradually released in 35 h for the samples at 25 and 30 °C, and then the release rate reached at an equilibrium level. As observed from the samples at 35 and 40 °C, only 70 % drug was released upon the temperature above LCST (Fig. 7a). Obviously, the release rate at the temperature above LCST was lower than that below LCST.
As described above, the grafted PNVCL chains transferred from hydrophilic into hydrophobic state as the temperature increased above the LCST. They accumulated onto the hydrophobic surfaces of the micelles. In the drug-release procedure, the accumulated hydrophobic PNVCL layer reduced the diffusion rate of MLX from the hydrophobic cores to the aqueous medium, which resulted in the decreased release rate of MLX.
Conclusion
Biocompatible thermo-sensitive polymeric surfactant PHCS-g-PNVCL was synthesized by grafting PNVCL–COOH chains onto the backbone of PHCS. They self-assembled into uniform micelles with a low CMC of 0.51 mg/L. MLX as a model NSAIDs was loaded into the hydrophobic cores of the micelles with 51.3 wt% loading capacity. As observed by TEM, the micelles loaded with MLX showed a well-defined spherical shape with a diameter of 220 nm, which were much bigger than the blank micelles (180 nm). The size of the prepared MLX-loaded micelles was constant at room temperature, while increased significantly when the temperature was heated above 32 °C. In vitro release study showed that the release rate of MLX decreased obviously as the temperature increased above 32 °C. It means that these micelles can work as a prolonged drug-release system at body temperature.
References
Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, Sieper J (1998) Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 41:58–67
Dougados M, Gueguen A, Nakache JP, Velicitat P, Veys EM, Zeidler H, Calin A (1999) Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology 38:235–244
Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W et al (2002) Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 359:1187–1193
Rosario-Meléndez R, Yu W, Uhrich KE (2013) Biodegradable polyesters containing ibuprofen and naproxen as pendant groups. Biomacromolecules 14:3542–3548
Luger P, Daneck K, Engel W, Trummlitz G, Wagner K (1996) Structure and physicochemical properties of meloxicam, a new NSAID. Eur J Pharm Sci 4:175–187
Dougados M, Paternotte S, Braun J, Burgos-Vargas R, Maksymowych WP, Sieper J, van der Heijde D (2011) ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 70:249–251
De Souza R, Zahedi P, Allen CJ, Piquette-Miller M (2009) Biocompatibility of injectable chitosan-phospholipid implant systems. Biomaterials 30:3818–3824
Shi W, Nie D, Jin G, Chen W, Xia L, Wu X et al (2012) BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials 33:3119–3126
Gautam S, Chou CF, Dinda AK et al (2014) Fabrication and characterization of PCL/gelatin/chitosan ternary nanofibrous composite scaffold for tissue engineering applications. J Mater Sci 49:1076–1089. doi:10.1016/j.msec.2012.12.015
Zang S, Dong G, Peng B, Xu J, Ma Z, Wang X et al (2014) A comparison of physicochemical properties of sterilized chitosan hydrogel and its applicability in a canine model of periodontal regeneration. Carbohydr Polym 113:240–248
Sudheesh Kumar PT, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG et al (2012) Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–2629
Tran NQ, Joung YK, Lih E, Park KD (2011) In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 12:2872–2880
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H (2010) Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym 82:227–232
Upadhyaya L, Singh J, Agarwal V, Tewari RP (2013) Biomedical applications of carboxymethyl chitosans. Carbohydr Polym 91:452–466
Deng D, Qu L, Zhang J, Ma Y, Gu Y (2013) Quaternary Zn-Ag-In-Se quantum dots for biomedical optical imaging of RGD-modified micelles. ACS Appl Mater Interfaces 5:10858–10865
Kumar MR, Muzzarelli R, Muzzarelli C, Sashiwa H, Domb AJ (2004) Chitosan chemistry and pharmaceutical perspectives. Chem Rev 104:6017–6084
Cheng C, Xia D, Zhang X et al (2015) Biocompatible poly(N-isopropylacrylamide)-g-carboxymethyl chitosan hydrogels as carriers for sustained release of cisplatin. J Mater Sci 50:4914–4925. doi:10.1007/s10853-015-9036-7
Wang H, Zhao P, Liang X, Gong X, Song T, Niu R, Chang J (2010) Folate-PEG coated cationic modified chitosan-cholesterol liposomes for tumor-targeted drug delivery. Biomaterials 31:4129–4138
Park K (2014) Controlled drug delivery systems: past forward and future back. J Control Release 190:3–8
Ping Y, Liu CD, Tang GP, Li JS, Li J, Yang WT, Xu FJ (2010) Functionalization of chitosan via atom transfer radical polymerization for gene delivery. Adv Funct Mater 20:3106–3116
Yang SJ, Lin FH, Tsai KC, Wei MF, Tsai HM, Wong JM, Shieh MJ (2010) Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjugate Chem 21:679–689
Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, Van Blitterswijk C et al (2012) Cationic polymers and their therapeutic potential. Chem Soc Rev 41:7147–7194
Carreira AS, Goncalves FAMM, Mendonca PV, Gil MH, Coelho JFJ (2010) Temperature and pH responsive polymers based on chitosan: applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr Polym 80:618–630
Maeda Y, Nakamura T, Ikeda I (2002) Hydration and phase behavior of poly (N-vinylcaprolactam) and poly (N-vinylpyrrolidone) in water. Macromolecules 35:217–222
Laukkanen A, Valtola L, Winnik FM, Tenhu H (2004) Formation of colloidally stable phase separated poly (N-vinylcaprolactam) in water: a study by dynamic light scattering, microcalorimetry, and pressure perturbation calorimetry. Macromolecules 37:2268–2274
Lau AC, Wu C (1999) Thermally sensitive and biocompatible poly (N-vinylcaprolactam): synthesis and characterization of high molar mass linear chains. Macromolecules 32:581–584
Prabaharan M, Grailer JJ, Steeber DA, Gong S (2008) Stimuli-Responsive chitosan-graft-poly (N-vinylcaprolactam) as a promising material for controlled hydrophobic drug delivery. Macromol Biosci 8:843–851
Hurtgen M, Liu J, Debuigne A, Jerome C, Detrembleur C (2012) Synthesis of thermo-responsive poly (N-vinylcaprolactam)-containing block copolymers by cobalt-mediated radical polymerization. J Polym Sci Polym Chem 50:400–408
Cavus S, Cakal E (2012) Synthesis and characterization of novel poly (N-vinylcaprolactam-co-itaconic acid) gels and analysis of pH and temperature Sensitivity. Ind Eng Chem Res 51:1218–1226
Cakal E, Cavus S (2010) Novel poly (N-vinylcaprolactam-co-2-(diethylamino) ethyl methacrylate) gels: characterization and detailed Investigation on their stimuli-sensitive behaviors and network structure. Ind Eng Chem Res 49:11741–11751
Moshaverinia A, Roohpour N, Darr JA, Rehman IU (2009) Synthesis and characterization of a novel N-vinylcaprolactam-containing acrylic acid terpolymer for applications in glass-ionomer dental cements. Acta Biomater 5:2101–2108
Boyko V, Pich A, Lu Y, Richter S, Arndt KF, Adler HJP (2003) Thermo-sensitive poly (N-vinylcaprolactam-co-acetoacetoxyethyl methacrylate) microgels: 1-synthesis and characterization. Polymer 44:7821–7827
Kurita K, Ikeda H, Yoshida Y, Shimojoh M, Harata M (2002) Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: facile preparation of a precursor useful for chemical modifications. Biomacromolecules 3:1–4
Opanasopit P, Ngawhirunpat T, Chaidedgumjorn A, Rojanarata T, Apirakaramwong A, Phongying S et al (2006) Incorporation of camptothecin into N-phthaloyl chitosan-g-mPEG self-assembly micellar system. Eur J Pharm Biopharm 64:269–276
Wang Y, Liu Y, Liu Y, Wang Y, Wu J, Li R et al (2014) pH-sensitive pullulan-based nanoparticles for intracellular drug delivery. Polym Chem 5:423–432
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic and intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Oh KT, Lee ES (2008) Cancer-associated pH-responsive tetracopolymeric micelles composed of poly (ethylene glycol)-b-poly (l-histidine)-b-poly (L-lactic acid)-b-poly (ethylene glycol). Polym Adv Technol 19:1907–1913
Liu P, Shi B, Yue C, Gao G, Li P, Yi H et al (2013) Dextran-based redox-responsive doxorubicin prodrug micelles for overcoming multidrug resistance. Polym Chem 4:5793–5799
Acknowledgements
We gratefully acknowledge NSFC Grants 51403062, 51273063, and 20774030, the Fundamental Research Funds for the Central Universities, the higher school specialized research fund for the doctoral program (20110074110003), China Postdoctoral Science Foundation (2013M541485), 111 Project Grant (B08021), and Firmenich Aromatics (China) Co., Ltd for the support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, J., Han, H. et al. Self-assembled micelles of N-phthaloylchitosan-g-poly (N-vinylcaprolactam) for temperature-triggered non-steroidal anti-inflammatory drug delivery. J Mater Sci 51, 1591–1599 (2016). https://doi.org/10.1007/s10853-015-9482-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9482-2