Abstract
Purpose
Recently, new catheter technologies have been developed for atrial fibrillation (AF) ablation. We investigate the diagnostic accuracy of a circular mapping and pulmonary vein ablation catheter (PVAC) compared with a standard circular mapping catheter (Orbiter) and the influence of filter settings on signal quality.
Methods
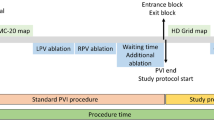
After reconstruction of the left atrium by three-dimensional atriography, baseline PV potentials (PVP) were recorded consecutively with PVAC and Orbiter in 20 patients with paroxysmal AF. PVPs were compared and attributed to predefined anatomical PV segments. Ablation was performed in 80 PVs using the PVAC. If isolation of the PVs was assumed, signal assessment of each PV was repeated with the Orbiter. If residual PV potentials could be uncovered, different filter settings were tested to improve mapping quality of the PVAC. Ablation was continued until complete PV isolation (PVI) was confirmed with the Orbiter.
Results
Baseline mapping demonstrated a good correlation between the Orbiter and PVAC. Mapping accuracy using the PVAC for mapping and ablation was 94% (74 of 79 PVs). Additional mapping with the Orbiter improved the PV isolation rate to 99%. Adjustment of filter settings failed to improve quality of the PV signals compared with standard filter settings.
Conclusions
Using the PVAC as a stand-alone strategy for mapping and ablation, one should be aware that in some cases, different signal morphology mimics PVI isolation. Adjustment of filter settings failed to improve signal quality. The use of an additional mapping catheter is recommended to become familiar with the particular signal morphology during the first PVAC cases or whenever there is a doubt about successful isolation of the pulmonary veins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice. It is associated with a significantly increased risk of thromboembolism, decreased cardiac output, and is often poorly tolerated [1]. The pulmonary veins (PV) are the most important source of ectopic activity for initiation and maintenance of paroxysmal AF [2], and isolation of PVs has become an effective method for curing patients from AF [2, 3]. During the last decade, different strategies for PV isolation have been established, and all of these techniques utilize a standard ablation catheter and PV isolation was achieved by a point-by-point ablation approach [4]. These techniques were effective but are time-consuming and require a high degree of operator skill. With the increasing demand for AF ablation procedures, new technologies with the ability of creating continuous linear lesions for antral PV isolation, so-called single shot devices, have been developed. A recently introduced ablation device is the pulmonary vein ablation catheter (PVAC™, Medtronic, Minneapolis, MN, USA). This novel 10-pole, circular catheter is used in combination with a multichannel, duty-cycled radio frequency (RF) generator (GENius, Medtronic, Minneapolis, MN, USA) for mapping and ablation all through a single catheter. In multiple clinical studies, the feasibility, efficacy, and safety of the PVAC have been demonstrated [5–8].
There are numerous challenges inherent in engineering a multipolar catheter for mapping and ablating PV potentials because the electrode shape and size has to be modified to accommodate the unique bipolar and unipolar energy delivery modes, in comparison to a standard multipolar catheter solely used for mapping, which in turn may result in an alteration of signal quality and accuracy.
Therefore, we investigate the mapping accuracy, signal quality, and the influence of filter settings using the PVAC catheter in comparison to a standard circular mapping catheter.
2 Methods
2.1 Study group
This study included 20 consecutive patients with highly symptomatic and drug refractory paroxysmal atrial fibrillation, who presented for pulmonary vein isolation. All patients included in this study had a history of recurrent arrhythmias for at least 6 months and were resistant to antiarrhythmic drug therapy. All patients provided informed, written consent before the study.
2.2 Catheter characteristics and generator
The PVAC™ catheter and the GENius™ multichannel RF generator have been described previously [5]. In brief, the PVAC catheter is a 9-F, over-the-wire, circular, decapolar mapping, and ablation catheter. The circular portion can be effectively manipulated to increase or decrease the diameter from 20 to 25 mm. Each platinum electrode (3 mm long, 1.5 mm outer diameter, 3 mm spacing) has a thermocouple under the surface on the anterior side, which directly contacts the endocardium.
The RF generator has five preset energy settings: bipolar, unipolar, and three ratios of bipolar/unipolar energy—4:1, 2:1, and 1:1. During ablation, each electrode is temperature-controlled and power-limited via its one dedicated generator, and a software algorithm modulates the power to reach the predefined target temperature. This prevents electrodes from overheating and temperature over-shoot. Power is limited to a maximum of 8 W per electrode when using 4:1 power setting or 10 W in all other settings.
The Orbiter PV catheter (Bard Electrophysiology) is a 14-electrode, 7.5-F circular mapping catheter with a variable loop design, which allows adjusting the diameter from 14.5 up to 25 mm. Each electrode has a 0.75-mm width and a 5-mm spacing (Table 1). The Orbiter was used with standard filter settings.
2.3 EP study
Electrophysiological studies and PV isolation were performed with patients in a fasting state. A pre-procedural transesophageal echocardiogram was performed in all patients before the procedure to rule out the presence of intracardiac thrombi. All patients received general anesthesia. Vascular access was obtained through the right femoral vein. After single transseptal puncture, systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time of ≥300 s.
2.4 Three-dimensional atriography for guiding PV isolation
Once access to the left atrium was gained, a rotational angiography during adenosine-induced ventricular asystole was performed. The left atrium and PVs were reconstructed by three-dimensional atriography (3-D-ATG) using specialized software (EP navigator, Philips Medical Systems). Three-dimensional atriography was used as a single navigation tool for guiding PV isolation.
2.5 Comparison of baseline PV potentials
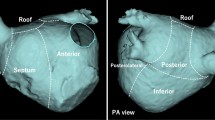
For evaluation of PVAC mapping accuracy, the signals from the Orbiter catheter were used as reference and baseline gold standard. Baseline PV potentials were first recorded with the Orbiter catheter, and the position of the catheter was carefully documented by 3-D-ATG as reference for maneuvering the PVAC catheter to a mapping position comparable to the Orbiter. To compare the recorded PV potentials with the PVAC, the pulmonary vein ostia were subdivided in four anatomical aspects: anterior superior, anterior inferior, posterior inferior, and posterior superior (Fig. 1). Templates of PV potentials with their corresponding fluoroscopic position were carefully annotated in the Bard EP recording system.
The Orbiter was then removed and the PVAC was advanced into the left atrium and was initially positioned in the antral region of the PV where signals were recorded during sinus rhythm. For precise signal comparison, the PVAC was then positioned within the PV to most closely match the position of the previously used Orbiter catheter, and the respective signals were then annotated in our Bard EP recording system. PVPs were recorded, paying special attention to their respective anatomic location within the PV and correlated to the Orbiter recordings.
For baseline recordings, the following filter settings, recommended by the company, were used for the PVAC: amplitude 5 mV, high pass 100 Hz, and low pass 500 Hz. After recording the potentials inside the PV, the PVAC catheter was repositioned to a more antral site which corresponds with the later ablation site (Fig. 2).
2.6 Ablation procedure
After obtaining baseline recordings, antral ablation of the PV was performed using the PVAC. RF energy was usually delivered for 60 s per application with a target temperature of 60°C. Ablation was always started with 4:1 bipolar/unipolar energy setting. Whenever sufficient tissue contact could be achieved with the PVAC, complete semi-circular ablation over all electrodes was given. If PVPs persisted post-ablation, only then the energy ratio was changed to 2:1 to achieve more lesion depth. After each RF application, the PVAC catheter was placed deeper within each PV to map and compare recordings with the baseline templates and to assess the presence or absence of PV potentials. PVAC ablation was continued until isolation of each PV was presumed.
2.7 Post-ablation comparison of PV potentials and differential pacing maneuvers
Upon isolating all four PVs, the PVAC was maneuvered to the initial baseline recording position in every vein. Signal assessment was performed in sinus rhythm in all patients. Differential pacing was performed to differentiate far-field signals from residual PV potentials using an octapolar catheter positioned in the coronary sinus (distal CS for left PVs, proximal CS for right PVs) and subsequently in the left atrial appendage for the left PVs and periostial for the right PVs. Far-field signals in the right superior pulmonary vein (RSPV) were also differentiated by pacing from the superior caval vein.
If isolation of the PVs was evident with the standard filter setting, the PVAC catheter was removed and the Orbiter catheter was reintroduced into the left atrium (LA). Again signal assessment of each PV was conducted during sinus rhythm and through differential pacing maneuvers. If residual PV potentials were uncovered with the Orbiter, the PVAC was reinserted at the same position documented by 3-D-ATG and filter settings were subsequently readjusted.
2.8 Adjustment of filter settings
The standard filter settings for PVAC procedures, which are recommended by the company, are high pass of 100 Hz and low pass of 500 Hz, which has shown in preclinical settings an optimal signal-to-noise ratio. Due to the electrode shape and surface which has to be smaller for proper current density when delivering bi- and unipolar energy for mapping and ablation with a single catheter, the PVAC may have some limitations detecting very low amplitude PV signals. In this study, we tried to investigate if there is a benefit in optimizing the filter settings to uncover minute or residual signals with the PVAC electrodes. Therefore, filter settings were changed from the standard configuration to four various settings (A, B, C, D) and if there were residual signals present with the Orbiter catheter which could not be detected with the PVAC (Table 2). If PV potentials were revealed using the modified filter settings, ablation was continued with the PVAC until all PV potentials were eliminated. If no PV potentials were recorded with the different filter setting, ablation was continued using the Orbiter alternately as signal reference for PV isolation. In all cases, isolation of the pulmonary veins was finally confirmed with the Orbiter.
2.9 Statistical analysis
Data are presented as mean ± standard deviation and percentages.
3 Results
3.1 Study group and imaging
Mapping and ablation was performed in 20 patients (12 males, eight females). The mean age of the study group was 60 ± 8 years (range 48–69 years). All patients were treated unsuccessfully with at least two antiarrhythmic drugs including classes I and III. Mean left atrial size was 42.4 ± 4.6 mm. Mean fractional shortening measured by transthoracic echocardiography was 39 ± 6%. Structural heart disease was present in five patients (coronary artery disease in two patients, left ventricular hypertrophy in three patients). Mild mitral insufficiency was present in six patients. No PV anomalies have been detected.
3.2 Ablation procedure
Total procedure time from vascular access to removal of sheaths was 119 ± 18 min. Mean fluoroscopy time was 35 ± 11 min. The mean RF application time for successful isolation of all PVs was 36.4 ± 11.3 min, in detail: left superior pulmonary vein (LSPV) 10 ± 5.8, left inferior pulmonary vein (LIPV) 10.2 ± 3.7, RSPV 10.1 ± 4.9, and right inferior pulmonary vein (RIPV) 6.1 ± 3.6 min, respectively. The mean number of PVAC applications for complete PV isolation in this study was 24 ± 10.6 for 4:1 energy ratio and 12 ± 9 for 2:1 ratio.
Using the PVAC, complete isolation could be achieved in 79 of 80 (99%) pulmonary veins at the end of the procedure, and both common ostia could be isolated successfully. One right inferior PV could not be isolated due to an acute vein take off angle which let the difficult catheter placement and repeated dislodgement. Therefore, an additional 4-mm irrigated tip catheter was used to complete PV isolation.
3.3 Baseline recordings
Baseline recordings with the Orbiter catheter could be performed in all veins. Visual comparison of PV signal quality (amplitude and morphology) showed only minor differences (Fig. 3(a), (b)). The PVAC has a tendency to record PV signals with lower amplitude, appearing less detailed and slightly smoothened compared to the Orbiter. Baseline mapping showed a good correlation between the Orbiter and PVAC with regard to the anatomical positional accuracy of recorded PVPs. A recording of a PVP with the PVAC, which was not recorded with the Orbiter, was found in two cases (PVAC superior). A recording of a PVP with the Orbiter, which was not recorded with the PVAC, was found in four cases (Orbiter superior). A recording of PVP at the same anatomical location with the PVAC and Orbiter was found in the residual recordings.
3.4 Recordings after ablation
Primary successful PV isolation with the PVAC and confirmed via the Orbiter was achieved in 74 of 79 (94%) PVs (Fig. 3(c), (d)). After PV isolation using the PVAC for mapping and ablation, in 6% (5 of 79 PVs), residual PV potentials could be revealed with the Orbiter, which were not detected due to the absence of signals or atypical PVP signals with the PVAC using standard filter settings. This results in a calculative mapping accuracy of 94% for the PVAC. There were no false-positive results using PVAC mapping (i.e., residual PV signals using PVAC mapping not detectable with the Orbiter). In detail, residual PV potentials could be unveiled with the Orbiter in 1 of 20 (5%) for LSPV, 2 of 20 (10%) for LIPV, and 2 of 20 (10%) for RIPV. Knowing that residual post-ablation PV potentials with a different morphology can be uncovered using PVAC than with standard circular mapping catheters, we could ablate another three PVs successfully without the need of an additional mapping catheter. Additionally, two PVs were finally isolated by using the Orbiter mapping catheter as signal reference.
3.5 Signal morphology and filter settings
In five PVs, remaining PV potentials were revealed with the Orbiter catheter. These tiny potentials were not able to be visualized with the PVAC alone or may have been overlooked due to signal morphology (Fig. 4). After reinserting the PVAC and maneuvering the catheter close to the position of the Orbiter, filter settings were modified as described above. The different filter setting (A, B, C, D) failed to improve quality of the PV signals and did not change signal morphology achieving a more PV-like potential (Fig. 5).
Post-ablation isolation of the LSPV was assumed using the PVAC without differential pacing (a). Differential pacing from the distal CS (b) reveals a blunt signal with two components but without a sharp PV signal separating from the LA signal, which can be observed commonly using the PVAC. A residual sharp PV potential could be uncovered with the Orbiter in lead 5/6 (c), which was also clearly separated using differential pacing (d). Surface leads in (c) and (d) are different from (a) and (b). AFF atrial far field, PVP pulmonary vein potential
Different filter settings after reinserting the PVAC and maneuvering the catheter close to the previous position of the Orbiter. Further adjustment of filter setting to (a) high pass of 0.05 Hz and low pass of 500 Hz, (b) high pass of 0.5 Hz and low pass of 500 Hz, (c) high pass of 10 Hz and low pass of 500 Hz, and (e) high pass of 30 Hz and low pass of 1,000 Hz failed to improve quality of the PV signals achieving a more PV-like potential. Sharpest deflection of the signals was observed with the standard filter setting: high pass of 100 Hz and low pass of 500 Hz (d)
4 Discussion
Isolation of PVs has turned out to be a cornerstone therapy in curing patients from AF [2–4]. Up to now, most EP labs still use a circular mapping catheter and a standard 4-mm-tip ablation catheter; however, PV isolation is often a time-consuming point-by-point ablation approach. With the increasing demand of AF ablation procedures, new catheter technologies were needed to make AF ablation faster, easier, and safer; therefore, “single shot devices” such as the PVAC have been developed to overcome this hurdle. In this study, we investigated the diagnostic accuracy of the PVAC in comparison with a standard circular mapping catheter and furthermore the influence of filter settings on signal quality. Baseline mapping demonstrated a good correlation between Orbiter and PVAC signals with respect to anatomical location and morphology. Calculative mapping accuracy of the PVAC catheter was 94%, and additional mapping via a conventional circular mapping catheter improved the mapping accuracy up to 99%. Adjustment of the filter settings failed to improve signal quality and morphology compared to the benchmark Orbiter recordings used for this mapping study.
Utilizing the PVAC for AF ablation is both feasible and safe [5–12]. Recent studies using the PVAC for mapping and ablation could show follow-up results comparable to conventional, point-by-point ablation studies [6–8, 11] but with shorter procedure time and reduced X-ray exposure [13]. However, a detailed analysis of the PVAC mapping accuracy has not been validated extensively. The unique electrode shape and size of the PVAC is different compared to a standard circular mapping catheter, which may influence the familiar PV signal morphology and amplitude. This may lead to difficulty in interpretation between PVPs and far-field signals, which are the essential information in any AF ablation procedure [14–17].
Duytschaever et al. compared PVAC electrograms with a decapolar circular mapping catheter (Optima, St. Jude Medical) where they found the isolation rate increased from 73% to 93% of the patients when adding a circular mapping catheter to the PVAC procedure [18]. We provide supplemental data demonstrating enhanced mapping accuracy for the PVAC. This could be improved by the addition of a circular mapping device with 14 electrodes.
When using the PVAC as a stand-alone ablation device, the operator has to concentrate on minute potentials which may be ignored due to different signal morphology as typically recorded with standard circular mapping catheters. Ignoring these signals will lead to mapping failure and reduced PV isolation rates. Importantly, compared to Duytschaever’s study group, the baseline and mapping accuracy was not superior employing a 3-D-ATG guided mapping strategy and using a 14-electrode catheter as reference.
Duytschaever et al. reported a 100% diagnostic accuracy in all PVs except the RIPV (81%), which they attributed to difficult positioning of the PVAC [18]. This is different from our data demonstrating false-negative results in different PVs. Positioning of the PVAC in the RIPV might have been facilitated by rotational angiography and 3-D-ATG in our study group.
Therefore, to reduce the noise generated with the low-frequency signals, the usual filtering frequency for intracardiac signals is between 30 and 40 Hz for the high-pass and 500 Hz for the low-pass filters. Although appropriately placed electrode catheters will record desired signals at any filtering frequency, filter settings between 30 and 40 Hz (high pass) and 500 Hz (low pass) are best suited for sharp intracardiac signals. As this will attenuate all parts of the signal below the high-pass and above the low-pass setting, PV-like potentials may have been possibly removed with the standard filter setting. Therefore, we investigated if a stepwise adjustment of the high-pass filter to 0.05 Hz or the low-pass filter up to 1,000 Hz amplifies an attenuated PVP. However, a change of filter settings failed to improve quality of the PV signals achieving a more PV-like potential.
This study has several limitations. First, our study group included only 20 patients; however, this allowed us to evaluate the mapping accuracy of the PVAC in a total of 80 pulmonary veins. Second, the follow-up period was only 3 months. Nevertheless, the primary endpoint of this study was the evaluation of mapping accuracy rather than the assessment of clinical outcome. Finally, obtaining exactly the same mapping position in subsequent recordings with two different catheters may be difficult, even with support of 3-D-ATG. However, this is the only way to compare the mapping accuracy using different catheters subsequently.
In conclusion, we investigated the diagnostic accuracy of the PVAC and the impact of filter settings on signal quality. Baseline mapping showed a good correlation between the Orbiter and PVAC with regard to the anatomical location of recorded PVPs and signal quality. Using the PVAC for mapping and ablation, the mapping accuracy was 94%. This might be due to different signal morphologies, less detailed and slightly smoothened compared to the Orbiter, mimicking successful PV isolation in some veins. Further adjustment of filter settings failed to improve quality of the PV signals to a more PV-like potential. Using the PVAC as a stand-alone strategy for mapping and ablation, one should be aware of a different signal morphology feigning successful isolation in some cases. The use of an additional mapping catheter is recommended when an institution experienced in AF ablation procedures performs the first PVAC cases to become familiar with the particular signal morphology of the PVAC or whenever there is a doubt about successful isolation of the pulmonary veins.
References
Kannel, W. B., & Abbott, R. D. (1982). Epidemiologic features of chronic atrial fibrillation: The Framingham Study. The New England Journal of Medicine, 306, 1018–1022.
Haissaguerre, M., Jais, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine, 339, 659–666.
Pappone, C., Rosanio, S., Oreto, G., Tocchi, M., Gugliotta, F., Vicedomini, G., et al. (2000). Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation, 102, 2619–2628.
Oral, H., Knight, B. P., Tada, H., Ozaydin, M., Chugh, A., Hassan, S., et al. (2002). Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation, 105, 1077–1081.
Fredersdorf, S., Weber, S., Jilek, C., Heinicke, N., von Bary, C., Jungbauer, C., et al. (2009). Safe and rapid isolation of pulmonary veins using a novel circular ablation catheter and duty-cycled RF generator. Journal of Cardiovascular Electrophysiology, 20, 1097–1101.
Boersma, L. V., Wijffels, M. C., Oral, H., Wever, E. F., & Morady, F. (2008). Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm, 5, 1635–1642.
Wieczorek, M., Hoeltgen, R., Brueck, M., Bandorski, D., Akin, E., & Reza, S. A. (2009). Pulmonary vein isolation by duty-cycled bipolar and unipolar antrum ablation using a novel multielectrode ablation catheter system: First clinical results. Journal of Interventional Cardiac Electrophysiology, 27, 23–31.
Scharf, C., Boersma, L., Davies, W., Kanagaratnam, P., Peters, N. S., Paul, V., et al. (2009). Ablation of persistent atrial fibrillation using multielectrode catheters and duty-cycled radiofrequency energy. Journal of the American College of Cardiology, 54, 1450–1456.
Wijffels, M. C., OM, V. A. N., Boersma, L. V., Werneth, R., Kunis, C., Hu, B., et al. (2009). Characterization of in vitro and in vivo lesions made by a novel multichannel ablation generator and a circumlinear decapolar ablation catheter. Journal of Cardiovascular Electrophysiology, 20, 1142–1148.
Ouyang, F., Bansch, D., Ernst, S., Schaumann, A., Hachiya, H., Chen, M., et al. (2004). Complete isolation of left atrium surrounding the pulmonary veins: New insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation, 110, 2090–2096.
Wieczorek, M., Hoeltgen, R., Akin, E., Salili, A. R., Oral, H., & Morady, F. (2009). Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar radiofrequency energy. Journal of Cardiovascular Electrophysiology, 21, 399–405.
Deneke, T., de Groot, J. R., Horlitz, M., Mugge, A., Grewe, P. H., Bunz, K., et al. (2009). Pulmonary vein isolation using a novel decapolar over-the-wire mapping and ablation catheter. Expert Review of Cardiovascular Therapy, 7, 1341–1347.
Bulava, A., Hanis, J., Sitek, D., Osmera, O., Karpianus, D., Snorek, M., et al. (2010). Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multielectrode catheter and point-by-point ablation. Pacing and Clinical Electrophysiology, 33, 1039–1046.
Ouyang, F., Antz, M., Ernst, S., Hachiya, H., Mavrakis, H., Deger, F. T., et al. (2005). Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: Lessons from double Lasso technique. Circulation, 111, 127–135.
Haissaguerre, M., Shah, D. C., Jais, P., Hocini, M., Yamane, T., Deisenhofer, I., et al. (2000). Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation, 102, 2463–2465.
Oral, H., Knight, B. P., Ozaydin, M., Chugh, A., Lai, S. W., Scharf, C., et al. (2002). Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: Feasibility and mechanistic insights. Circulation, 106, 1256–1262.
Haissaguerre, M., Jais, P., Shah, D. C., Garrigue, S., Takahashi, A., Lavergne, T., et al. (2000). Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation, 101, 1409–1417.
Duytschaever, M., Anne, W., Papiashvili, G., Vandekerckhove, Y., & Tavernier, R. (2009). Mapping and isolation of the pulmonary veins using the PVAC catheter. Pacing and Clinical Electrophysiology, 33, 168–178.
Acknowledgment
We like to thank Brian Yunker for his helpful review of the manuscript.
Disclosures
There are no ethical problems related to the material. This study was not supported by industry. Christian von Bary receives grants <5,000 €/year from Medtronic for consultation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Bary, C., Fredersdorf-Hahn, S., Heinicke, N. et al. Comparison of PV signal quality using a novel circular mapping and ablation catheter versus a standard circular mapping catheter. J Interv Card Electrophysiol 31, 131–139 (2011). https://doi.org/10.1007/s10840-011-9546-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-011-9546-4