Abstract
Background
High-density (HD) mapping of the pulmonary vein (PVs) has been hypothesized to improve the detection of conduction gaps in the radiofrequency ablation lesions set after pulmonary vein isolation (PVI) for the treatment of atrial fibrillation (AF). We aimed to compare the incidence of gaps after PVI with a standard 20-pole circumferential mapping catheter (CMC-20) and an HD mapping catheter (HD Grid).
Methods
This prospective study included patients scheduled for high-power short-duration PVI. Acute PVI was defined as an entrance and exit block using the CMC-20 after ≥ 20 min waiting period. The left atrium was then remapped using the HD Grid high-density mapping catheter to identify residual conduction gaps in the PVI lines by voltage and activation criteria. The primary endpoint was the number of gaps identified per patient by the HD Grid catheter.
Results
A total of 20 patients were included (mean age 59.9 ± 10.8 years, 15% female, 70% paroxysmal AF). The new map with the HD Grid identified 6 gaps in 4 patients (20%) or 0.3 ± 0.7 gaps per patient (p = 0.055 when compared to CMC-20). Five gaps (83%) were located at the right PVs. There was no difference in mapping time (CMC-20 12.2 ± 2.6 min vs HD Grid 11.7 ± 3.4 min, p = 0.452); however, the number of points was significantly higher in the HD Grid map (1662.7 ± 366.1 vs 1171.6 ± 313.6, p < 0.001).
Conclusions
HD mapping during AF ablation identified PVI gaps in 1 out of 5 patients. Therefore, HD mapping may have the potential to improve AF ablation success rates in the long term.
Trial registration
ClinicalTrials.gov NCT04850508 on April 20, 2021.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Catheter ablation for atrial fibrillation (AF) has evolved from a specialized, experimental procedure into a common treatment to prevent recurrent, symptomatic AF [1]. The main target remains the electrical isolation of the pulmonary veins (PVI), which requires a complete isolation of the left atrium (LA) from the triggers in the pulmonary veins (PVs) that typically initiate AF episodes. When using point-by-point radiofrequency catheter ablation, PVI is achieved by creating 2 continuous lesion sets, one around each pair of veins. Despite technological advances, the 1-year success rate in large randomized trials of a first radiofrequency catheter ablation in paroxysmal AF was approximately 65%, and about 15% of patients required at least one repeat ablation [2, 3]. During these repeat ablations, a mean of 2.1 ± 1.4 PVs showed reconduction, most frequently in the right and left superior veins [2]. Efforts to improve the success rate of the first procedure have been made by studying different ablation settings, such as high-power short-duration (HPSD), and lesion spacing, to ensure contiguous ablation lesions [4, 5]. Further, it has been hypothesized that high-density (HD) electro-anatomical mapping of the PV ostium might improve the detection of gaps in the radiofrequency lesions set [6].
Currently, the 20-pole circumferential catheter (CMC-20) is considered the standard for mapping the PV ostia during AF ablation. The CMC-20 is designed with 20 electrical poles, grouped 2 by 2 to allow bipolar recordings. HD mapping catheters have a different electrode configuration, such as the Advisor™ HD Grid catheter (HD Grid; Abbott, Minneapolis, MN, USA) which has a total of 16 electrodes configured in a 4 by 4 matrix [7]. With each electrical wavefront being decomposed to 2 orthogonal vectors, every direction of a local wavefront should be detected by these configurations and thus overcoming the limitations of perpendicular bipoles [7]. A direct comparison between the CMC-20 and the HD Grid has not been published. Therefore, we conducted a prospective study to investigate the acute superiority of the HD Grid catheter to identify acute gaps in the ablation lesion set after a standard PVI procedure using the CMC-20 catheter.
2 Methods
2.1 Study design
This single center, single arm, investigator-initiated, prospective study enrolled patients scheduled for atrial fibrillation ablation between October 2021 and April 2022 to compare the acute incidence of gaps after PVI between a CMC-20 and the Advisor™ HD Grid mapping catheter (Abbott, Minneapolis, MN, USA). The study followed ICH Good Clinical Practice guidelines and the Declaration of Helsinki, including approval of the study protocol by the University of Calgary’s Research Ethics Board. Participants provided written informed consent. The study is registered at ClinicalTrials.gov (NCT04850508). The study data are available upon reasonable request direct to the corresponding author.
2.2 Study population
Patients aged ≥ 18 years who were scheduled for a first clinically indicated PVI for symptomatic, paroxysmal or persistent AF were eligible for participation. Exclusion criteria included: prior left-sided catheter or surgical ablation for AF or atypical atrial flutter, New York Heart Association class III or IV, severely reduced left ventricular ejection fraction (≤ 35%), LA diameters ≥ 55 mm on transthoracic echocardiogram, severe pulmonary hypertension, recent myocardial infarction or coronary intervention ≤ 3 months, contraindication to oral anticoagulation therapy, pregnancy, and active enrollment in another interventional clinical study.
2.3 Atrial fibrillation ablation procedure
2.3.1 Preprocedural considerations
Patients on direct oral anticoagulants (DOAC) were instructed to take their last dose 24 h prior to the procedure. Vitamin-K antagonists were not interrupted before the procedure with a target INR of 2.0–2.5. Interruption of antiarrhythmic drugs (AAD) was left to the discretion of the operator. All patients underwent a transthoracic echocardiogram (TTE), resting 12-lead electrocardiogram (ECG), and cardiac magnetic resonance imaging (MRI) scan in the months preceding the AF ablation procedure. The LA morphology was segmented from the cardiac MRI and used during the procedure to guide geometry collection. A transesophageal echocardiography (TEE) for the exclusion of LA appendage thrombus was left to the discretion of the operator.
2.3.2 Procedural considerations
All AF ablations were performed by experienced investigators under general anesthesia. The initial PVI was performed according to institutional standard practice. Ultrasound-guided vascular access was obtained. A steerable decapolar catheter (Inquiry™, Abbott, USA; Polaris X™, Boston Scientific, USA, or Livewire™ Duo-Decapolar, Abbott, USA) was guided from the left femoral vein and inserted into the coronary sinus. In the absence of a patent foramen ovale, a single transseptal puncture was performed using a Swartz™ SL-1™ sheath (Abbott, USA) and a BRK™ transseptal needle (Abbott, USA) with the ablation catheter located at the His and fluoroscopic guidance. The ablation catheter (TactiCath™ Contact Force Sensor Enabled™, Abbott, USA) was guided to the LA through the transseptal puncture. The use of a steerable sheath for the ablation catheter (Agilis™ NxT, Abbott, USA) was left to the discretion of the operator. After the transseptal puncture a heparin bolus was administered, followed by continuous infusion targeting an activated clotting time of 300–350 s.
Next, a 3-dimensional electroanatomic map (EnSite™ Precision™, Abbott, USA) of the LA was performed with a CMC-20 (Lasso™, Biosense Webster, USA). If the patient was in atrial fibrillation, external electrical cardioversion was performed prior to LA mapping. The map was obtained during coronary sinus pacing at a cycle length of 800 ms. A local activation timing (LAT) map and bipolar voltage map were obtained with both bipolar (high‐pass filter 30 Hz, low‐pass filter 300 Hz, noise filter) and unipolar (high‐pass filter 0.5 Hz, lowpass filter 100 Hz, and noise filter) filters switched on. Peak-to-peak bipolar voltage < 0.05 mV and 0.05–0.5 mV were defined as scar and low voltage areas, respectively. Interior projection, exterior projection, and interpolation were set at 5 mm, 5 mm, and 7 mm respectively. Then, point-by-point radiofrequency ablation of each pair of veins was performed using HPSD settings at 50 Watts with a target contact force between 10 and 25 g. Irrigation pump settings were 30 mL/min during ablation and 2 mL/min otherwise. Lesion duration was 8 s on the posterior wall and 10 s elsewhere.
After completion of the circumferential lesion set, acute isolation of each PV was assessed by the entrance and exit block using the CMC-20. Entrance block was confirmed by the absence of local PV potentials inside the PV antra in sinus rhythm and/or from atrial pacing outside of the vein. If a PV was not accessible for the CMC-20 after ablation, the entrance block was assessed by placing the ablation catheter at different positions in this PV. Exit block was confirmed by the absence of LA capture when pacing every pole of the CMC-20 catheter inside the PVs at 10-mA output and 2.0-ms pulse duration. In case of residual conduction, additional ablation was performed, and operators were allowed to use low-power, long-duration settings of 30 Watts with a target force–time-integral (FTI) of 400. The procedural endpoint was PVI as determined by the entrance and exit block in all PVs after a 20-min waiting period. Adenosine to check for dormant conduction was not administered.
2.3.3 Study protocol
The study protocol was initiated upon verification of PVI with the CMC-20 catheter after the mandatory minimal 20-min waiting period. The CMC-20 catheter was exchanged for the HD Grid catheter and a new geometry, LAT and bipolar voltage map of the LA and PVs was made. All EnSite™ Precision™ settings and CS pacing during HD Grid mapping were identical to these of the CMC-20 map. If the PV was not accessible with the HD Grid, the ostium of this vein was extensively mapped. The new map was inspected carefully for the presence of signals of interest, which may reflect a gap. A gap was defined as the presence of residual PV potentials within the ablation lesion set (not necessarily inside the veins) and was confirmed by the presence of exit conduction when pacing the location of interest with the ablation catheter. Near-field and far-field electrograms were discriminated by differential pacing maneuvers, for example pacing the left atrial appendage or superior vena cava. If the presence of a gap was confirmed, additional ablation was performed until PVI was achieved, electrograms of interest were eradicated, and/or pacing within the lesion set no longer captured the left atrium.
2.3.4 Postprocedural care
At the end of the procedure, catheters were removed from the heart and standard procedure protocols were resumed. Protamine was administered, and Z-sutures were used for maintaining hemostasis. All patients underwent a limited TTE to exclude pericardial effusion prior to same-day discharge. All patients were prescribed 1 month of proton pump inhibitors as a precautionary measure against atrio-esophageal fistula. AAD were continued for 6 weeks after the procedure, except amiodarone which was stopped on the day of the procedure. Anticoagulation was restarted on the same day and continued for at least 3 months.
2.4 Data collection
The following data were collected prior to the procedure: clinical characteristics, medical history, current medical treatment, detailed atrial fibrillation history, and the results of cardiac MRI, TTE, and ECG. During the procedure, time intervals were recorded, as presented in the procedural timeline (Fig. 1). Detailed procedural data on ablation lesions, mapping results, and study protocol findings were collected. As subject participation ended at the end of the PVI ablation procedure, this study did not include any follow-up on AF recurrence. The medical record was reviewed 1 month after the procedure to collect significant adverse events.
2.5 Endpoints
The primary objective was to determine the number of gaps identified by the HD Grid catheter after a standard PVI procedure, performed with the CMC-20 catheter. Presence and locations of gaps were recorded during the procedure on a graphical representation of the PVs (Fig. 2). The primary endpoint was the number of gaps per patient identified by the HD Grid catheter after a standard PVI procedure with the CMC-20 catheter and ≥ 20-min waiting time.
Secondary endpoints included the number of patients with ≥ 1 gap, the location of gaps (left versus right PVs), LA mapping time, the number of points used in the map, discrepancies in low voltage areas defined as voltage < 0.50 mV obtained by the CMC-20 and HD Grid catheter during atrial pacing, and the incidence of clinically significant adverse events requiring prolonged hospital stay or emergency department presentation.
2.6 Power calculation
Power calculation was based on Porterfield et al. [8], the only study with a comparison in number of gaps between CMC-20 and HD Grid, who reported a mean number of gaps per patient of 1.44 gaps per patient for the procedure with the CMC-20 catheter and 2.15 gaps per patient with the HD Grid catheter [8]. Given the design of our study, where each patient serves as her/his own control, a sample size of 13 would achieve 84% power to detect a mean paired difference of 0.7 gaps per patient with an estimated standard deviation of paired differences of 0.8 and a significance level (a) of 5% using a two-sided paired t-test. After correction for an expected prevalence of 20% for PV abnormalities, the study required a minimum of 17 patients to be included. Due to the limitations of the available data, such as the absence of direct comparison and standard deviations, the investigators agreed to include a minimum of 20 patients.
2.7 Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median with quartiles (interquartile range, IQR), depending on the result of the Kolmogorov–Smirnov test for normality. Categorical variables are presented as absolute number and percentage. Analysis of paired data, including the primary endpoint, was performed with a paired t-test or a Wilcoxon signed-rank test, if applicable. Comparison of characteristics by the presence of gaps was performed with an unpaired Student t-test or Mann–Whitney U test, if applicable, for continuous variables and Fisher’s exact test for categorical variables. A two-tailed p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Patient population
A total of 21 patients was consented; however, in 1 patient, the study protocol was not performed due to technical issues. Therefore, results are presented for 20 analyzable patients (Table 1). The mean age was 59.9 ± 10.8 years and 3 (15%) were female. Fourteen patients (70%) underwent ablation for paroxysmal AF, 12 (60%) underwent at least 1 electrical cardioversion, and the median CHA2DS2-VASC score was 1.0 (IQR 0.0–2.0). Only 1 patient (5%) was on a vitamin-K antagonist, while the remaining were on a DOAC. Seventeen patients (85%) were on AAD, of whom 3 were prescribed AAD pill-in-the-pocket. The mean LA diameter was 39.9 ± 5.7 mm, and 4 patients (20%) had variant PV anatomy (3 with a common left PV trunk, and 1 patient with a middle right PV).
3.2 Procedural characteristics
The procedural characteristics are presented in Table 2. In 1 patient, the CMC-20 map included only geometry due to recurrent atrial fibrillation within seconds after electrical cardioversion. Additional cavo-tricuspid ablation was performed in 2 patients (10%), and a roof line was performed in 1 patient (5%). These ablations were performed prior to the study protocol during the additional ablation window (Fig. 1). The mean total procedural time was 170.2 ± 24.0 min, and LA dwelling time was 140.7 ± 23.0 min. Performing the study protocol added 17.7 ± 6.5 min.
3.3 Study endpoints
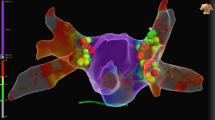
All patients underwent successful PVI. First-pass isolation was achieved in 67.5% of vein pairs (left PVs 75%, right PVs 60%). Additional ablation for early reconnection was performed in 17.5% of vein pairs (left PVs 20%, right PVs 15%). After a median waiting time of 33.5 min (IQR 22.0–45.8), all PVs were isolated without evidence of any gaps when assessed with the CMC-20 catheter. Results are presented in Table 3 and Fig. 2. Remapping with the HD Grid catheter identified a total of 6 gaps in 4 unique patients (20%). This results in 0.3 ± 0.7 gaps per patient with the HD Grid catheter versus 0 gaps with the CMC-20 catheter (p = 0.055). Two gaps were considered PV reconnection with electrograms clearly visible inside the PV, while the remaining 4 gaps showed local electrograms just within the ablation lesion set without any PV potentials visible when positioning the HD Grid catheter in the PV. Five gaps (83%) were located at the right PVs, predominantly located at the septum and carina. Only 1 gap (17%) was located at the posterior carina of the left PV. Post-ablation high-density mapping examples are presented in Fig. 3 and in cases presented as supplement 1, supplement 2, supplement 3, and supplement 4.
Post-ablation high density mapping examples. A Bipolar voltage map of a PVI with no gaps identified. A: Posterior-anterior view of a PVI procedure with no gaps identified on post-ablation HD mapping. B: Left-lateral view. C: Right-anterior view. B Case with gap at the posterior carina of the left PVs. A: Posterior-Anterior view of a bipolar voltage map showing a gap at the posterior carina of the left PVs. The sparkle map is available as supplement 3.A. B: Ablation lesions projected on the surface of the same map resulting in isolation. C: Intracardiac electrograms with the CMC-20 located in the LIPV showing entrance block. D: The HD Grid catheter showed small electrograms at the location of the gap with 2 components (lead C1-D1). First, a low frequency component corresponding to far-field atrial electrograms (white arrow). The second sharp component (red arrow) corresponds to the local electrogram and became later when moving the HD Grid towards the LIPV. E: The HD Grid catheter in the LIPV showing reconnection (red arrow). C Case with 2 gaps visualized on the bipolar voltage map. A: Two separate gaps visible at the right PVs. The first located at the anterior carina, the second gap mid-carina. B: Mid-carina electrograms visualized on the HD Grid catheter, later confirmed with the ablation catheter. C: The 2 gaps required separate ablation lesions
After ablation, the left inferior PVs were not accessible in 2 patients for neither the CMC-20 nor the HD Grid catheter. The right inferior PV was not accessible for the CMC-20 in 1 patient, but the HD Grid could be positioned inside. Patients with gaps did not differ significantly from those without gaps with respect to AF type or baseline characteristics.
The mapping time was not significantly different between the CMC-20 and HD Grid catheter (Table 4, 12.2 ± 2.6 min vs. 11.7 ± 3.4 min, p = 0.452). However, maps with the HD Grid included significantly more points (1662.7 ± 366.1 vs. 1171.6 ± 313.6, p < 0.001).
All identified gaps were isolated again with the first lesion, after which a median of 4 (IQR 3–7) consolidation lesions was made. As all patients had a normal bipolar voltage map with both the CMC-20 and HD Grid catheter, no differences in the presence or extent of low voltage areas were noted. One patient presented to the emergency department with pericarditis 2 days after the procedure. There was no significant pericardial effusion, and symptoms were managed with non-steroidal anti-inflammatory drugs. No other adverse events were noted.
4 Discussion
In this prospective study, where each patient served as her/his own control, mapping with the HD Grid catheter identified residual gaps in 20% of patients after standard PVI was verified with a CMC-20 catheter. The majority of gaps were located at the septal side of the right PVs and in the carina. There was no significant difference in mapping time between the CMC-20 and HD Grid catheter, but the number of points in the map was significantly higher in the HD Grid map.
Porterfield et al. [8] retrospectively compared the performance of 3 diagnostic catheter technologies, including the CMC-20 and HD Grid catheter in 99 AF ablation cases. They showed that the HD Grid detected an average of 49.0% more gaps per patient than the CMC-20 catheter, corresponding to 2.15 gaps per patient for the HD grid and 1.44 gaps per patient by the CMC-20 catheter. With a mean of 0.3 gaps per patient identified by the HD Grid, our study did not reach statistical significance (p = 0.055). The HD grid found fewer gaps after PVI than the reported 2.15 gaps per patient by Porterfield et al. [8] which served as the basis for the power calculation. However, in the study by Porterfield et al. [8], there was a high heterogeneity in the waiting time and how PVI was confirmed. Further, only 3 of 33 patients in Porterfield et al. [8] underwent ablation with comparable HPSD settings, while this has been shown to be superior to low-power long-duration settings with regard to lesion durability and AF outcome [4, 9]. Despite these differences, our rigorously designed study still found residual gaps in 20% of patients. This may well be the result of the detailed HD mapping, while Porterfield et al. [8] did not report on any mapping settings.
The resolution of bipolar electrocardiogram mapping is dependent on the number of electrodes, as well as electrode size, spacing, and orientation of the bipole [7]. Far-field electrograms were more common using the HD Grid mapping catheter, in part due to the 3-mm interelectrode spacing. While a detailed analysis of the multidimensional signals could help discriminating far-field from near-field electrograms, electrogram morphology and differential pacing maneuvers were always used in this study to confirm appropriate electrogram interpretation. Due to the bipole configuration, bipolar mapping of the PVs may be limited in the detection of myocardial sleeves. A comparison of standard bipolar mapping and HD mapping showed that HD mapping was able to identify significantly more myocardial sleeves, which altered their ablation strategy, and resulted in delivering more lesions [10]. The majority of clinical data on PV reconnection and gaps has been obtained during repeat procedures. Given that the majority of AF triggers originate from the PVs, AF recurrence after PVI is often considered secondary to PV reconnection [11]. In our study, most of the gaps were located at the septal side and in the carina of the right PVs. Previous reports have found a similar patterns of gaps, particularly when using an HPSD approach [12,13,14,15]. Predisposition of the carina regions has been linked to a higher incidence of non-endocardial gaps, particularly at the right PVs due to intercaval bundle fibers [15]. The predisposition of the septal side of the right PVs has been related to increased tissue thickness and the presence of interatrial conduction tissue [12]. It is well known that HPSD ablation results in wider, more superficial ablation lesions when compared to low-power long-duration ablation [9]. Hansom et al. showed that compared to low-power low-duration settings, patients required more application at the right carina to achieve isolation when using HPSD ablation [12].
In a non-randomized study including 108 patients with PV reconnections, HD mapping identified the specific location of the gap in 61% of cases, while this was only 40% with a CMC [16]. Further, when using HD mapping, fewer RF applications were required to obtain isolation of the PVs (12.5 ± 6.1 vs. 15.6 ± 7.7, p = 0.02) [16]. Crandall et al. [17] conducted a non-randomized study comparing the 10-pole circumferential catheter (n = 402) and the HD Grid (n = 70). The HD Grid was associated with shorter procedure and fluoroscopy times, but more important, the 1-year recurrence rate of any atrial arrhythmia was significantly lower in the HD Grid group (13% vs. 25%, p < 0.001) [17].
Our study illustrates that HD mapping may detect some of these gaps during the initial PVI procedure, even in the presence of conventional definitions of complete PVI by the entrance and exit block with a CMC-20. Therefore, our study adds to the evidence that HD mapping has the potential to improve the short-term and long-term success rate of AF ablation. Multicenter randomized clinical trials to assess the impact of HD mapping on PVI outcome are needed to confirm reproducibility and validate these initial findings.
4.1 Limitations
The present study has several limitations related to its design. First, it was a single-center study with predominantly male patients with paroxysmal AF and a low median CHA2DS2-VASC score. This may limit the reproducibility of our study in larger trials and persistent AF. Second, electrical PVI was confirmed by demonstrating the entrance and exit block, which is our standard institutional practice. Whether a new LA voltage map obtained with the CMC-20 would have detected the gaps identified by the HD Grid was not studied. Finally, this study was a proof-of-concept study aimed at detecting gaps acutely after a standard PVI procedure. Due to the low number of study participants required after power calculation, the study protocol did not include long-term follow-up of AF recurrence.
5 Conclusions
HD mapping with the HD Grid catheter after a routine RF PVI procedure performed with a CMC-20 catheter identified residual gaps in 20% of patients. Gaps were predominantly found at the carina and the septal side of the right PVs, where the more superficial lesions with HPSD ablation may be insufficient due to increased tissue thickness. HD mapping may have the potential to further improve long-term AF ablation success rates.
Abbreviations
- AF:
-
Atrial fibrillation
- CMC-20:
-
20-Pole circumferential mapping catheter
- DOAC:
-
Direct oral anticoagulant
- ECG:
-
Electrocardiogram
- HD:
-
High-density
- HPSD:
-
High-power short-duration
- IQR:
-
Interquartile range
- LA:
-
Left atrium
- LAT:
-
Local activation timing
- LIPV:
-
Left inferior pulmonary vein
- LSPV:
-
Left superior pulmonary vein
- MRI:
-
Magnetic resonance imaging
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- SD:
-
Standard deviation
- RSPV:
-
Right superior pulmonary vein
- RIPV:
-
Right inferior pulmonary vein
- TEE:
-
Transesophageal echocardiogram
- TTE:
-
Transthoracic echocardiogram
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020. https://doi.org/10.1093/eurheartj/ehaa612
Kuck KH, Albenque JP, Chun KJ, Furnkranz A, Busch M, Elvan A, et al. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE trial. Circ Arrhythm Electrophysiol. 2019;12(6):e007247. https://doi.org/10.1161/CIRCEP.119.007247.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45. https://doi.org/10.1056/NEJMoa1602014.
Ravi V, Poudyal A, Abid QU, Larsen T, Krishnan K, Sharma PS, et al. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: a systematic review and meta-analysis. Europace : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Cardiac Cell Electrophysiol Eur Soc Cardiol. 2021;23(5):710–21. https://doi.org/10.1093/europace/euaa327.
Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ’CLOSE’-protocol. Eur : Eur Pacing Arrhythmias Card Electrophysiol : J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2018;20(FI_3):f419–27. https://doi.org/10.1093/europace/eux376.
Garcia-Bolao I, Ramos P, Ballesteros G, Vives E. New mapping tools to assess lesion in atrial fibrillation. Eur : Eur Pacing Arrhythmias Card Electrophysiol: J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2019;21(Supplement_3):iii2–4. https://doi.org/10.1093/europace/euz110.
Takigawa M, Relan J, Kitamura T, Martin CA, Kim S, Martin R, et al. Impact of spacing and orientation on the scar threshold with a high-density grid catheter. Circ Arrhythm Electrophysiol. 2019;12(9):e007158. https://doi.org/10.1161/CIRCEP.119.007158.
Porterfield C, Gora PJ, Wystrach A, Rossi P, Rillo M, Sebag FA, et al. Confirmation of pulmonary vein isolation with high-density mapping: comparison to traditional workflows. J Atr Fibrillation. 2020;12(6):2361. https://doi.org/10.4022/jafib.2361.
Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa M, et al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol. 2018;29(11):1570–5. https://doi.org/10.1111/jce.13724.
Papageorgiou N, Karim N, Williams J, Garcia J, Creta A, Ang R, et al. Initial experience of the high-density grid catheter in patients undergoing catheter ablation for atrial fibrillation. J Interv Card Electrophysiol. 2022;63(2):259–66. https://doi.org/10.1007/s10840-021-00950-y.
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66. https://doi.org/10.1056/NEJM199809033391003.
Hansom SP, Alqarawi W, Birnie DH, Golian M, Nery PB, Redpath CJ, et al. High-power, short-duration atrial fibrillation ablation compared with a conventional approach: Outcomes and reconnection patterns. J Cardiovasc Electrophysiol. 2021;32(5):1219–28. https://doi.org/10.1111/jce.14989.
Bologna F, Bordignon S, Perrotta L, Dugo D, Nagase T, Chen S, et al. Incidence and pattern of conduction gaps after pulmonary vein isolation with the endoscopic ablation system. J Interv Card Electrophysiol. 2020;57(3):465–71. https://doi.org/10.1007/s10840-019-00556-5.
Nair GM, Yeo C, MacDonald Z, Ainslie MP, Alqarawi WA, Nery PB, et al. Three-year outcomes and reconnection patterns after initial contact force guided pulmonary vein isolation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28(9):984–93. https://doi.org/10.1111/jce.13280.
Nakamura K, Sasaki T, Minami K, Take Y, Inoue M, Sasaki W, et al. Prevalence, characteristics, and predictors of endocardial and nonendocardial conduction gaps during local impedance-guided extensive pulmonary vein isolation of atrial fibrillation with high-resolution mapping. J Cardiovasc Electrophysiol. 2021;32(8):2045–59. https://doi.org/10.1111/jce.15152.
Garcia-Bolao I, Ballesteros G, Ramos P, Menendez D, Erkiaga A, Neglia R, et al. Identification of pulmonary vein reconnection gaps with high-density mapping in redo atrial fibrillation ablation procedures. Eur : Eur Pacing Arrhythmias Card Electrophysiol: J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. 2018;20(FI_3):f351–8. https://doi.org/10.1093/europace/eux184.
Crandall B, Kanuri S, Cutler M, Osborn J, Miller J, Mallender C, et al. High power ultra short duration ablation with HD grid improves freedom from atrial fibrillation and redo procedures compared to circular Mapping Catheter. J Atr Fibrillation. 2020;13(2):2414. https://doi.org/10.4022/jafib.2414.
Funding
Abbott provided in-kind support, including the provision of HD Grid mapping catheters used in study procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the University of Calgary’s Research Ethics Board.
Informed consent
Participants provided written informed consent.
Conflict of interest
SBW: Research grants from Abbott, Boston Scientific, Medtronic Canada; consulting from Boston Scientific, unrelated to the manuscript. VK has research support, honoraria, or Ad board from Medtronic, Servier, Novartis, BMS Pfizer, and Libin Cardiovascular Institute.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vandenberk, B., Quinn, F.R., Barmby, J. et al. High-density mapping improves detection of conduction gaps after pulmonary vein isolation ablation with a circular mapping catheter. J Interv Card Electrophysiol 66, 1401–1410 (2023). https://doi.org/10.1007/s10840-022-01434-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01434-3