Abstract
Reported mapping procedures of left atrial (LA) low-voltage areas (LVAs) vary widely. This study aimed to compare the PentaRay®/CARTO®3 (PentaRay map) and Orion™/Rhythmia™ (Orion map) systems for LA voltage mapping. This study included 15 patients who underwent successful pulmonary vein isolation (PVI) for atrial fibrillation. After PVI, PentaRay and Orion maps created for all patients were compared. LVAs were defined as sites with ≥ 3 adjacent low-voltage points < 0.5 mV. LVAs were indicated in 8 (53%) among 15 patients, and the average values of the measured LVAs was comparable between the systems (PentaRay map = 5.4 ± 8.7 cm2; Orion map = 4.3 ± 6.4 cm2, p = 0.69). However, in 2 of 8 patients with LVAs, the Orion map indicated LVAs at the septum and posterolateral sites of the LA, respectively, whereas the PentaRay map indicated no LVAs. In those patients, sharp electrograms of > 0.5 mV were properly recorded at the septum and posterolateral sites during appropriate beats in the PentaRay map. The PentaRay map had a shorter procedure time than the Orion map (12 ± 3 min vs. 23 ± 8 min, respectively; p < 0.01). Our study results showed a discrepancy in the LVA evaluation between the PentaRay and Orion maps. In 2 of 15 patients, the Orion map indicated LVAs at the sites where > 0.5-mV electrograms were properly recorded in the PentaRay map.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary vein isolation (PVI) has become a well-established treatment for paroxysmal atrial fibrillation (AF) [1]. In addition to PVI, several ablation methods for the atrial substrate that maintains AF in patients with persistent AF have been reported in the past [2, 3]; however, it is unclear as to which techniques other than PVI can improve the outcomes of ablation for persistent AF [4]. As previously described, an atrial fibrotic substrate, as measured from atrial voltage mapping, may be involved in the maintenance of AF, and extensive fibrosis of the left atrium (LA) is a significant predictor of AF ablation failure [5,6,7]. Furthermore, an individualized approach for AF ablation based on low-voltage areas (LVAs) has been developed, and several studies have reported that LVA ablation in addition to PVI improve the outcome of ablation for persistent AF [6, 8,9,10]. However, the reported procedures of LVA mapping vary widely among studies [6, 8, 10, 11], and the methodology for defining LVAs has not been standardized [6]. Moreover, if the configuration of the electrodes is different, the recorded electrograms may be different as well [12], although no studies that compared the procedures of LVA mapping had been reported. We hypothesized that different mapping systems, especially if the configurations of the catheter and electrodes are different, might lead to different LVA assessments. Therefore, we compared LVAs delineated by the PentaRay®/CARTO® (Boisense Webster, Diamond Bar, CA, USA) and Orion™/Rhythmia™ (Boston Scientific, Washington, DC, USA) systems, which are the most experienced in our institution, and examine whether there are differences in LVA assessments owing to the different mapping systems.
Methods
Study population
This study included 15 patients with drug-refractory AF in whom a successful PVI was achieved by radiofrequency (RF) catheter or cryoballoon (CB) ablation. After PVI, two voltage maps of the LA were created by using the PentaRay®/CARTO®3 (PentaRay map) and Orion™/Rhythmia™ (Orion map) systems in all patients for comparison. This study was approved by the ethics committee of our institution and fully complied with the guidelines of the Declaration of Helsinki. The study was registered in the Universal Hospital Medical Information Network Clinical Trials Registry (UMIN000023845), and all patients gave a written informed consent before participation.
Voltage mapping and LVA measurement
All procedures were performed under conscious sedation with dexmedetomidine. Following femoral access and transseptal puncture, PVI was performed by cryothermal or RF ablation in all patients. After PVI, two voltage maps of the LA were created during constant pacing from the coronary sinus ostium with a 600-ms cycle length by using the PentaRay/CARTO3 (version 6) system and Orion/Rhythmia system in each patient. The PentaRay catheter was inserted into the LA via an 8- or 8.5-Fr long sheath (SL0, Abbott, St. Paul, MN, USA) according to circumstances, and the Orion catheter was inserted via an 8.5-Fr long sheath (SL0). To prevent the influence of the first-created voltage map to the later-created map, the sequence of creating PentaRay and Orion maps was alternated for each case. If AF was sustained after PVI, electrical cardioversion was performed before LVA mapping. Heparin was administered before the transseptal catheterization to maintain an activated clotting time of 300–350 s during the procedure.
The PentaRay is a 20-pole steerable mapping catheter arranged in five soft radiating spines, and one spine has four ring electrodes. Each electrode is 1 mm with an interelectrode spacing of 2–6–2 mm. The Orion is a mini-basket catheter consisting of eight splines, each containing eight flat electrodes. Each electrode is 0.4 mm2 with the interelectrode spacing of 2.5 mm measured from center to center. Appropriate beats and electrograms were automatically selected by each mapping system, and each point was classified according to the peak-to-peak bipolar voltage. Bipolar electrograms were filtered at 16–500 Hz and 30–300 Hz in the PentaRay and Orion maps, respectively. An impedance-based algorithm (Tissue Proximity Indicator [TPI]) for the exclusion of mapping points with low tissue–electrode contact was not used in the PentaRay map for comparison with the Orion map. Mapping points located within 4 mm from the external surface in the PentaRay map and within 2 mm in the Orion map were analyzed. Mapping point density was set at 1 mm in the PentaRay map.
In accordance with the previous studies [6, 8, 13], LVAs were defined as sites with ≥ 3 adjacent low-voltage points < 0.5 mV. When LVAs were delineated, we attempted to reposition the mapping catheter with appropriate contact and reconfirmed the voltage. All mapping points and the recorded electrograms were reviewed to reach a consensus by two experienced observers. The extent of the LVAs was quantified by manually measuring the encircled areas on the LA side of the border of the LVA created by the CB or RF ablation for PVI by using the measuring software available with each mapping system. The LA was categorized into six areas, and the locations of LVAs were classified as anterior, septal, roof, posterior, inferior, and posterolateral (Fig. 1). These areas were manually measured as with the measurement of LVAs, and the LA surface area was calculated by adding together each area other than the isolated PV/LA antrum, LA appendage, and mitral orifice area.
Statistical analysis
Continuous variables are expressed as the mean ± SD and compared by performing the Student’s t test. Categorical variables were compared using the Fisher’s exact test. A p value < 0.05 was considered to be indicative of statistical significance.
Results
Patient characteristics
Baseline patient characteristics are presented in Table 1. The mean age was 71 ± 9 (range 54–81) years, and 5 (33%) patients were female. Three (20%) patients had paroxysmal AF, and the remaining 12 (80%) had persistent AF. One patient (7%) had cryoablation, and 2 (13%) patients were redo cases. Echocardiography revealed a mean left ventricular ejection fraction of 59 ± 7% (range 47–68%), and the mean diameter of the LA was 44 ± 4 mm (range 37–50 mm). One (7%), 10 (67%), 4 (27%), 1 (7%) patient had chronic heart failure, hypertension, diabetes, and coronary artery disease, respectively.
LVA measurements
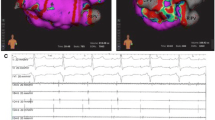
The results of the PentaRay and Orion maps are presented in Tables 2 and 3. The Orion map (7389 ± 1755 points) had a larger number of mapping points than the PentaRay map (3141 ± 761 points, p < 0.01). The shell volumes (PentaRay map = 183 ± 31 ml; Orion map = 176 ± 30 ml, p = 0.55) and LA surface areas (PentaRay map = 128 ± 17 cm2; Orion map = 125 ± 21 cm2, p = 0.66) were comparable between the two maps. LVAs were indicated in 8 (53%) patients in both maps and measured similarly by both systems (PentaRay map = 5.4 ± 8.7 cm2; Orion map = 4.3 ± 6.4 cm2, p = 0.69). With respect to each classified location, the measured LVAs were also comparable. In both maps, LVAs were delineated at the roof in 3 patients, posterior site in 3 patients, inferior site in 3 patients, and anterior site in 5 patients. However, at the septum, the LVA was delineated in 2 and 3 patients in the PentaRay and Orion maps, respectively. Similarly, at the posterolateral site, the LVA was delineated in 1 and 2 patients in the PentaRay and Orion maps, respectively. In each site, no significant difference was observed in the number of the patients between the two maps; however, in 2 patients, a discrepancy in LVA evaluation between the PentaRay and Orion maps was observed. When LVAs were delineated, we attempted to reposition the mapping catheter and reassessed the voltage in both maps. In these 2 patients, the LVAs were not altered after changing the shape of the Orion catheter to maneuver the catheter to the desired location. Figure 2 shows the discrepancy between the two voltage maps of the LA after PVI (Patient No. 6). No low-voltage point was mapped at the septum of the LA in the PentaRay map, whereas the LVA at the septum was delineated in the Orion map, and in that LVA, > 0.5-mV (purple) points were observed. Figure 3 shows the discrepancy at the posterolateral site of the LA (Patient No. 2). No LVA was delineated in the PentaRay map, although it was delineated in the Orion map.
Discrepancy between the two voltage maps of the left atrium (LA) by the PentaRay/CARTO (a) and Orion/Rhythmia (b) systems in one patient after pulmonary vein (PV) isolation (patient no. 6). A low-voltage area (LVA) was defined as a site with ≥ 3 adjacent low-voltage points of < 0.5 mV. In the PentaRay/CARTO map, no low-voltage point was mapped at the septum of the LA, and at the site indicated by the arrow, a 1.20-mV electrogram was recorded. In contrast, the Orion/Rhythmia map delineated an LVA at the septum. In that LVA, > 0.5-mV (purple) points were also observed, and a 1.71-mV electrogram was recorded within the LVA (arrow). AP anteroposterior, LI (S) left inferior (superior), RI (S) right inferior (superior)
Discrepancy between the two voltage maps of the left atrium (LA) by the PentaRay/CARTO (a) and Orion/Rhythmia (b) systems in one patient after pulmonary vein (PV) isolation (patient no. 2). A low-voltage area (LVA) was defined as a site with ≥ 3 adjacent low-voltage points of < 0.5 mV. In the PentaRay/CARTO map, an LVA was not detected at the posterolateral site of the LA, and a 2.33-mV electrogram was recorded at the site indicated by the arrow. In contrast, an LVA was delineated at the posterolateral site in the Orion/Rhythmia map, and a low-voltage electrogram was recorded at the posterolateral site (arrow). LI (S) left inferior (superior), LPO left posterior oblique, RI (S) right inferior (superior)
The PentaRay map (12 ± 3 min) had a shorter time taken to create the voltage map than the Orion map (23 ± 8 min, p < 0.01).
Discussion
Major findings
The study results showed the following: (1) the average values of the measured LVAs were comparable between the PentaRay and Orion maps; (2) in 2 patients, the Orion map delineated the LVA at the site where no LVA was delineated in the PentaRay map. There was a discrepancy in the LVA evaluation between these maps.
Electrograms recorded by the PentaRay and Orion catheters
Electrograms recorded by the PentaRay and Orion catheters are not expected to have exact same meanings because of different sizes, forms, and spacing of the electrodes. However, in this study, the average values of the measured LVAs were similar between the two maps. If catheters have appropriate contact with the LA endocardium, the results may not differ between two maps for the assessment of LVA in the LA. Any potential differences in recording when using these catheters, such as a tiny potential for identifying reentry circuits or channels of tachycardia, were not investigated in the study.
Discrepancy between the PentaRay and Orion maps
The Orion map had a greater number of mapping points and mapping time than the PentaRay map (Table 2). In both maps, the mapping time was defined as the minimum time for tracing the LA evenly and reconfirming the delineated LVAs by the catheter in each system; the operator did not consciously create a more detailed Orion map. In the CARTO (version 6) system, because there is a limit on the data volume and number of mapping points, only mapping points with an interval > 1 mm were analyzed by the default settings. Therefore, a high-density map was created in the Orion map. Moreover, in patients with LVAs, the number of mapping points was relatively higher (Table 3). An increased number of mapping points may also increase the likelihood for LVA detection. However, as previously stated, when LVAs were delineated, we reassessed them carefully in both maps. We consider that the mapping point inevitably increased in patients with LVAs. Therefore, our data do not suggest that the number of mapping points leads to an altered LVA recognition.
We considered some potential causes for the longer mapping time in the Orion map. Because multiple mapping points are automatically collected in both mapping systems, several of these may be recorded with low or no contact, which might detect false LVAs [14]. Whether the PentaRay electrodes are in adequate contact with the LA endocardium can be easily determined by the change in shape of the soft radiating spines by CARTO or fluoroscopy despite a normal-voltage electrogram is not recorded. On the other hand, in the Orion map, it is difficult to determine how much distortion of the basket is caused by the tissue–electrode contact, and the Rhythmia system does not have a TPI-like feature. Therefore, it might take more time to decide that an Orion catheter traced the LA evenly and whether or not the low-voltage electrogram and LVAs are true. Furthermore, this tissue–electrode contact judgement difficulty may have affected the increase in the number of mapping points in the Orion map, especially in patients with LVAs.
In 2 patients, the Orion map indicated an LVA at a site where no LVA in the PentaRay map was delineated (Figs. 2 and 3). In those patients, sharp > 0.5-mV electrograms were properly recorded at the septum and posterolateral sites during appropriate beats in the PentaRay map. Therefore, LVAs are probably not present there, and it is deemed appropriate to assess the LVAs on the Orion maps of those two patients as false. The Orion map may sometimes delineate false LVAs, which may be caused by the low contact between tissues and electrodes. In the patient who delineated the LVA at the septum (Fig. 2, patient no. 6) despite the LVA contained mapping points with a normal electrogram, several inappropriate points with a low contact falsely identified the non-LVA as the LVA, as determined by a majority decision of the Rhythmia system. In the other patient who delineated the LVA at the posterolateral site (Fig. 3, patient no. 2), the Orion electrodes might not have contacted the LA endocardium.
No significant difference was found between the two maps in terms of the comparisons of the measured LVAs and the number of the patients with LVAs by each classified location. Therefore, our data did not suggest that false LVAs due to impaired contact were likely delineated at specific sites. Moreover, we quantified areas where < 0.1-mV electrograms were recorded in the two maps. If the Orion catheter frequently recorded electrograms with low tissue–electrode contact and the PentaRay catheter recorded more accurate electrograms with appropriate contact, the < 0.1-mV areas in the Orion map may be shown wider than that in the PentaRay map. However, the < 0.1-mV areas were comparable between the two maps (PentaRay map = 0.7 ± 1.5 cm2; Orion map = 0.6 ± 1.0 cm2, p = 0.78). These results suggest that the Orion catheter was not generally manipulated with low contact, although mapping with appropriate contact was difficult only in the confined areas in some patients. After the threshold of LVAs was altered from < 0.5 to < 0.1 mV, the false LVA that delineated at the septum (Fig. 2, patient no. 6) disappeared, and the false LVA that delineated at the posterolateral site (Fig. 3, patient no. 2) was reduced (from 3.39 to 1.16 cm2). The posterolateral site in Patient No. 2 might be mapped with lower or no tissue–electrode contact.
The number of patients is very small in this study; therefore, we cannot determine the frequency of discrepancy and inferiority of one over the other. In addition, if the configuration of the electrodes is different between the two mapping catheters, the recorded electrograms may be different as well [12]. However, in some cases, it may be difficult to create a contact between the electrodes of the Orion catheter and the LA endocardium, which could lead to LA voltage underestimation. Furthermore, the inaccurate assessment of LVAs can affect the assessment of fibrosis, ablation based on LVAs [6, 8,9,10], and linear ablation design. The Orion map should be mapped with more attention to the tissue–electrode contact.
Features to avoid low-contact mapping points in CARTO and Rhythmia systems
The CARTO system has a TPI feature to avoid mapping points with low tissue–electrode contact [14]; however, the Rhythmia system does not have such feature. Therefore, the Orion electrode–tissue contact is determined only by the shape of the external surface delineated by the Orion catheter or recorded electrograms. In 2 patients who had the false LVA in this study, we could not determine out the unnaturalness of the extra surface and could not improve the low contact by repositioning the Orion catheter. Moreover, it is difficult to determine whether the recorded low-voltage electrogram is due to a correct LVA evaluation or due to a low contact. In this study, mapping points located within 4 mm from the external surface in the PentaRay map without using a TPI module and within 2 mm in the Orion map were analyzed. The false LVAs, which may be caused by a low contact, were delineated in the Orion map with settings in which low-contact points were more likely to be excluded. As another possible cause for the false LVAs, the external surface itself, which is the basis of the distance filter, may not be appropriately delineated because of the low contact of the basket-shaped inflexible spline in the Orion map. Consequently, mapping points determined to be within 2 mm from the external surface may be > 2 mm away from the true LA endocardium.
Study limitations
First, this was a single-center study with a limited number of patients. The number of patients was too small to prove significant differences between the two methods. More patient data are needed to yield a statistically meaningful analysis. Second, we defined LVAs according to the previous studies that described the association between atrial fibrosis and AF ablation. However, there are various grades in atrial fibrosis and a measured voltage depends on mapping system, electrode size and spacing, wave front direction, mapping density, tissue–electrode contact, and other variables. The definition of LVAs is not absolute in all mapping procedures for LVAs and is still under investigation. Third, the LVAs were measured after PVI on the LA side of the PVI line. However, local voltage around the LA antrum can change due to the instability of ablation lesion or PV reconnection, and the PVI line was assessed with reference to the LVA around the LA antrum or ablation tags. Therefore, there might be a slight error in the LVA measurement if the measured LVA was in contact with the PVI line. Fourth, two maps were not compared with the MRI findings of fibrosis. Fifth, two maps were continuously created by one operator; thus, the first map might have affected the second map. Sixth, while both the PentaRay and Orion maps were created by experienced operators, the tissue–electrode contact could be influenced by the skills of the operator. Finally, this study aimed to absolutely evaluate the voltage of electrograms recorded by the two systems; however, we obtained no data on possible differences in detection, such as tiny potentials for identifying reentry circuits or channels of arrhythmia, between the electrograms generated by the two systems.
Conclusions
Our study results showed a discrepancy in the LVA evaluation between the PentaRay and Orion maps. In 2 of 15 patients, the Orion map indicated LVAs at the sites where > 0.5-mV electrograms were properly recorded in the PentaRay map.
References
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A (2005) Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 111:1100–1105
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T (2004) A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 43:2044–2053
Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clémenty J, Jaïs P (2005) Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 16:1138–1147
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, Investigators STARAFII (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372:1812–1822
Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A (2005) Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 45:285–292
Kottkamp H, Schreiber D, Moser F, Rieger A (2017) Therapeutic approaches to atrial fibrillation ablation targeting atrial fibrosis. JACC Clin Electrophysiol 3:643–653
Williams SE, Linton N, O’Neill L, Harrison J, Whitaker J, Mukherjee R, Rinaldi CA, Gill J, Niederer S, Wright M, O’Neill M (2017) The effect of activation rate on left atrial bipolar voltage in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 28:1028–1036
Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, Hindricks G, Piorkowski C (2014) Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 7:825–833
Yamaguchi T, Tsuchiya T, Nakahara S, Fukui A, Nagamoto Y, Murotani K, Eshima K, Takahashi N (2016) Efficacy of left atrial voltage-based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 27:1055–1063
Sim I, Bishop M, O’Neill M, Williams SE (2019) Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol 56:213–227
Hohendanner F, Romero I, Blaschke F, Heinzel FR, Pieske B, Boldt LH, Parwani AS (2018) Extent and magnitude of low-voltage areas assessed by ultra-high-density electroanatomical mapping correlate with left atrial function. Int J Cardiol 272:108–112
Anter E, Tschabrunn CM, Josephson ME (2015) High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythm Electrophysiol 8:537–545
Huang D, Li JB, Zghaib T, Gucuk Ipek E, Balouch M, Spragg DD, Ashikaga H, Tandri H, Sinha SK, Marine JE, Berger RD, Calkins H, Nazarian S (2018) The extent of left atrial low-voltage areas included in pulmonary vein isolation is associated with freedom from recurrent atrial arrhythmia. Can J Cardiol 34:73–79
Asvestas D, Vlachos K, Bazoukis G, Martin CA, Letsas KP, Sakellaropoulou A, Kossyvakis C, Saplaouras A, Prappa E, Goga C, Vassilikos V, Valkanas K, Deftereos S, Sideris A, Efremidis M (2018) Left atrial voltage mapping using a new impedance-based algorithm in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 41:1447–1453
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has a real or perceived conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaseno, K., Hasegawa, K., Miyazaki, S. et al. Discrepancy between CARTO and Rhythmia maps for defining the left atrial low-voltage areas in atrial fibrillation ablation. Heart Vessels 36, 1027–1034 (2021). https://doi.org/10.1007/s00380-021-01773-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01773-7