Abstract
Spirulina (Arthrospira) is a cyanobacterium and an excellent source of natural compounds. The dried biomass can be applied in functional foods or in pharmaceutical products; however, its biochemical and physicochemical properties are affected by the drying operation. In this work, the drying effect in Spirulina biomass in a spouted bed at different air temperatures (80, 90, 100, and 110 °C) was studied and was compared with conventional tray drying (55 °C). The dried products and the in natura sample were analyzed for total phenolic compounds, antioxidant activity, protein solubility, phycocyanin content, thiobarbituric acid (TBA) value, and color parameters. DSC, TGA, FTIR, and MEV of the samples were also performed. The results showed that the spouted bed dryer at 80 °C and the tray dryer at 55 °C were more suitable for Spirulina drying in relation to the pigments and the lipid oxidation, due to the lowest losses of phycocyanin and at the lowest TBA values. However, the highest values of antioxidant activity and proteins solubility and the lowest losses on total phenolic compounds were found in the spouted bed dryer at 100 °C, resulting in a product with the greatest thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of microalgae has received increasing attention due to their great potential as a nutritional source and bioactive compounds that can enhance the nutritional value of food products (Batista et al. 2013). Spirulina (Arthrospira) is a multicellular, filamentous cyanobacterium which grows naturally in alkaline water (Lee 1997). This microalga is rich in group B vitamins, minerals, unsaturated fatty acids, proteins (55–70% w/w, dry basis) of high biological value and pigments, such as carotenoids, chlorophyll-a, and phycobiliproteins (Belay 2002). Phycocyanin is one of the more important photosynthetic pigments of the phycobiliproteins family and can constitute up to 20% of dry weight of the Spirulina biomass. This pigment is a natural blue dye and has been used mainly as a food dye and in cosmetics (Chaiklahan et al. 2011). Researchers also have reported that the Spirulina is natural source of antioxidants and phenolic compounds, being used in food preservation and in human health (Kepekçi et al. 2013; Nuhu 2013).
The drying operation is a traditional process that has been used to preserve microalgal biomass. However, for heat-sensitive materials, the quality of dried product depends mainly on the air conditions (Chua et al. 2003; Costa et al. 2016). In addition, the selection of drying method depends on the operation scale and application for which the dried product is intended (Show et al. 2013). The traditional methods that have been used for the microalgae drying are spray drying, freeze-drying, and tray drying (Desmorieux and Decaen 2006; Tello-Ireland et al. 2011). In relation to the drying operation of Spirulina biomass, there are few studies about the effects of the air conditions on product quality reported in the literature (Desmorieux and Decaen 2006; Oliveira et al. 2010; Costa et al. 2016). Another technique that can be used for Spirulina drying is the spouted bed, which presents low operation cost, and it is still little explored for microalgae drying (Oliveira et al. 2008). This dryer is appropriate to the drying of pastes, solutions, and suspensions using inert particles, which promotes high heat and mass transfer rates due to the gas–solid contact. Spouted bed dryer is a potential alternative to the flash and spray dryers, because the dried product presents the same quality (Epstein and Grace 2011; Fujita et al. 2013).
The aim of this work was to evaluate the effects of spouted bed drying and tray drying on the characteristics of Spirulina biomass. The products dried were characterized according to the total phenolic compounds, antioxidant activity, protein solubility, phycocyanin content, lipid oxidation (TBA value), and color parameters. The effect of temperature was also verified through analyses of thermogravimetric (TGA, DSC) and infrared transform (FTIR). The powder size characterization was evaluated by scanning electron microscopy (SEM).
Material and methods
Cultivation and characterization of Spirulina LEB-18
Spirulina strain LEB-18 was isolated from the Mangueira Lagoon in southern Brazil, and the water was supplemented with 20% Zarrouk medium for maintenance of the inoculum and biomass production (Morais et al. 2008). The cultivation was carried out in raceway-type open bioreactors, which were mechanically stirred at 18 rpm, according to the procedure of Morais et al. (2009). At the end of cultivation (when the biomass concentration reached 0.5 g L−1), the biomass was recovered by filtration and pressed to recover the material, which had around 0.20 g g−1solid content (wet basis).
The chemical composition of the biomass samples of in natura Spirulina were characterized, and the results showed (w.b.): moisture content of 0.780 ± 0.013 g g−1; ash content of 0.013 ± 0.004 g g−1; protein content of 0.149 ± 0.023 g g−1; lipid content of 0.022 ± 0.002 g g−1; and carbohydrate content of 0.036 ± 0.012 g g−1. Thus, it can be observed that Spirulina biomass is a rich source of proteins, which corresponded to around 68% (w/w) on a dry basis.
Biomass drying
Spouted bed dryer
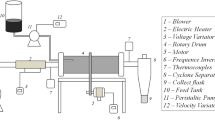
The drying assays in spouted bed were carried out in an equipment of conical conventional geometry, which was developed in previous work (Larrosa et al. 2015), and Fig. 1 shows the equipment schema. The height and the diameter of cell were of 0.15 and 0.175 m, respectively, being a glass base with enclosed angle of 60°. The drying air was supplied by a radial blower (Ibram, model CR0850, Brazil) with 6 kW, heated by three electric resistances (2.4 kW). The air flow measurements were realized by an orifice plate meter, and the drying temperatures were measured with thermocouples. The biomass was introduced into the drying cell in a semi-continuous form using a syringe, by atomization with compressed air (2 atm abs). The dried powder was transported by drying air and was collected in a cyclone. Polyethylene particles with load of 0.5 kg (diameter of 0.0032 m, sphericity of 0.7, and density of 960 kg m−3) were used as inert material to assist the biomass drying.
Schema of the spouted bed equipment: (1) radial blower, (2) valves, (3) electric resistances, (4) temperature control, (5) orifice plate meter, (6) glass manometer, (7) spouted cell, (8, 9) dry bulb thermocouples, (10) wet bulb thermocouple, (11) collector cyclone, (12) suspension tank, (13) air compressor
The drying operation conditions, in order to guarantee stability in the bed, were defined in preliminary tests as: inlet air temperatures from 80 to 110 °C (absolute humidity of 0.013 ± 0.001 kg kg−1), biomass suspension flow rate of 0.4 kgbiomass kginert −1 h−1, and biomass suspension concentration of 5% (w/w). The fluid dynamics tests were performed in all drying temperatures (80, 90, 100, and 110 °C). The values of minimum spouting air velocity, which represent the point where bed collapse occurred, were found at around 0.33 ± 0.01 m s−1 for all temperatures. The air circulation rate used in each assay was double the minimum spouting velocity, which is recommended for suspensions/pastes drying (Epstein and Grace 2011). The biomass accumulation rate in the spouted bed dryer was determined using the total bed mass at the end of the operation, the total bed mass before the operation, the total solid mass introduced into the dryer, and the final moisture content of the dried powder. All assays in spouted bed drying were performed in 210 min, afterwards, the dried products were analyzed. The dried biomass samples were ground in a knife mill (Willey model no. 3, Philadelphia, USA) and were passed/retained on 150/250 mesh sieves (Tyler standard), which corresponded a size range from 63 to 106 μm. The drying assays were carried out in duplicate.
The evaporative thermal efficiency (η E) of drying operation is defined as the ratio of the heat used in evaporation to the heat of saturated inlet air, and global thermal efficiency (η G) is the ratio of the heat used in evaporation to the total heat input of the drying air, according to Eqs. 1 and 2, respectively.
Where m Ain and m Aout are the inlet and outlet dry airflow rates, respectively (kg s−1), c Sin and c Sout are the humid heat of the inlet and outlet drying air, respectively (J kg−1 K−1), T Ain and T Aout are the inlet and outlet drying air temperatures, respectively (°C), T WB is the wet bulb temperature of drying air (°C) and T AMB is the ambient air temperature.
In drying process supposed as adiabatic, the heat losses are negligible; thus, Eqs. 1 and 2 can be approximated for the relations shown in Eqs. 3 and 4 (Mujumdar 2007).
Conventional tray drying
The tray drying assays were performed according to Oliveira et al. (2010). The biomass was dried at 55 °C with hot air velocity at 2.5 m s−1 and absolute humidity of 0.013 ± 0.001 kg kg−1. The sample load in tray was 4 kg m−2, and the tray thickness was 4 mm (samples were in cylindrical pellet forms with the same tray thickness). The duration of drying assays was until the samples reached a moisture content about of 0.10 kg kg−1 (wet basis), which corresponded the commercial moisture content of the microalgae. The dried microalgae samples were ground in a knife mill (Willey model no. 3, Philadelphia, USA) and were passed/retained on 35/150 mesh sieves (Tyler standard), representing the size range from 106 to 425 μm. The assays were carried out in duplicate.
Analyses of the biomass and of the dried products
Composition centesimal analysis
The samples in natura and the dried products were analyzed for moisture content, ash content, and protein content by AOAC methods (AOAC 1995). The lipid content was analyzed by the Folch et al. method (Folch et al. 1957), and the carbohydrate content was estimated by difference.
Total antioxidant activity analysis
The antioxidant activity was determined by DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging according to Brand-Williams et al. (1995), with some modifications. Each sample of methanol phenolic extract (0.5 mL) was mixed with 3.5 mL of 0.06 mM DPPH methanol solution and incubated for 60 min at room temperature in the dark, and the decrease in absorbance at 515 nm was measured. The results were expressed as ability to scavenge the DPPH radical, following Eq. 5:
where AA is antioxidant activity (%inhibition DPPH), A 0 is the absorbance of the control reaction, and A 1 is the absorbance of the sample.
Total phenolic compounds analysis
The total phenolic compounds were determined by extraction with methanol through the precipitation of the non-phenolics with Ba(OH)2 and ZnSO4 (Assis et al., 2014). The spectrophotometric method (SP-220, Bioespectro, Brazil) was carried out to estimate the total phenolic content, using Folin–Ciocalteau reagent. The absorbance of methanol extracts was measured at 750 nm using gallic acid as standard. The results were expressed as mggallic acid equivalent (GAE) (100 gdry sample)−1.
Protein solubility analysis
The protein solubility in water was determined according to Morr et al. (1985). Samples of 2.5 g and 50 mL of distilled water were placed in rotary shaker for 15 min and then centrifuged at 3500×g for 15 min. The contents of the tubes were filtered, and the supernatants were used for protein content determination by the micro-Kjeldahl method (AOAC 1995). The protein solubility was calculated as the percentage of the soluble protein content in relation to the total protein content in the dry matter (g (100 gdry sample)−1).
2.3.5 Phycocyanin content analysis
The phycocyanin content was evaluated according to Moraes et al. (2010). The samples were mixed with distilled water in a biomass to solvent ratio of 0.16 g mL−1 (dry matter). The solutions were mixed in shaker for about 4 h and then centrifuged. The optical densities of the samples were measured at 620 and 652 nm, and the phycocyanin concentration was determined according to Bennet and Bogorad (1973) as shown in Eq. 6.
Where Pc is the phycocyanin concentration (mg mL−1), OD620 is the optical density of the sample at 620 nm, and OD652 is the optical density of the sample at 652 nm. The results of phycocyanin content were expressed in all assays in mg g−1, in relation to the in natura sample.
Thiobarbituric acid analysis
The thiobarbituric acid (TBA) value for lipid oxidation determination was evaluated according to Tiburcio et al. (2007), with some modifications. The Spirulina powder (1 g) was mixed with 20 mL of chloroform and filtered. The filtrate (10 mL) was placed in centrifuge tubes with 10 mL of trichloroacetic acid 7.5% (w/v) and centrifuged at 2000×g for 15 min. The supernatants (4 mL) and 1 mL of 0.02 M TBA solution were stirred for 5 min and then incubated in boiling water bath for 40 min to observe development of the color. The supernatant absorbance was determined at 530 nm. The TBA value was calculated using a standard curve obtained by reacting of tetramethoxypropane solution 0.01 M with TBA; the value was expressed as milligram of malonyldialdehyde (MDA) per kg of sample on a dry basis.
Color analysis
The color was measured by a Minolta Chroma system (CR-300, Minolta Corporation, USA). Each sample was put on aluminum plates and randomly measured at 3 spots. The color system was calibrated against a standard calibration plate of a white surface and a set to CIE standard illuminant C. The color parameter values of lightness (L), greenness/redness (a), and blueness/yellowness (b) were recorded for each sample. The Hue angle (Hue), chroma index (C), and color difference (ΔE) were determined according to Larrosa et al. (2015).
Thermal analyses
The thermogravimetric analysis (TGA) and the differential scanning calorimetry (DSC) analysis were evaluated on the dried powders (Rivero et al. 2010 ). In both analyses, a small sample (around 4–6 mg) was loaded in a silver pan. An empty pan was used as a reference material. The dynamic assays of thermogravimetry (TGA) were performed using a thermobalance (Shimadzu, model TGA, Japan). Temperature programs for the assays were from 25 to 500 °C at a heating rate of 10 °C min−1, under nitrogen flow (50 mL min−1). In DSC analyses (Shimadzu, model DSC60, Japan), the pans were sealed and scans were run at a heating rate of 10 °C min−1 from 15 to 200 °C.
Fourier-transform infrared spectroscopy analysis
The presence of groups characteristic of phenolic compounds in methanolic extracts were analyzed by Fourier-transform infrared (FTIR) spectroscopy (Shimadzu, Prestige 21, model The-210045, Japan), according to horizontal attenuated total reflectance (HATR) through plate crystal with an aperture angle of 45° (Silverstein et al. 2007). The analysis was recorded at room temperature (20 °C) using scanning over the frequency range of 4000–400 cm−1 at a resolution of 4 cm−1, with 45 scans per aliquot.
Scanning electron microscopy
The morphologies of products obtained in spouted bed drying and in tray drying were observed using a scanning electron microscope (Jeol, model JSM-6610LV, Japan) (Goldstein et al. 1992). The sample surfaces were coated with carbon in a sputter coater (Sputtering Denton Vacuum Desk V, USA) before SEM examination.
Statistical analysis
The biochemical and physicochemical properties (phenolic compounds content, antioxidant activity value, protein solubility value, phycocyanin content, TBA value) and color parameters were determined by three repetitions, and they were compared to evaluate the significant differences between the values by Tukey’s test at level of 95% (p < 0.05) (Box et al. 2005).
Results
The values of the outlet air temperatures (T Aout), wet bulb temperatures of the inlet drying air (T WB), absolute humidity of outlet drying air, evaporative thermal efficiency (η E), and global thermal efficiency (η G) in the spouted bed are shown in Table 1 for the inlet drying air temperatures from 80 to 110 °C. The moisture contents of all products obtained from spouted bed dryer were around of 0.09 g g−1 (w.b.). Already for the conventional tray dryer, the final moisture content of samples was about 0.10 g g−1 (w.b.), in a drying total time of 210 min.
The values of total antioxidant activity for the samples in the tray drying and in natura microalgae were 34.6 ± 1.1 and 80.4 ± 1.0%, respectively. The values of total antioxidant activity increased with the inlet drying air temperature increase for the spouted bed as shown in Fig. 2a. The total phenolic compounds are shown in Fig. 2b. The protein solubility in water was evaluated in the dried samples and in natura sample, and the results for the drying assays in spouted bed are presented in Fig. 2c. The phycocyanin content of the in natura Spirulina (30.3 ± 0.3 mg g−1 db) was affected by the temperature in spouted bed drying as shown in Fig. 3a. The TBA value is an indicator to lipid oxidation degree, and the results are presented in Fig. 3b. The color difference for samples dried in spouted bed was affected by air temperature, as shown in Fig. 3c.
The thermal analyses (TGA and DSC) were evaluated for the samples and the dried products. The TGA curves are shown in Fig. 4a, and the maximum temperatures of weight loss (T peak DTG) were calculated by the derivative of the thermogravimetric curves in Fig. 4b, and these values are shown in Table 2, which indicate the decomposition stages of the different constituents in the sample. The DSC analysis can be observed through the DSC curves of the dried samples in Fig. 5a and in natura Spirulina sample in Fig. 5b.
FTIR spectral analyses of the methanol extracts of in natura sample, dried products in spouted bed at 100 °C (most suitable condition for total phenolic compounds and protein solubility), and in tray drying were performed. The functional groups of phenolic compounds were identified according to Fig. 6. The morphological properties of the powders obtained in spouted bed and in tray drying (milled in a knife mill) are shown in Fig. 7, using a scanning electron microscope (SEM).
Discussion
Drying operations in spouted bed and in tray
The residence time values of Spirulina biomass into the spouted bed for all experimental assays were approximately 13 ± 1 min. Tacon and Freitas (2007) found residence time values in range from 12.2 to 17.7 min for paste drying in the spouted bed. The residence time is a very important parameter because it determines the relation between the drying conditions and the material degradation. This parameter is considered as the necessary operation time so that the equipment reaches the permanent regime. The outlet drying air temperature is another important parameter to obtain the best product quality (Benali and Amazouz 2006), being that the stable spouted regime is found when the outlet drying air temperature becomes constant. The spouted bed dryer showed good performance because in all inlet drying air temperatures had low values of accumulated mass (< 5%, w/w). In addition, bed collapse was not observed in all experimental assays.
Thermal drying is an energy-intensive operation which accounts for up to 15% of all industrial energy consumed. The evaporation capacity is proportional to the temperature difference between the inlet air and the outlet air in the spouted bed dryer. The values of global thermal efficiency (η G) and the evaporative thermal efficiency (η E) were in the range of 25 and 30%, respectively, in spouted bed drying, with the highest values in inlet drying air at 100 °C (Table 1). The conventional dryers often operate at low thermal efficiency, typically between 25 and 50%, but this may be also lower than 10% (Mujumdar 2007). Thus, it is desirable to reach the higher possible values, using the highest inlet air temperatures. However, there are some limitations to be considered, because an increase in the inlet air temperature can lead to serious damage in the dried biomass, and a decrease in the outlet air temperature can lead to higher moisture content in the product. In addition, a decrease in heat input could also cause an increase in the global thermal efficiency; on the other hand, the heat consumption is proportional to the evaporation rate, and for a given rate, it depends on the biomass suspension concentration of dryer feed.
Physicochemical and biochemical analyses of biomass and dried products
The antioxidant activity for the inlet air temperatures at 100 and 110 °C in spouted bed drying (Fig. 2a), showed no significant differences at 95% level (p > 0.05) in relation to in natura Spirulina. In addition, the results showed that the antioxidant activity of the samples presented a similar behavior at the total phenolic compounds, indicating that these compounds could be the major contributor to the antioxidant activity of the Spirulina biomass.
The total phenolic compounds showed that the drying operation led to a reduction in range from 10 to 76%, presenting significant differences at 95% level (p < 0.05) in relation to in natura Spirulina (862.5 ± 12.0 mgGAE (100 gdb)−1). The highest losses were observed in tray drying (205.9±7.9 mgGAE (100 gdb)−1), and the lowest losses were observed in spouted bed at 100 and 110 °C (Fig. 2b). The phytochemicals degradation can occur in drying assays and may be associated with oxidative reactions or decomposition of thermolabile compounds induced by the drying hot air and by losses of volatile substances. Depending of the food processing, the phenolic compounds released from the disruption of the cell walls can accelerate oxidation and enzymatic hydrolysis. However, at the highest temperatures, the enzyme activities probably would be deactivated and could avoid losses of phenolics. Therefore, at the highest inlet air temperatures (100–110 °C) in the spouted bed, the highest outlet air temperatures (around 80 and 90 °C, respectively) were reached, leading to a reduction in the residence time of the material in the equipment (around 13 min), and so, decreasing the losses of phenolic compounds. Besides, the lowest inlet air temperatures led to the greater losses of bioactive compounds, and therefore, the enzyme activities were not totally inhibited. Losses of phenolic compounds were also found by Fujita et al. (2013) in a range from 33 to 42% on frozen pulp of camu-camu dried in spouted bed.
The results of the protein solubility in water indicate that the proteins of dried Spirulina biomass have a great interaction with water, which is a good functional property in food applications. The difference in the values can be associated to the residence time and the outlet air temperature, as spouted bed drying at 100 °C gave the best results (Fig. 2c). Although the outlet air temperature in this condition was 80 °C (Table 1), the temperature of the dried product was around 5 °C above of inlet air wet bulb temperature (which was in the range of 37 °C), and this temperature is in the range that protein solubility increases (from 40 to 50 °C) (Maciel et al. 2012). The protein solubility in water showed that the drying assays in spouted bed, with exception at 80 and 110 °C, and in tray drying (35.9 ± 2.6 g (100gdb)−1) increased this functional property of the proteins in relation to in natura Spirulina (28.6 ± 0.7 g (100gdb)−1). In spouted bed drying at 100 °C, the values of the protein solubility in water were higher than the values of Oliveira et al. (2008), which were found in the range of 35 to 38%.
The increase of inlet air temperature in spouted bed drying decreased the phycocyanin content significantly (p < 0.05) (Fig. 3a). The percentage losses of the phycocyanin contents were in range of 32 to 70% in relation to in natura. However, in spouted bed at 80 °C (20.1 ± 1.2 mg g−1 db) and in tray drying (19.8 ± 1.5 mg g−1 db), no significant differences were shown (p < 0.05). During the tray drying, the thickness of Spirulina biomass provided some protection to degradation, while in spouted bed the biomass was more exposed to the drying air due to smaller particles produced by atomization of the suspension. Phycocyanin is a blue pigment of the phycobiliprotein family with great commercial interest, and this pigment is very sensitive to heat, and its losses might be associated to the denatured proteins. According to Patel et al. (2004), thermal denaturation leads to modification of the structure in the native protein, giving rise to definite changes in the chemical, physical, and biological properties. Oliveira et al. (2010) reported a loss around 37% of the phycocyanin content of Spirulina in convective tray drying. Tello-Ireland et al. (2011) found that dehydration resulted in a decrease in phycocyanin content at higher temperatures in Gracilaria chilensis.
The increase of the inlet air temperature from 80 to 110 °C in spouted bed led to a significant increase of the TBA value (p<0.05) (Fig. 3b. In the tray drying (0.20 ± 0.02 mgMDA kg−1 db), the TBA value was a little higher than the in natura sample (0.14 ± 0.03 mgMDA kg−1 db). In spouted bed drying, the biomass atomization on the bed increased its superficial area to hot air exposure, thus becoming more susceptible to oxidation; however, in the tray dryer, the biomass thickness provided some protection from lipid oxidation. Tiburcio et al. (2007) in sun drying and oven drying, found values ranging from 0.47 to 0.56 mgMDA kg−1, and Oliveira et al. (2010) for different air temperatures (50–70 °C) in convective tray drying reported values in range from 0.65 to 2.27 mgMDA kg−1.
Tray dried samples showed lower values of color difference (5.07 ± 0.63) than the samples in spouted bed drying (Fig. 3c). This can be explained due to their similar values of lightness (L) and coordinates (a, b) in relation to the in natura biomass values. L values between in natura sample and the dried samples in spouted bed were significantly different (p < 0.05), but there were no significant differences (p > 0.05) between the in natura and the tray-dried samples. However, the results did not show browning in the dried samples, and the lightest values were in spouted bed drying. The a and b values showed significant difference between in natura and the dried samples. The lowest values for tray drying can be associated to the greatest drying time (± 210 min). The decrease of a and b coordinates can be affected due to pigment decomposition, like chlorophylls and carotenoids and non-enzymatic reactions. The chroma (C) value is a measure of the degree of color saturation, and spouted bed drying showed a significant difference (p < 0.05) in relation to the in natura sample, presenting highest values. Hue angles of the all samples showed predominantly green-yellowness color.
Thermal analyses
TGA curves in Fig. 4a show the first stage of decomposition process up to 120 °C, which represents loss of free and loosely bound water. The second stage of decomposition, between 180 and 350 °C, is attributed to volatility of the proteins and carbohydrates. In this range, the weight losses of the samples were similar, except in natura samples due to the high amount of water. The third stage from 350 to 500 °C can be associated with lipid degradation (Campanella et al. 2012). The in natura sample showed a pronounced peak in the range from 25 to 100 °C, due to 72% loss of mass (free water). The main peak temperature (approximately 300 °C) suggests protein decomposition, which is the main nutrient of Spirulina biomass. The in natura sample showed minor mass loss with highest DTG peak temperature, T peak DTG, because this sample had a higher moisture content. The highest temperatures (DTG peaks) were obtained in tray drying and in spouted bed drying at 100 °C (Fig. 4b and Table 2), indicating major thermal stability of its proteins.
In DSC analysis, it can be observed that the sample curves showed endothermic peaks. This technique is used to measure enthalpy changes as a function of temperature or time according to changes of physical and chemical properties (Lin and Wang 2012). Analyzing Fig. 5a, the sample dried in spouted bed at temperature of 100 °C (line C) showed the highest peak temperature and one of the highest enthalpy changes (see values in Table 2). This fact is due to the higher thermal stability of their components, which can be associated to proteins and phenolic compounds. The enthalpy defines the amount of thermal energy required to cause structural changes in the microalgae; thus, the sample dried in spouted bed at 100 °C needs more energy to promote protein denaturation and phenolics degradation. In Fig. 5b, the in natura sample showed a highest endothermic peak with a higher enthalpy variation than the other samples (Table 2), due to the highest moisture content (80% w/w, w.b.), protecting its bioactive compounds in relation to thermal degradation.
Fourier-transform infrared spectroscopy analysis
The infrared spectrum in spouted bed at 100 °C (Fig. 6b) was similar to in natura spectrum (Fig. 6a). The bands in the range from 3550 cm−1 are related to O-H stretching; 3150 cm−1 shows N-H presence and from 2850 cm−1 to 2700 cm−1 represents the aldehyde group. The 1700 cm−1 range is related to vibrational stretching of C=O, and the 1300 cm−1 band is relative to deformation angular of C–O–H. The range from 1100 to 1000 cm−1 indicates a stretching of C–O, and the 800 cm−1 band corresponds to the angular deformations of aromatic rings (Silverstein et al. 2007). The range of the 800 cm−1 band showed higher transmittance of the in natura and the spouted bed samples than the samples in tray drying (Fig. 6c). In addition, the 1020 cm−1 band appeared only in tray-drying sample, which can represent some division of the 800 cm−1 band reducing its transmittance. Thus, these differences observed in the samples could be associated to minor concentration on total phenolic compounds in tray drying.
Scanning electron microscopy analysis
The powder of Spirulina biomass obtained by spouted bed showed no difference in shape and particle size in the different temperatures. Besides, rough surface particles with small diameter were observed in Fig. 7b (< 100 μm) and different structure than the powder of the tray drying in Fig. 7a (> 100 μm). In spouted bed, the biomass is atomized and covers the polyethylene surface of the inert bodies, forming a thin layer around them; thus, during the drying operation of this layer, the interparticle friction from inert bodies leads to particles with small size and irregular surfaces. The SEM of the dried powder in tray drying milled in a knife mill showed a compact, rigid, and non-porous surface, which could lead the minor losses in the phycocyanin content and lower increase in TBA value in relation to the spouted bed drying.
Conclusions
The effects of the drying methods of the Spirulina biomass in spouted bed and in tray drying were investigated. Spouted bed drying at 80 °C and tray drying (55 °C) were the more suitable techniques to obtain the highest phycocyanin contents and minor lipid oxidation. However, spouted bed drying at 100 °C reached greater thermal stability in relation to the proteins, due to the highest values of total phenolic compounds, antioxidant activity, and protein solubility. All samples showed predominantly green-yellowness color, and the tray dried samples showed lower color difference than the samples in spouted bed drying. The infrared spectrum (FTIR) of the samples in spouted bed at 100 °C was similar to the spectrum of in natura sample. However, in the tray-drying samples some differences in the spectrum were observed, and this can be associated to minor concentration of total phenolic compounds. The SEM of the dried powder in tray drying, milled in knife mill, showed a compact, rigid, and non-porous surface, which can lead to the minor losses in the phycocyanin content and TBA value in relation to spouted bed drying.
References
Antelo FS, Costa JAV, Khalil SJ (2008) Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem Eng J 41:43–47
AOAC (1995) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Washington, DC
Assis LM, Machado AR, Motta AS, Costa JAV, Souza-Soares LA (2014) Development and characterization of nanovesicles containing phenolic compounds of microalgae Spirulina strain LEB-18 and Chorella pyrenoidosa. Adv Mater Phys Chem 4:6–12
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173
Belay A (2002) The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management (review). J Am Nutraceut Assoc 5:26–48
Benali M, Amazouz M (2006) Drying of vegetable starch solutions on inert particles: quality and energy aspects. J Food Eng 74:484–489
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Box GEP, Hunter JS, Hunter WG (2005) Statistics for experiments: design, innovation, and discovery, 2nd edn. John Wiley & Sons, Hoboken
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT – Food Sci Technol 28:25–30
Campanella A, Muncrief R, Harold MP, Griffith DC, Whitton NM, Weber RS (2012) Thermolysis of microalgae and duckweed in a CO2-swept fixed-bed reactor: bio-oil yield and compositional effects. Bioresour Technol 109:154–162
Chaiklahan R, Nattayaporn C, Loha V, Tia S, Bunnag B (2011) Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour Technol 102:159–7164
Costa BR, Rodrigues MCK, Rocha SF, Pohndorf RS, Larrosa APQ, Pinto LAA (2016) Optimization of Spirulina sp. drying in heat pump: effects on the physicochemical properties and color parameters. J Food Process Preserv 40:934–942
Chua KJ, Mujumdar AS, Chou SK (2003) Intermittent drying of bioproducts – an overview. Bioresour Technol 90:285–295
Desmorieux H, Decaen N (2006) Convective drying of Spirulina in thin layer. J Food Eng 77:64–70
Epstein N, Grace JR (2011) Spouted and spout-fluid beds: fundamentals and applications. Cambridge University Press, New York
Folch J, Lees M, Stanley GHS (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fujita A, Borges K, Correia R, Franco BDGM, Genovese MI (2013) Impact of spouted bed drying on bioactive compounds, antimicrobial and antioxidant activities of commercial frozen pulp of camu-camu (Myrciaria dubia McVaugh). Food Res Int 54:495–500
Goldstein JI, Newbury DE, Echil P, Joy DC, Romig AD Jr, Lyman CE, Fiori C, Lifshin E (1992) Scanning electron microscopy and X–ray microanalysis. Plenum Press, New York
Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem 141:1972–1979
Larrosa APQ, Cadaval TRS Jr, Pinto LAA (2015) Influence of drying methods on the characteristics of a vegetable paste formulated by linear programming maximizing antioxidant activity. LWT – Food Sci Technol 60:178–185
Lee YK (1997) Commercial production of microalgae in the Asia-Pacific rim. J Appl Phycol 9:403–411
Lin SY, Wang SL (2012) Advances in simultaneous DSC-FTIR microspectroscopy for rapid solid-state chemical stability studies: some dipeptide drugs as examples. Adv Drug Deliver Rev 64:461–478
Maciel LR, Miyasaki EK, Feisther VA, Pinto LAA (2012) Statistical evaluation of the protein enrichment of rice bran using spouted bed. Dry Technol 30:733–738
Moraes CC, Burkert JFM, Kalil SJ (2010) C-phycocyanin extraction process for large-scale use. J Food Biochem 34:133–148
Morais MG, Reichert CC, Dalcanton F, Durante AJ, Marins LFF, Costa JAV (2008) Isolation and characterization of a new Arthrospira strain. Z Naturforsch C 63c:144–150
Morais MG, Radmann EM, Andrade MR, Teixeira GG, Brusch LRF, Costa JAV (2009) Pilot scale semicontinuous production of Spirulina biomass in southern Brazil. Aquaculture 294:60–64
Morr CVB, German J, Kinsela JM, Regenstein JP, Van Buren A, Kilara BA (1985) A collaborative study to develop a standardized food protein solubility procedure. J Food Sci 50:1715–1718
Mujumdar AS (2007) Handbook of industrial drying, 3rd edn. Taylor & Francis Group, New York
Nuhu AA (2013) Spirulina (Arthrospira): an important source of nutritional and medicinal compounds. J Mar Biol 1:1–8
Oliveira EG, Rosa GS, Moraes MA, Pinto LAA (2008) Phycocyanin content of Spirulina platensis dried in spouted bed and thin layer. J Food Process Eng 31:34–50
Oliveira EG, Duarte JH, Moraes K, Crexi VT, Pinto LAA (2010) Optimisation of Spirulina platensis convective drying: evaluation of phycocyanin loss and lipid oxidation. Int J Food Sci Technol 45:1572–1578
Patel A, Pawar R, Mishra S, Sonawane S, Ghosh PK (2004) Kinetic studies on thermal denaturation of C-phycocyanin. Indian J Biochem Biophys 41:254–257
Rivero S, García MA, Pinotti A (2010) Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov Food Sci Emerg Technol 11:369–375
Show KY, Lee DJ, Chang JS (2013) Algal biomass dehydration. Bioresour Technol 135:720–729
Silverstein RM, Webster FX, Kiemle DJ (2007) Spectrometric identification of organic compounds. John Wiley & Sons, New York
Tacon LA, Freitas LAA (2007) Paste residence time in a spouted bed dryer. III: effect of paste properties and quality interactions. Dry Technol 25:841–852
Tello-Ireland C, Lemus-Mondaca R, Vega-Gálvez A, López J, Di Scala K (2011) Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT – Food Sci Technol 44:2112–2118
Tiburcio PC, Galvez FCF, Cruz LJ, Gavino VC (2007) Optimization of low-cost drying methods to minimize lipid peroxidation in Spirulina platensis grown in the Philippines. J Appl Phycol 19:719–726
Acknowledgements
Authors gratefully acknowledge the CAPES/Brazil (Coordination for the Improvement of Higher Education Personnel) for the financial support, the Biochemical Engineering Laboratory (LEB/FURG/Brazil) for the biomass Spirulina Leb-18, and CEME-SUL/FURG/Brazil (Electron Microscopy Center of South/Rio Grande/RS/Brazil) for the scanning electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larrosa, A.P.Q., Camara, Á.S., Pohndorf, R.S. et al. Physicochemical, biochemical, and thermal properties of Arthrospira (Spirulina) biomass dried in spouted bed at different conditions. J Appl Phycol 30, 1019–1029 (2018). https://doi.org/10.1007/s10811-017-1265-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1265-5