Abstract
The high content of bioactive compounds in the microalga Spirulina platensis has recently attracted attention from food and pharmaceutical industries. However, for its application an effective preservation technique must be developed. In this paper, we investigated the use of a non-conventional rotary dryer (with an inert bed) for drying the microalga Spirulina biomass and the effects of the operational conditions (air temperature, intermittent feeding interval, filling degree of inert particles, and rotation speed) on its bioactive compounds. The results indicated that this non-conventional drying system offers an effective alternative for expanding the use of this biomass in an adequate form. We identified the conditions in which the dried material had maintained satisfactory contents of phenolics (air temperature of 70 °C and intermittent feeding interval of 10 min), flavonoids (intermittent feeding interval of 17.4 min), and phycocyanin compounds (air temperature of 40 °C), which were near to those present in fresh microalga.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spirulina platensis is a single-cell blue-green microalga (cyanobacteria) which is a natural source of a wide variety of essential nutrients, such as proteins, vitamins, amino acids, minerals, and unsaturated fatty acids. This microalga also contains important antioxidant and anti-inflammatory compounds, including carotenoids, phenolic acids, flavonoids and phycocyanin, whose therapeutic and health properties are well documented in several studies. Given these exceptional characteristics, this microalga has potential applications in various industrial processes, including those used in agriculture, food and pharmaceuticals [1,2,3,4].
The dehydration of Spirulina is an essential step in the effective use of this material, since its moisture content after harvesting can exceed 90% (wet basis) [5,6,7]. However, the drying technique and operating conditions can significantly affect the functional properties and nutritional value of this microalga. Therefore, the correct choice of equipment and operating conditions is critical to product quality. Some studies have shown that conventional drying systems may be ineffective in preserving the quality of microalga [8,9,10]. Thus, alternative methods that better preserve the functional and nutritional properties of the dried product must be identified.
Rotary dryers are widely used in various industrial processes, due to their flexibility in handling a wider range of materials than is possible with other types of dryers and their high processing capacity. The conventional configuration of the rotary dryer consists of a cylindrical rotary drum slightly inclined from the horizontal, which is equipped with flights that lift the solids and cause them to fall in cascades across the interior of the dryer [11,12,13,14,15]. However, this device is generally used for granular material, so to process pastes like this microalga biomass, an alternative equipment configuration is necessary. The use of inert particles inside the drum offers an interesting possibility for drying pasty materials, since an inert bed can increase the degree of surface contact between hot air and the material, and prevent loss of material on the walls and dryer structure [16,17,18]. Compared with other devices that use inert beds for drying pasty materials, such as fluidized and spouted beds, a rotary dryer with an inert bed will experience a lower pressure drop and higher moisture removal efficiency, due to its use of larger inert particles that prevent instability or bed agglomeration [19,20,21].
In this study, we investigated the performance of a rotary dryer with an inert bed in the dehydration of S. platensis and analyzed the effects of various process variables (air temperature, filling degree, rotation speed and intermittent feeding interval) on the quality of the final product, as expressed by the content of bioactive compounds: phenolics, flavonoids, and phycocyanin.
Materials and methods
Raw material
The microalga Spirulina platensis used in this work was supplied by the Brasil Vital Company, located in the state of Goias, Brazil. Prior to use, the material was filtered in vacuum conditions, then divided and packaged into small portions and stored in a freezer until its use in the experiments.
Experimental apparatus
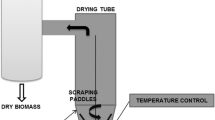
The experimental apparatus, as presented in Fig. 1, consisted of a 5-HP blower (Kepler-Weber, 112 M), an air heating system comprising electric resistances controlled by a variac, a rotary drum coupled to a rotation system comprising a 0.75-HP motor (WEG, W22) and a frequency inverter (WEG, CF-08), and a Stairmand cyclone separator (diameter of 10 cm) with a flask to collect the dried material in the underflow. Temperatures were measured by thermocouples, positioned in front of and behind the rotary drum. The fresh Spirulina was fed by a system equipped with a peristaltic pump (Masterflex, 7553-70) connected to a velocity variator.
The rotary drum used in the experiments was made of stainless-steel (Fig. 2a) with an inner diameter of 12 cm, length of 36 cm and three flights with 2.5 cm in height (Fig. 2b). The inert particles (Fig. 2c) were porcelain spheres with a diameter of 1.9 cm and density of 2.32 g/cm3.
Experimental design
The experimental conditions were chosen based on a central composite design (CCD) [22]. To analyze the four independent variables, we conducted a total of 26 experiments with two replicates at the central levels. The independent variables included air temperature (T), intermittent feeding interval (FI), filling degree of the inert particles (FD), and rotation speed (RS). Table 1 shows the coded and real values for the independent variables.
In each test, about 110 g of fresh Spirulina was fed in cycles, with intermittent feeds of 10 g of material and rest periods. The rest time was the variable Intermittent feeding interval (FI). The filling degree (FD) was calculated based on the dimensions of the rotary drum and the properties of the particles bed (size, density and porosity) and expressed the volumetric fraction of inert particles inside the rotary drum during the dehydration process. We used a digital tachometer (MINIPA, MDT-2238A) to measure the Rotation speed (RS). To maximize the collection efficiency of the cyclone separator, we used a feed air flow rate corresponding to the highest capacity of the blower (0.92 m3/min).
Moisture and water activity (a w )
The moisture content of the samples was determined using the oven method: 105 ± 3 °C per 24 h [23], and the water activity (aw) was measured using a LabSwift (Novasina) device that measures the aw of the material in a scale from 0.000 to 1.000 with a precision of ± 0.001. All measurements were repeated three times.
Analysis of bioactive compounds
The analyzes of the bioactive compounds were repeated three times and the total contents were expressed as a mean value ± standard deviation. We used the Student’s t test to evaluate the statistical significance of the difference between mean values. The quality responses analyzed were the contents of total phenolics, total flavonoids, and phycocyanin. We quantified the effects of the independent variables on these quality responses using regression techniques.
Total phenolics content (TPC) To determine the TPC we used the Folin–Ciocauteau method [24], with gallic acid as the standard and spectrophotometric reading at 622 nm. The results are expressed in milligrams of gallic acid per 100 g of sample (dry matter).
Total Flavonoids Content (TFC) To determine the TFC we used the colorimetric method described by Zhishen et al. [25], using rutin as the standard and a spectrophotometric reading at 450 nm. The results are expressed in milligrams of rutin per 100 g of sample (dry matter).
Phycocyanin Content (PC) We extracted phycocyanin based on the method reported by Costa et al. [26], using water as the solvent extractor and spectrophotometric reading at 620 nm and 652 nm. We calculated the phycocyanin content using Eq. (1) [27], and converted the results to grams of phycocyanin per 100 g of the sample (dry matter):
where PC is the phycocyanin content (mg/mL) and OD620 and OD652 are the optical densities of the samples at 620 nm and 652 nm, respectively.
Scanning electron microscopy (SEM)
Physical changes that occur during dehydration may affect the product quality [28, 29]. As such, after drying the Spirulina biomass, we examined it by scanning electron microscopy (SEM) technique, using a Carl Zeiss microscope, EVO MA 10, to evaluate the microstructural characteristics of the material [30]. We attached the material to the microscope supports using a conductive carbon tape and, then, metallized it with gold (Leica, SCD 050). We used a 10-kV acceleration voltage in the SEM analysis.
Results and discussion
Moisture and water activity (a w )
After dehydration in the rotary dryer with an inert bed, the dried Spirulina obtained was a fine and homogeneous powder. Figure 3 shows images of the fresh (a) and dried (b) microalga and Table 2 shows the final moisture and water activity (aw) values obtained in each test of the experimental design.
The fresh microalga had a moisture content of 0.827 ± 0.01 g water/g wet matter (wet basis) and the aw value of 0.967. The moisture content of the microalga after drying process ranged from 0.036 to 0.078 g water/g wet matter and the aw ranged from 0.233 to 0.361. This means that the material reached moisture content and water activity levels suitable for storage and transportation in all the operating conditions used in this work. Most bacteria, fungi, and yeast are inhibited in their activity and growth when the aw is lower than 0.600 [31]. The greatest moisture removals were obtained in the tests with the highest temperature (Exp. 16) and the highest filling degree (Exp. 22), showing the relevance of these variables (T and FD) in the heat and mass transfer process. Thus, the non-conventional rotary dryer used in this work was effective for processing S. platensis. However, we still had to verify whether the equipment and operating conditions used in this work enabled the production of a product with good quality.
Bioactive compounds
Figures 4, 6, and 7 show the respective contents of total phenolics (TPC), total flavonoids (TFC), and phycocyanin (PC) after drying in each experimental test (see conditions in Table 2). Figure 5 shows response surfaces of these quality responses as a function of the main independent variables (T and FI). We obtained these response surfaces using regression equations fitted to the experimental data, to enable quantification of the effects of the studied independent variables (T, FI, FD, and RS) on the quality responses (Eqs. 2, 4 and 5).
The phenolic compounds present in this microalga have been reported to exhibit pharmacological properties such as being anticarcinogenic, antiviral, antimicrobial, anti-inflammatory, and antitumoral [1]. Figure 4 shows the total phenolic compounds (TPC) present in Spirulina after drying for each experimental test. We can see that in some operating conditions the dried microalga had a TPC value close to that of the fresh material (462.12 mg gallic acid/100 g samples in dry matter). These best results were obtained in experiments in which various variables were at the central levels (intermediate values). For example, Exps. 26 and 21 (see Table 2) led to TPC values of 433.38 and 400.13 mg gallic acid per 100 g samples, respectively. We also note that several studies [1, 8, 32] have reported that conventional drying techniques have led to a degradation of these compounds after drying, which reinforces the potential of this new rotary dryer for Spirulina dehydration.
Equation (2) shows the fitted equation (R2 = 0.84) for TPC as a function of the significant parameters related to the independent variables considering their linear, quadratic and interaction effects. This equation for TPC, as well as Eqs. (4) and (5), for TFC and PC, respectively, are presented with the independent variables in coded form. In these regressions, we coded the studied variables (T, FI, FD and RS) using Eq. (3):
For T in °C, FI in min, FD in % and RS in rpm.
Figure 5a shows the response surface of TPC as a function of T and FI, with the other independent variables at central levels. In the figure, we can clearly see the non-linear effects of T and FI on the TPC and that the highest TPC values are obtained at intermediate levels of the independent variables, as noted above.
A variety of biological activities, including antioxidant, anti-inflammatory, estrogenic, antimicrobial, and antitumor abilities have been reported for flavonoids compounds [8, 33]. Figure 6 shows the TFC of S. platensis after drying in each of the experimental tests, as well as the TFC of a fresh sample. We can also see that in some tests the TFC values were closer to the value of the fresh Spirulina (9.86 mg rutin/100 g sample in dry matter). The TFC of Spirulina dried in the conditions used in test 20, for example, was 8.33 mg rutin per 100 g sample in dry matter. This experiment (test 20) was performed at a high level of FI with the other variables at central levels.
Equation (4) shows the fitted equation (R2 = 0.80) for TFC as a function of the significant parameters with the independent variables in coded form. Figure 5b shows the response surface of TFC as a function of T and FI, with the other independent variables at central levels, in which we can confirm the best conditions for the TFC are those identified above, i.e., high FI periods and the other variables at intermediate levels:
Among the compounds present in the Spirulina biomass, phycocyanin is the main pigment produced, which reach 20% of the dry weight of cell protein. This compound has been used in food coloring and cosmetics, but its significant therapeutic value, due to its high antioxidant and anti-inflammatory properties, has attracted attention for its application in pharmaceutical and functional food industries [34,35,36]. Figure 7 shows the PC in microalga after dehydration. We can see that some of the experiments led to PC in the dried Spirulina close to that of fresh microalga (14.55 g Spirulina/100 g in dry matter). Most of these experiments were performed at lower temperatures, for example the Experiments 6 and 2, were performed at 50 °C, and resulted in PC values of 13.85 and 13.64 g phycocyanin per 100 g (dry matter), respectively. On the other hand, Experiments 18 and 11, were performed at 99.7 °C and 90 °C, respectively, and resulted in PC values of 6.55 and 6.82 g phycocyanin per 100 g sample (dry matter), respectively, which represent a significant degradation of this compound. Desmorieux and Decaen [5], Doke [6] and Sarada et al. [37] also observed the thermosensibility of phycocyanin during the drying of this microalga when using conventional techniques.
Equation (5) shows the fitted equation (R2 = 0.84) for PC as a function of T and FI (coded form), which we identified as significant independent variables for this compound. Figure 5c shows the response surface of PC as a function of these independent variables, from which we can confirm the thermosensibility of this compound and the highest PC value at the lowest drying temperature:
Scanning electronic microscopy (SEM) images
Figure 8 shows SEM images of Spirulina samples before and after dehydration, which was performed at central levels of the experimental design (Exp. 25 of Table 2). We can see that the microalga suffered a considerable alteration in its physical structure after moisture removal process. The spiral or helical filaments, which give this species its name to Spirulina, are visible in the fresh samples, but disappear after drying. In addition, the biomass surface had melted with clogging of the formed pores. This “smooth” and “solid leaf” aspect was also reported by Desmorieux et al. [32] after convective drying of S. platensis. However, the different operating conditions had no influence on this aspect of the dried microalga. Figure 9 shows SEM images of the microalga after drying in different experimental conditions, in which we can see no significant changes in the morphology of the dried samples, even after very different operating conditions.
Conclusions
In this work, we used a novel rotary dryer with an inert bed, to successfully dehydrate the microalga S. platensis. The operating conditions used led to a moisture content of the microalga after drying that was lower than 0.080 g water/g wet matter and a water activity lower than 0.400. These results mean that the shelf life of this microalga can be extended by minimizing microbial growth. We also quantified the effect of operating variables (Temperature, Intermittent feeding interval, Filling degree and Rotation speed) on the bioactive compounds present in microalga after drying. We then derived equations for predicting the total phenolic, total flavonoid, and phycocyanin as a function of these independent variables and identified the conditions that promote high levels of these bioactive compounds after drying. Based on our results, we can conclude that the rotary dryer with an inert bed is a very good option for the efficient drying of S. platensis, while also preserving the contents of the main bioactive compounds.
References
Agustini TW, Suzery M, Sutrinanto DWF, Ma’ruf, Hadyanto (2015) Comparative study of bioactive substances extracted from fresh and dried Spirulina sp. Procedia Environ Sci 23:282–289
Chauhan VS, Ramamurthy V (1996) Enhanced Spirulina growth in outdoor ponds correlates with daily reduction in oxygen production rate. Bioprocess Eng 15:9–12
Dissa AO, Desmorieux H, Savadogo PW, Segda BG, Koulidiati J (2010) Shrinkage, porosity and density behavior during convective drying of spirulina. J Food Eng 97:410–418
Hultberg M, Lind O, Birgersson G, Asp H (2017) Use of the effluent from biogas production for cultivation of Spirulina. Bioprocess Biosyst Eng 40:625–631
Desmorieux H, Decaen N (2005) Convective drying of spirulina in thin layer. J Food Eng 66:497–503
Doke JM Jr (2005) An improved and efficient method for the extraction of phycocyanin from Spirulina sp. Int J Food Eng 1(5):1–13
Silva NC, Duarte CR, Barrozo MAS (2019) Dehydration of microalgae Spirulina platensis in a rotary drum with inert bed. Powder Technol 351(1):178–185
Nakagawa K, Ritcharoen W, Sri-Uam P, Pavasant P, Adachi S (2016) Antioxidant properties of convective-air-dried Spirulina maxima: evaluation of phycocyanin retention by a simple mathematical model of air-drying. Food Bioprod Process 100:292–302
Oliveira EG, Rosa GS, Moraes MA, Pinto LAA (2009) Characterization of thin layer drying of Spirulina platensis utilizing perpendicular air flow. Bioresour Technol 100:1297–1303
Show KY, Lee DJ, Tay JH, Lee TM, Chang JS (2015) Microalgal drying and cell disruption—recent advances. Bioresour Technol 184:258–266
Santos DA, Petri IJ, Duarte CR, Barrozo MAS (2013) Experimental and CFD study of the hydrodynamic behavior in a rotating drum. Powder Technol 250:52–62
Santos DA, Barrozo MAS, Duarte CR, Weigler F, Mellmann J (2016) Investigation of particle dynamics in a rotary drum by means of experiments and numerical simulations using DEM. Adv Powder Technol 27:692–703
Zhang L, Jiang Z, Weigler F, Mellmann J, Tsotsas E (2020) PTV measurement and DEM simulation of the particle motion in a flighted rotating drum. Powder Technol 363:23–37
Gu C, Zhang X, Li B, Yuan Z (2014) Study on heat and mass transfer of flexible filamentous particles in a rotary dryer. Powder Technol 267:234–239
Tada EFR, Bück A, Tsotsas E, Thomeo JC (2020) Mass transport in a partially filled horizontal drum: modelling and experiments. Chem Eng Sci 214:115448
Honorato GC (2006) Design of a rotary dryer for shrimp cephalothorax drying. Doctoral Thesis, Federal University of Rio Grande do Norte, Brazil, p 185
Moura BD (2016) Study of drying dynamics in a rotary dryer with intermittent feeding. Doctoral Thesis, Federal University of Rio Grande do Norte, Brazil, p 123
Pallai E, Szentmarjav T, Mujumdar AS (2007) Spouted bed drying. In: Mujumdar AS (ed) Handbook of industrial drying, 3rd edn. CRC Press-Taylor & Francis Group, Boca Raton, pp 363–385
Almeida ARF, Freire FB, Freire JT (2010) Transient analysis of pasty material drying in a spouted bed of inert particles. Dry Technol 28:330–340
Cunha FG, Santos KG, Barrozo MAS (2013) Mechanical extraction of natural dye from Bixa orellanna seeds in spouted bed. Ind Crop Prod 45:279–282
Freire JT, Ferreira MC, Freire FB, Nascimento BS (2012) A review on paste drying with inert particles as support medium. Dry Technol 30:330–341
Oliveira MS, Queiroz GM, Guimaraes RC, Ataide CH, Barrozo MAS (2007) Selectivity in phosphate column flotation. Min Eng 20:197–199
AOAC, Association of Official Analytical Chemists (1995) Official methods of analysis of the Association of Official Analytical Chemists, vol 1, 16th edn. AOAC, Gaithersburg
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Method Enzymol 299:152–178
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Costa BR, Rodrigues MCK, Rocha SF, Pohndorf RS, Larrosa APQ, Pinto LAA (2016) Optimization of Spirulina sp. Drying in heat pump: effects on the physicochemical properties and color parameters. J Food Process Pres 40:934–942
Bennett A, Bogorad L (1973) Complimentary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Barrozo MAS, Mujumdar AS, Freire JT (2014) Air-drying of seeds: a review. Dry Technol 32:1127–1141
Sablani SS, Rahman MS (2008) Fundamentals of food dehydration. In: Hui YH, Clary C, Farid MM, Fasina OO, Noomhorm A, Welti-Chanes J (eds) Food drying science and technology. DEStech Publication Inc, Lancaster-USA, pp 1–42
Fortes MCB, Silva AAM, Guimaraes RC, Ataide CH, Barrozo MAS (2007) Pre-separation of siliceous gangue in apatite flotation. Ind Eng Chem Res 46:7027–7029
Barrozo MAS, Murata VV, Costa SM (1998) The drying of soybean seeds in countercurrent and concurrent moving bed dryers. Dry Technol 16:2033–2047
Desmorieux H, Madiouli J, Herraud C, Mouaziz H (2010) Effects of size and form of Arthrospira Spirulina biomass on the shrinkage and porosity during drying. J Food Eng 100:585–595
Shibasaki-Kitakawa N, Iizuka Y, Takahashi A, Yonemoto T (2017) A kinetic model for flavonoid production in tea cell culture. Bioprocess Biosyst Eng 40:211–219
Silveira ST, Quines LKM, Burkert CAV, Kalil SJ (2008) Separation of phycocyanin from Spirulina platensis using ion exchange chromatography. Bioprocess Biosystem Eng 31:477–482
Oliveira EG, Duarte JH, Moraes K, Crexi VT, Pinto LAA (2010) Optimization of Spirulina platensis convective drying: evaluation of phycocyanin loss and lipid oxidation. Int J Food Sci Tech 45:1572–1578
Silveira ST, Burkert JFM, Costa JAV, Burkert CAV, Kalil SJ (2007) Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresource Technol 98:1629–1634
Sarada R, Pillai MG, Ravishankar GA (1999) Phycocyanin from Spirulina sp.: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem 34:795–801
Acknowledgements
The authors are grateful for the financial support of the Brazilian research funding agencies CNPq, Finep, Capes and Fapemig.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, N.C., Duarte, C.R. & Barrozo, M.A.S. Analysis of the use of a non-conventional rotary drum for dehydration of microalga Spirulina platensis. Bioprocess Biosyst Eng 43, 1359–1367 (2020). https://doi.org/10.1007/s00449-020-02329-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02329-1