Abstract
Spray drying is a very popular method for microalgal biomass drying; however, systematic research on the response of the biochemical composition during the process of spray drying has not been addressed thus far. This study investigated the influence of the inlet temperature and the initial solid content on the biochemical composition of spray-dried Scenedesmus acuminatus biomass. The fatty acid composition and contents of CHNS, lipids, carbohydrates, protein, starch, and pigments were analyzed to characterize the quality and bioactivity of the dried product. The results showed that the moisture content of the dried microalgal powder decreased with increasing inlet temperature and initial solid content, and the lowest moisture content of 2.37%, with a higher drying yield of 84%, was achieved at an optimized inlet temperature of 220 °C and an initial solid content of 16%. The biochemical compositions of CHNS, total lipids, carbohydrates, protein, starch, and fatty acids in the spray-dried biomass were similar to those in the freeze-dried biomass and were barely altered throughout the spray drying process. The pigment partially degraded as the inlet temperature increased; however, this degradation could be alleviated by increasing the initial solid content of the microalgal suspension because cell aggregates provided protection. Thermogravimetric analysis (TGA) further confirmed that spray drying did not affect the quality of proteins, lipids, or carbohydrates, suggesting that the spray drying technique could be applied to S. acuminatus for the production of both biofuel and nutritional supplements. These results may serve as a reference for the selection of the drying method, the utilization of the nutritional components in S. acuminatus, and the selection of biochemical parameters for spray drying performance evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Through highly efficient photosynthesis, microalgae convert carbon dioxide and other nutrients into biological molecules such as lipids, proteins, sugars, and pigments [1]. Thus, microalgae are widely used in the production of feed, food, fine nutraceuticals, and pharmaceutical products and in environmental remediation applications [2]. In recent years, studies on biofuel production using microalgal biomass have attracted much interest due to the numerous notable advantages of microalgae, such as their much higher biomass productivity than terrestrial plants, high lipid content (up to 20–50% of triacylglycerol), short growth period, and less or no requirement for arable land [3]. Biomass is currently the most widespread form of renewable energy, which could bring about positive impacts on economic, environmental, and health. Biomass from microalgae is considered as an ideal feedstock for the third-generation biodiesel, as it is not in competition with food crops, along with the advantage of considerably areal biomass yield compared to land crops. The development of microalgal biomass could be regarded as an efficient means to meet some of the main Sustainable Development Goals (SDGs) set by the United Nations General Assembly in 2015 for the year 2030, in particular, Affordable and Clean Energy [4].
Generally, microalgae production processes involve cultivation, harvesting, drying, extraction, and transformation stages. However, almost 60% of the total energy consumed in microalgal production is the drying process, which is necessary to avoid spoilage and facilitate transportation and storage [5]. Thus, after the microalgal biomass is harvested, the microalgal slurry (5–15% solid content) must be dried rapidly up to 90–95% (solid content) [6]. Moreover, to avoid affecting the subsequent biomass biorefineries, the drying process should ensure the quality of biomass in addition to the rapid reduction of moisture content [5], which may contribute to the overall sustainability of microalgal multiproduct production and accelerate the commercialization of microalgae-based bioproducts [7].
Currently, the common drying methods are rotary drying, freeze drying, flash drying, solar drying, convective drying vacuum shelf drying, and spray drying [5]. Among these methods, spray drying has become a very popular method for microalgal biomass drying. Spray drying has several advantages: flexible and continuous operation, fast drying speed, high throughput and yield, but more importantly, inexpensive operation [8]. Additionally, spray drying also presents excellent properties of protection, stabilization, solubility and controlled release of bioactive compounds, and is suitable for polysaccharides, lipids, and proteins, especially heat-sensitive ingredients [8, 9]. Generally, spray drying has advantages over freeze drying in terms of drying speed, continuous operation, and economic cost for the extraction of microalgae active components [10, 11]. Thus, spray drying has been widely used for different microalgal species, such as Spirulina [12], Chlorella [13], and Dunaliella [14] at the production scale and Nannochloropsis [15] at the laboratory scale.
Liu [16] found that the yield and moisture content of Chlorella powder and Chrysophyte powder were significantly affected by the inlet temperature and feed concentration during the spray drying process. Castejon et al.[9] reported that spray drying had little effect on the omega-3 lipids extracted from Nannochloropsis gaditana (no significant difference was observed at the 5% level). Palabiyik et al. [15] investigated chlorophyll-a and total carotenoid quantities of spray-dried Isochrysis galbana and Nannochloropsis oculata biomass at different inlet temperatures (150–200 °C) and found that higher pigment contents were obtained at 170 °C and 180 °C for I. galbana and N. oculate, respectively. Leach el al. [17] applied spray drying technology to dry Dunaliella salina to produce a β-carotene-rich powder; they indicated that a lower outlet temperature yielded higher carotenoid recoveries and that microencapsulation would significantly increase the storage stability. Foo et al. [18] successfully extracted fucoxanthin from Chaetoceros calcitrans using spray drying, and the produced fucoxanthin microcapsule showed good bioactivity. Loch-Neckel et al. [19] found that the extraction of dry biomass extracts from Haematococcus pluvialis using spray drying was especially promising for the production of raw pharmaceutical materials, with high total carotenoid values, and they did not observe a significant loss of antioxidant activity. Lin and Huang [11] compared the microstructures of spray-dried and freeze-dried microalgal powders and found that the total chlorophyll content was higher in the spray-dried powders than in freeze-dried Chiarella powders, and no coliforms were found in the spray-dried powders, though they ranged from 102 to 103 cells per gram in the freeze-dried powders.
Thus, spray drying technology can rapidly reduce the water content of microalgae biomass and protect the bioactive components, which indicates that this method could become the preferred drying technology for microalgae production. However, there is still a lack of systematic research on the influence of spray drying conditions on changes in biochemical composition, especially for Scenedesmus acuminatus biomass (Table 1), which may hinder the understanding of the biological activity and quality of microalgal powder after spray drying and thus affect the quality of subsequent microalgae products.
The objective of this study was to determine the influence of the inlet temperature and initial solid content of the spray drying process on the moisture content and drying yield. The contents of CHNS, lipids, carbohydrates, proteins, starch, fatty acids, and pigments under various drying conditions were contrasted to characterize the quality and bioactivity of the dried product, revealing the responsiveness of the biochemical composition of the products during the process of spray drying. The results may serve as a reference for the selection of drying method, utilization of the nutritional components in Scenedesmus acuminatus, and selection of biochemical parameters for spray drying performance evaluation.
Materials and Methods
Solvents and Reagents
Organic solvents (acetone, methanol, chloroform, dimethyl sulfoxide, diethyl ether, hexane, etc.) from Merck (Germany) were used. Chemicals for the culture media preparation (NaNO3, K2HPO4, Na2CO3, MgSO4·7H2O, CaCl2·2H2O, etc.) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Four pigment standards—lutein, zeaxanthin, chlorophyll b, and chlorophyll a—and one fatty acid methyl ester (FAME) standard mixture were purchased from Sigma-Aldrich (USA).
Microalgal cultivation and harvesting
S. acuminatus (GT-2, State Development & Investment Corp., China) was grown in tubular photobioreactors with a 5-cm light path and a culture volume of 13 m3. A modified BG-11 culture medium with a reduced nitrogen concentration of 16 mg L−1 NO3−-N was used to enhance lipid accumulation. The daily maximum solar light intensity inside the greenhouse was approximately 1200 μmol m−2 s−1, as measured with a light meter (LI-250A, Lincoln, USA). The average culture temperature was approximately 25 °C. During cultivation, air enriched with 2–4 L min−1 pure CO2 was provided intermittently to maintain the pH between 6.5 and 7.0. The concentration of S. acuminatus was initially 0.2 g L−1 of dry weight and reached 1 g L−1 within 13 days.
The S. acuminatus suspension was harvested by a membrane filtration unit with a filtration area of 180 m2, and the microalgal suspension was condensed to a 30 g L−1 slurry. Following centrifugation at 7600 × g with a disc stack centrifuge, 160 g L−1 S. acuminatus paste was collected, which was used as the raw material for the spray drying experiment. S. acuminatus suspensions with different concentrations of 80 g L−1, 120 g L−1, and 160 g L−1 were obtained by dilution of the centrifuge-collected paste with the centrifugation supernatant. The viscosities of the S. acuminatus suspensions of different concentrations were tested using a viscometer (NDJ-9S, Shanghai Pingxuan Scientific Instrument Co., Ltd., China), as shown in Figure S1. To understand how the drying process affects the quality of the dried powder, a control group was dried to form a powder using a freeze dryer (FreeZone 10 L, Labconco Corp., USA) at − 50 °C and 0.021 MPa for 72 h.
Spray Drying Experiment
The spray drying experiment was conducted with a laboratory-scale mini-spray dryer (B-290, Buchi, Switzerland) equipped with an air compressor (WSC 22140B, Huifeng, China). A fixed feed flow rate of 8 mL min−1 was used, and the atomizing air velocity was 473 L h−1. Therefore, the average residence time of microalgal cells in the chamber was 1.23 s according to the operating manual. Two key factors affecting the spray drying performance, the inlet temperature (120 °C, 170 °C, and 220 °C) and solid content (8%, 12%, and 16%), were evaluated for their influence on the drying performance and changes in the biochemical composition of the spray-dried S. acuminatus.
Analytical Methods
The moisture content, CHNS, total lipids, carbohydrates, protein, starch and FAMEs, pigment, morphology, particle size distribution, and thermal stability of S. acuminatus biomass after spray drying were analyzed to investigate the effect of the spray drying process on the quality of microalgal biomass. These analytic methods were as follows.
Moisture Content
The moisture content in the spray-dried biomass was analyzed gravimetrically using a heat-generating halogen analyzer (MB35, Ohaus, Switzerland). Approximately 1.0 g of the biomass was loaded onto an aluminum plate, and the temperature inside the weight chamber was increased to 105 °C. The measurement was completed when the weight reading stabilized, and the scale was accurate to 0.001 g. The results were expressed as the weight percent (w w−1) [20].
CHNS Analysis
The CHNS content in the spray-dried S. acuminatus biomass was determined using a CHNS/O analyzer (PE2400II, PerkinElmer Inc., USA) operated at a combustion temperature of 975 °C and a reduction temperature of 500 °C. The loaded samples (2–7 mg) were weighed with an autobalance (AD-6000, Perkin Elmer Inc., USA), which was accurate to 0.1 μg.
Total Lipids, Carbohydrates, Protein, Starch, and FAMEs
Carbohydrates were measured by a phenol–sulfuric acid method [21]. The protein content was analyzed using the Bradford method [22], which consisted of measurement of the A595 of the samples and standards against a reagent blank and then comparison to a standard curve for quantitation. The starch content was quantified using an assay kit [23] (STA20, Sigma-Aldrich) based on the catalytic hydrolysis of starch into glucose by α-amylase and amyloglucosidase, which involved measurement of the A540 using an ultraviolet spectrophotometer (Hach DR6000, USA) and calculation of the starch content according to the equation %Starch = (△ATEST)(900)/(△ASTD) (mg sample).
The total lipids were measured using a gravimetric method [24] after lipid extraction from the biomass using an accelerated solvent extraction system (Dionex 350, Thermo Fisher Scientific, CA, USA). Solvent A (methanol:DMSO, 9:1) and solvent B (hexane:diethyl ether, 1:1) were used to extract the total lipids. The results were expressed as a percentage: total lipid weight/algal weight (w w−1).
FAMEs produced from S. acuminatus oil were quantified using gas chromatography-mass spectrometry (GC–MS) (7890B-5977A, Agilent) with flame ionization detection. The GC column was a silica capillary column (HP-88 column; 60 m × 0.25 mm × 0.2 μm). All standards and samples were injected in split mode (split/column flow ratio of 20:1). The injection temperature was 250 °C. The initial oven temperature of 50 °C was held for 2 min, increased at a rate of 25 °C min−1 to 175 °C, held for 5 min, increased at 7 °C min−1 to 210 °C, held for 2 min, increased at 2 °C min−1 to 230 °C, and held for 1 min. The mass spectrometer was operated in electron impact (EI) mode at 70 eV over a scan range of m/z 50–650. The injected sample volume was 1.0 μL [25].
Pigment Extraction and Analysis
Spray-dried S. acuminatus powder was extracted using the same Dionex 350 solvent extraction system. A total of 50 mg of algal biomass was extracted with 5 mL of extraction solvent at 1500 psi and 100 °C for 3 min. The preheating time was set to 5 min, and the static cycle included two cycles and three methanol extractions. The flush volume at the end of the extraction was 45% of the cell volume, and the purge time was set to 30 s. After extraction, the pigment solutions were collected in a 50-mL brown volumetric flask.
The extracted pigments were determined using high-performance liquid chromatography (HPLC) (Waters Alliance e2695, Waters, USA). A Waters Spherisorb C18 column (250 × 5 mm, 4.6 μm) was installed. The pigment-extracted solution was subjected to HPLC analysis. The pigments were separated using a solvent mixture of 0.1 M Tris–HCl with a pH of 8.0, pure acetonitrile, methanol, and ethyl acetate [26]. The flow rate was maintained at 1.2 mL min−1, and the sample injection volume was 10 μL.
Based on the pigment concentrations in S. acuminatus, six working standard solutions of the pigment were prepared: 0, 0.001 mg mL−1, 0.002 mg mL−1, 0.005 mg mL−1, 0.01 mg mL−1, and 0.02 mg mL−1. Good linear relationships between the mass concentrations and the peak areas of the four pigment standards, i.e., lutein, zeaxanthin, chlorophyll b, and chlorophyll a, were observed at retention times of 9.443 min, 9.700 min, 10.343 min, and 11.392 min, respectively. The pigment contents in S. acuminatus were identified according to the retention times of the standards and quantified using the external standard method. The pigment chlorophyll a was detected at 664 nm, and the other pigments were detected at 445 nm using a diode array detector (2996, Waters, USA).
Morphology and Particle Size Distribution
The spray-dried powder was resuspended in water, and the morphology of the particles was examined using a microscope (CX31, Olympus Corp., Japan). The particle size distribution of the initial cells and spray-dried powder of S. acuminatus was measured using a laser diffraction particle size analyzer (Mastersizer 3000, Malvern UK), and the particle size distribution of the initial S. acuminatus cells with an average size of 6.95 ± 0.11 μm is shown in Figure S2.
Thermogravimetric Analysis (TGA)
The thermal stability of S. acuminatus biomass was measured by TGA using a thermogravimetric analyzer (TG 209C, NETZSCH Gerätebau GmbH, Germany). Approximately 26 mg of each sample was loaded into a 40 μL Al2O3 crucible for each measurement. The temperature inside the chamber was increased to 700 °C with a heating rate of 10 °C min−1 and a nitrogen gas flow rate of 50 mL min−1.
Statistical Analysis
All experiments were carried out in triplicate. The results are reported as the average values ± standard deviations. One-way analysis of variance (ANOVA) with a least significant difference (LSD) post hoc test was used to evaluate the statistical significance of the differences between the control and experimental groups. In all data analyses, a P value of 0.05 was considered statistically significant.
Results and Discussion
Influence of the Spray Drying Conditions on the Moisture Content and Drying Yield
The influence of the spray drying conditions on the moisture content of the powder is shown in Fig. 1A. The moisture content decreased from 3.95 to 2.37% as the inlet temperature increased from 120 to 220 °C at an initial solid content of 16%. The same trend was obtained for initial solid contents of 12% and 8%. In addition, an increase in the initial solid content from 8 to 16% led to a decrease in the moisture content from 3.97 to 3.22% at 170 °C. These results indicated that the moisture content of the spray-dried product was closely associated with both the inlet temperature of the drying air and the solid content of the feed suspension. Specifically, a higher inlet temperature and a higher initial solid content were beneficial for obtaining microalgal powder with higher solid content.
The outlet temperature and powder yield under different drying conditions are shown in Fig. 1B. With increasing inlet temperature, the outlet temperature increased significantly for all different treatments; however, the powder yield presented different trends of change. At an initial solid content of 8%, the yield of the microalgal powder increased rapidly from 65 to 92% when the inlet temperature was increased from 120 to 220 °C, whereas at an initial solid content of 16%, a high yield of 84% was maintained even at lower inlet temperatures. The water loading rate was relatively high at the lower initial solid content (8%), while the driving force was low at the lower inlet temperature (120 °C). As a result, S. acuminatus biomass cannot be fully dried at low inlet temperatures and initial solid contents, and thus, the yield was lower than those at higher inlet temperatures and higher initial solid contents.
The drying yield increased as the initial solid content in the feed and the inlet temperature also increased, and the highest dry mass production was obtained at an initial solid content of 16%, with a value of 76.8 g powder per hour at an inlet temperature of 220 °C. In general, increasing the initial solid content of the feed and increasing the inlet temperature are preferable over other procedural changes to obtain a higher yield [27]. The higher the initial solid content is, the more energy-efficient the spray drying process. However, according to Figure S1, the viscosity of the S. acuminatus suspension increased exponentially (R2 = 0.98) with increasing solid content, resulting in difficulty in spraying from the nozzle as the initial solid content exceeded 16%. In our recent study [28], the dewatering cost of membrane filtration increased by approximately 20% when an S. acuminatus suspension was concentrated from 8 to 16%. Therefore, the drying performance of an S. acuminatus suspension with initial solid contents of 8% and 12% was also studied to explore the integration of the dewatering process with the spray drying process.
Changes in the Biochemical Composition of the Spray-dried Biomass
The quality of dried microalgal biomass is also important because it affects the bioactivity of the main components and the feasibility of subsequent multiproduct applications. Therefore, changes in the biochemical composition, including CHNS, total lipid, carbohydrate, protein and starch, and fatty acid compositions of the spray-dried biomass were investigated and compared with those of freeze-dried biomass. The results are discussed as follows.
The CHNS Content
Microalgal cells mainly consist of carbon, hydrogen, nitrogen, and sulfur. The elemental composition of spray-dried S. acuminatus products at different inlet temperatures and initial solid contents is shown in Fig. 2. The C, H, N, and S contents of S. acuminatus in the freeze-dried biomass were 46.1 ± 0.25, 6.15 ± 0.54, 1.74 ± 0.04, and 0.95 ± 0.06, respectively. The C, H, N, and S contents in the spray-dried biomass at different inlet temperatures and initial solid contents varied over ranges of 43.8–45.6%, 5.06–5.62%, 1.81–2.47%, and 0.74–0.85%, respectively. These results were in accord with the literature, which shows that the carbon content of microalgal biomass ranges between 44.6 and 47.7%, and the nitrogen content ranges between 2.33 and 11.3% [29]. Additionally, no significant difference in the C, H, N, and S contents between the spray-dried samples and freeze-dried samples was observed, which demonstrated that spray drying under these conditions could achieve high-quality products. Hosseinizand et al. [30] also found that the contents of C, H, N, S, and O in dried Chlorella vulgaris biomass were almost constant with those obtained by freeze-drying as the temperature increased.
The Total Lipid, Carbohydrate, Protein, and Starch Contents
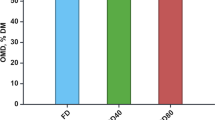
Table 2 lists the total lipid, carbohydrate, protein, and starch contents in the S. acuminatus biomass under different drying conditions. In the spray-dried biomass, the total lipid, carbohydrate, and protein contents were 33.6 ± 1.18, 37.4 ± 0.81, and 5.72 ± 0.39, respectively, and these three components accounted for 72.8–79.9% of the total biomass. Starch and FAMEs accounted for 16.7% and 27.8% of the S. acuminatus dry weight, respectively. Therefore, the total lipid, carbohydrate, protein, and starch contents, as well as the FAME contents, did not change obviously with different inlet temperatures and initial solid contents during the spray drying process, and these biological components were similar to those obtained by freeze drying. These results indicated that spray drying did not change the composition of these biological components under the conditions investigated in this paper.
Ryckebosch et al. [31] also found that spray and freeze-drying did not affect the total lipid content. However, Hosseinizand et al. [30] reported that carbohydrates and proteins in convection-dried Chlorella significantly decreased as the temperature increased from 60 to 140 °C and were obviously lower than those in freeze-dried Chlorella. These results indicated that spray drying has an advantage over convection drying in terms of protecting the biochemical composition because spray drying took only a few seconds, while convection drying consumed hundreds of minutes.
The fatty acid composition
Knothe et al. [32] reported that the FAME content in fuel directly corresponds to the fatty acid composition of the biomass feedstock, and the FAME content, in turn, determines the properties of the fuel. The FAMEs in S. acuminatus lipids mainly consisted of C18:1 (34.2%) and C16:0 (29.7%), and medium-chain fatty acids (≤ C18) were the predominant fatty acids in the biodiesel, as shown in Table 3. The results also showed that unsaturated fatty acids (UFAs) were the dominant components, comprising 68.1–68.7% of the total fatty acids in the biodiesel, and this result was similar to that obtained by Chen et al.[33]. Fuels rich in monounsaturated fatty acids (MUFAs) would have adequate cetane numbers (CNs), cold flow parameters, and viscosities [34], indicating that S. acuminatus is an ideal biodiesel feedstock.
At a fixed feed rate of 8 mL min−1, there were no significant changes in the carbohydrate, protein, lipid, starch, or FAME contents when the inlet temperature ranged from 120 to 220 °C, and the initial solid content varied from 8 to 16% during spray drying. These results implied that this spray drying technique could be applied to S. acuminatus for the production of both biofuel and nutritional supplements.
Influence of Spray Drying Conditions on the Pigment Content
The chlorophyll a, chlorophyll b, lutein, and zeaxanthin contents in spray-dried S. acuminatus under different drying conditions are shown in Fig. 3. At an initial solid content of 16%, the chlorophyll-a content significantly decreased as the inlet temperature increased: from 1.24 at an inlet temperature of 120 to 0.74 mg g−1 at 170 °C and then to 0.41 mg g−1 at 220 °C (Fig. 3A). Similar temperature-dependent trends of chlorophyll a were also observed at initial dry weights of 12% and 8%. Interestingly, at the same inlet temperature, chlorophyll a decreased with decreasing initial dry weight. The highest chlorophyll-a content (1.24 mg g−1) was achieved at an inlet temperature of 120 °C and an initial solid content of 16%.
Chlorophyll b also decreased obviously as the inlet temperature increased from 120 to 220 °C (Fig. 3B); however, the initial solid content did not have a significant effect on the chlorophyll b content. The trends of lutein with the inlet temperature for various initial solid contents were identical to that of chlorophyll a, but there was a smaller range of variation (Fig. 3C). Additionally, there were no significant changes in the zeaxanthin content under different drying conditions (Fig. 3D), potentially because zeaxanthin is somewhat resistant to changes in heat and light, but direct sunlight and high temperature applied for long periods negatively impact its amenability to spray drying [35].
In summary, with an increase in inlet temperature from 120 to 220 °C, the total contents of pigments significantly decreased as a result of pigment degradation at high temperatures, which was in accordance with the result reported by Leach et al. that β-carotene recovery of Dunaliella salina decreased as the inlet temperature increased from 200 to 265 °C [17]. Palabiyik et al. [15] found that chlorophyll a and carotenoids of N. oculate increased first as the temperature increased from 150 to 180 °C and then decreased as the temperature increased to 200 °C, while they were lowest at 180 °C for I. galbana. These results may indicate that pigments extracted from different microalgae present various thermal sensitivities. When the inlet temperature was maintained at 120 °C, the contents of all pigments except chlorophyll b decreased obviously with the reduction in the algal biomass from 16 to 8%. A possible reason for this result is that as the algal biomass in the raw material increases, more agglomeration of the product occurs, resulting in the protection of the pigments by the agglomerates. The results suggested that when spray drying is used for microalgal-based pigment production, the pigment content might be a useful indicator for the optimization of the spray drying process at the industrial scale, and the pigments could be protected through the formation of large particles.
The particle size distributions of S. acuminatus powder dried with different initial solid contents at 120 °C are shown in Fig. 4A. The fraction of large particles in the dried S. acuminatus powder increased obviously as the initial solid content increased. Specifically, aggregates formed more easily when the initial solid content was high during the spray drying process due to the high viscosity of the sprayed slurry. This phenomenon may be similar to the artificial microencapsulation that occurs during the spray drying process; it is used to protect, transport, or control the release of active compounds [9]. Leach et al. [17] found that microencapsulation of β-carotene-rich powder produced from Dunaliella salina using spray drying technology would significantly increase the storage stability.
Figure 4B shows a microscopy image of spray-dried S. acuminatus powder at an initial dry weight of 16%. In addition to normal single S. acuminatus cells (5 μm), an increasing number of aggregates (20–30 μm) were generated with increasing initial solid content, which agreed with other authors’ observations of microcapsules that were produced by spray drying and ranged up to 30 μm [9]. Studies have shown that artificial microcapsules can achieve high physical protection of bioactive compounds from degradation [18]. The SEM images of the spray-dried cells and the freeze-dried S. acuminatus are shown in Fig. S3. The surface of the spray-dried cells looks smoother than that of the freeze-dried, indicating that the shape of the cell is kept well during the spray drying process. This may be due to its fast process of several seconds, compared with the more than 24 h of drying due to the freeze-drying. It is unknown whether the permeabilities of cell walls after these drying methods are different or not, and whether this will affect the subsequent extraction of the bioproducts. More studies are needed to reveal the changes in cellular characteristics after different drying methods, which would benefit the development of the biorefinery process.
Under the same drying conditions, the increase in particle size of spray-dried products at the higher initial dry weight may be caused by the increased droplet size as a result of the higher viscosity [36]. The viscosities of S. acuminatus slurries with 8%, 12%, and 16% initial solid contents were 3.83 cP, 10.2 cP, and 108.65 cP, respectively, which also indicated that the change in viscosity, rather than the initial solid content, affected the size distribution of S. acuminatus in the dried biomass. The shrinkage ratio decreased with increased viscosity, implying that the droplets produced by spraying slurries with higher initial solid contents and consequently higher viscosity may form a crust at an earlier stage of drying because they were more easily saturated, preventing further shrinkage upon drying [37]. As a result, the degradation of pigments inside the aggregates was reduced at higher initial solid contents, which contributed to the higher pigment content in the dried biomass.
Analysis of the Thermal Decomposition Process
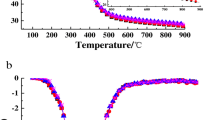
The TGA and the rate of weight loss-derivative thermogravimetry (DTG) curves of both the freeze-dried and spray-dried samples (16% solid content, dried at 220 °C) are shown in Fig. 5. The rate of temperature increase was set at 10 K min−1 under a N2 atmosphere. Three individual stages were distinguished during the combustion process [38]. The first stage extended from room temperature to 150 °C and corresponded to the loss of moisture and highly volatile compounds [39], resulting in nearly 5% weight loss. The second stage extended from 150 to 485 °C and was attributed to the decomposition of most organic compounds [40], leading to approximately 14% solid residue formation. During the second stage, three strong peaks at approximately 250.8 °C, 300.5 °C, and 385.6 °C were observed; these were attributed to the decomposition of lipids, carbohydrates, and proteins, respectively [41]. The third stage extended from 485 to 700 °C, with the TGA curve decreasing slowly but the DTG curve remaining almost horizontal. The weight loss was much smaller than that observed in stage two, which could be a result of the continued decomposition of carbon through further breakage of C–C and C–H bonds [42]. Similar results for other microalgal species have been reported in the literature [43,44,45,46].
The outlet temperatures were below 130 °C, which is below the degradation temperatures of the nutritional ingredients indicated by the TGA results. In addition, both freeze drying and spray drying produced almost the same TGA curve (Fig. 5), suggesting that spray drying had no noticeable influence on the gravimetric curve of the dried biomass compared to freeze drying. As the temperature remained below the wet-bulb temperature of the drying gas until drying was almost complete [47], the spray drying conditions did not affect the quality of proteins, lipids, or carbohydrates, confirming previous conclusions.
Thus, spray drying can achieve the rapid drying of microalgal biomass and has no effect on the biological components except the pigment, and the degradation of pigments would be alleviated by increasing the initial solid content of the microalgal suspension. Additionally, for biodiesel and feed production, higher inlet temperatures and relatively higher dry weight concentrations are recommended to obtain lipids, carbohydrates, and proteins in higher yields. However, for pigment production, reducing the inlet temperature and increasing the solid content during spray drying will yield high-quality pigment products. The concept of microalgae biorefineries of multiproduct microalgae systems has been proposed recently [7], Studying the influence of spray drying on the quality of biomass would be critical to systematically understand the effects of spray drying conditions on the biochemical composition of microalgae biomass, and thus contribute to the process development of advanced and sustainable microalgae biorefineries.
This study investigated the influence of spray drying conditions (inlet temperature and initial solid content) on the biochemical composition of Scenedesmus acuminatus biomass on an experimental scale, and the results were compared with those of freeze drying. Effects on the extraction and biorefinery of active substances, bioactivity analysis for subsequent specific products, and a larger scale of validation are also required. Although spray drying is one of the most commonly used drying techniques for many microalgae genera, especially for heat-sensitive components and has been applied in practical production [48], future studies should also consider its economic and sustainability features in the entire microalgae production process [49, 50]. It needs to be mentioned that the parameters obtained in this study may need to be fine-tuned when using industrial-scale spray dryer as particle formation, as well as mass and heat transfers inside the dryer are different.
Conclusions and Future Directions
This study investigated the influence of the inlet temperature and initial solid content of the spray drying process on the moisture content, drying yield, and biochemical composition of the products. The results showed that the moisture content and drying yield presented obvious differences under various spay drying conditions; specifically, the moisture content of the dried microalgal powder decreased with increasing inlet temperature and initial solid content, while the drying yield showed the opposite trend. The lowest moisture content of 2.37% with a higher drying yield of 84% was achieved at an inlet temperature of 220 °C and an initial solid content of 16%. A comparison of the biochemical composition with that obtained by freeze-drying demonstrated that there were almost no significant differences in the total lipids, carbohydrates, proteins, starch, and fatty acids in spray-dried microalgal powder. However, the contents of chlorophyll a, chlorophyll b, and lutein decreased with increasing inlet temperature and increased as the initial solid content increased, which indicated that pigments were sensitive to the spray drying conditions. Pigment degradation could be alleviated by increasing the initial solid content, resulting from the protection of cell aggregates. TGA also suggested that spray drying did not affect the quality of proteins, lipids, and carbohydrates.
These results may help us to understand the response of the biochemical composition of microalgal biomass during the process of spray drying; and provide a reference for the selection of drying methods, utilization of nutritional components in S. acuminatus, and selection of biochemical parameters for spray drying performance evaluation. For spray drying applied to microalgae to produce a specific product, effects of spray-dried biomass on the extraction and biorefinery of active substances, bioactivity analysis for subsequent specific products, and a larger scale of validation are also required in future studies. Additionally, the economic and sustainability features of spray drying in the entire microalgae production process should be also considered.
Abbreviations
- S. acuminatus :
-
Scenedesmus acuminatus
- FAME:
-
Fatty acid methyl ester
- CHNS:
-
Carbon, hydrogen, nitrogen, sulfur
- UFA:
-
Unsaturated fatty acid
- TGA:
-
Thermogravimetric analysis
- MUFA:
-
Monounsaturated fatty acid
- CN:
-
Cetane number
- DTG:
-
Derivative thermogravimetry
References
Khan SA, Rashmi HMZ, Prasad S, Banerjee UC (2009) Prospects of biodiesel production from microalgae in India. Renew Sustain Energy Rev 13(9):2361–2372. https://doi.org/10.1016/j.rser.2009.04.005
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. https://doi.org/10.1263/jbb.101.87
Paone E, Tursi A (2021) Production of biodiesel from biomass. Advances in Bioenergy and Microfluidic Applications 165–192 https://doi.org/10.1016/b978-0-12-821601-9.00006-6
Tursi A (2019) A review on biomass: importance, chemistry, classification, and conversion. Biofuel Res J 6(2):962–979. https://doi.org/10.18331/brj2019.6.2.3
Villagracia ARC, Mayol AP, Ubando AT, Biona JBMM, Arboleda NB, David MY, Tumlos RB, Lee H, Lin OH, Espiritu RA, Culaba AB, Kasai H (2016) Microwave drying characteristics of microalgae (Chlorella vulgaris) for biofuel production. Clean Technol Environ Policy 18(8):2441–2451. https://doi.org/10.1007/s10098-016-1169-0
Hosseinizand H, Lim CJ, Webb E, Sokhansanj S (2017) Economic analysis of drying microalgae Chlorella in a conveyor belt dryer with recycled heat from a power plant. Appl Therm Eng 124:525–532. https://doi.org/10.1016/j.applthermaleng.2017.06.047
Potijun S, Jaingam S, Sanevas N, Vajrodaya S, Sirikhachornkit A (2020) Improving the co-production of triacylglycerol and isoprenoids in Chlamydomonas. Biofuel Res J 7(4):1235–1244. https://doi.org/10.18331/brj2020.7.4.2
Ray S, Raychaudhuri U, Chakraborty R (2016) An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci 13:76–83. https://doi.org/10.1016/j.fbio.2015.12.009
Castejón N, Luna P, Señoráns FJ (2021) Microencapsulation by spray drying of omega-3 lipids extracted from oilseeds and microalgae: effect on polyunsaturated fatty acid composition. Lwt 148https://doi.org/10.1016/j.lwt.2021.111789
Aliyu A, Lee JGM, Harvey AP (2021) Microalgae for biofuels via thermochemical conversion processes: a review of cultivation, harvesting and drying processes, and the associated opportunities for integrated production. Bioresour Technol Rep 14:100676. https://doi.org/10.1016/j.biteb.2021.100676
Lin LP (1985) Microstructures of spray-dried and freeze-dried microalgal powders. Food Microstructure 4(2):341–348
Dong J, Wu B, Xiang W, Xia D, He M (1996) Spray drying technology in large-scale production of Spirulina platensis SCS powder. J China Agric Univ 1:40–44
Englmaierová M, Skřivan M, Bubancová I (2013) A comparison of lutein, spray-dried Chlorella, and synthetic carotenoids effects on yolk colour, oxidative stability, and reproductive performance of laying hens. Czech J Anim Sci 58(No. 9):412–419. https://doi.org/10.17221/6941-cjas
Orset S, Leach GC, Morais R, Young AJ (1999) Spray-drying of the microalga Dunaliella salina: effects on beta-carotene content and isomer composition. J Agric Food Chem 47(11):4782–4790. https://doi.org/10.1021/jf990571e
Palabiyik I, Durmaz Y, Öner B, Toker OS, Coksari G, Konar N, Tamtürk F (2017) Using spray-dried microalgae as a natural coloring agent in chewing gum: effects on color, sensory, and textural properties. J Appl Phycol 30(2):1031–1039. https://doi.org/10.1007/s10811-017-1324-y
Liu Q (2015) Optimization of spray drying in processing two kinds of microalgae and production of microalgae bread. Dalian, Dalian Ocean University: 1–53
Leach G, Oliveira G, Morais R (1998) Spray-drying of Dunaliella salina to produce a beta-carotene rich powder. J Ind Microbiol Biotechnol 20(2):82–85. https://doi.org/10.1038/sj.jim.2900485
Foo SC, Khong NMH, Yusoff FM (2020) Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Research 51. https://doi.org/10.1016/j.algal.2020.102061
Loch-Neckel G, Schutz FE, Derner RB, Lemos-Senna E (2018) Obtaining dried extracts from the biomass of the microalga Haematococcus pluvialis by spray drying. Materia-Rio De Janeiro 23 (4). https://doi.org/10.1590/s1517-707620180004.0555
Beringhs AO, Souza FM, de Campos AM, Ferraz HG, Sonaglio D (2013) Technological development of Cecropia glaziovi extract pellets by extrusion-spheronization. Rev Bras 23(1):160–168. https://doi.org/10.1590/s0102-695x2012005000123
Jia J, Han D, Gerken HG, Li Y, Sommerfeld M, Hu Q, Xu J (2015) Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res 7:66–77. https://doi.org/10.1016/j.algal.2014.11.005
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Tan KW, Lin H, Shen H, Lee YK (2016) Nitrogen-induced metabolic changes and molecular determinants of carbon allocation in Dunaliella tertiolecta. Sci Rep 6:37235. https://doi.org/10.1038/srep37235
Wen X, Jiang L, Geng Y, Shen X, Li Y (2012) Comparative study of quantitative analysis methods for microalgae total lipid. China Oils and Fats 37:80–85
Musharraf SG, Ahmed MA, Zehra N, Kabir N, Choudhary MI, Rahman A-u (2012)Biodiesel production from microalgal isolates of southern Pakistan and quantification of FAMEs by GC-MS/MS analysisChem Cent J 6https://doi.org/10.1186/1752-153x-6-149
Baek K, Kim DH, Jeong J, Sim SJ, Melis A, Kim JS, Jin E, Bae S (2016) DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci Rep 6:30620. https://doi.org/10.1038/srep30620
Lin Y, Liu Y, Wang L, Xie Y, Gao Z, Wang S (2018) Optimization of drying conditions and components to reduce wall sticking during spray drying of infant formula milk. Int J Agric Biol Eng 11(2):214–218. https://doi.org/10.25165/j.ijabe.20181102.2788
Wang L, Pan B, Gao Y, Li C, Ye J, Yang L, Chen Y, Hu Q, Zhang X (2019) Efficient membrane microalgal harvesting: pilot-scale performance and techno-economic analysis. J Clean Prod 218:83–95. https://doi.org/10.1016/j.jclepro.2019.01.321
Makareviciene PdV, Skorupskaitė V, Andrulevičiūtė V (2013) Biomass and oil production of green microalgae Scenedesmus sp. using different nutrients and growth. Environ Res Eng Manag 62(4):9. https://doi.org/10.5755/j01.erem.62.4.2318
Hosseinizand H, Sokhansanj S, Lim CJ (2018) Studying the drying mechanism of microalgae Chlorella vulgaris and the optimum drying temperature to preserve quality characteristics. Drying Technol 36(9):1049–1060. https://doi.org/10.1080/07373937.2017.1369986
Ryckebosch E, Muylaert K, Eeckhout M, Ruyssen T, Foubert I (2011) Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J Agric Food Chem 59(20):11063–11069. https://doi.org/10.1021/jf2025456
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels 22(2):1358–1364. https://doi.org/10.1021/ef700639e
Chen L, Liu T, Zhang W, Chen X, Wang J (2012) Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour Technol 111:208–214. https://doi.org/10.1016/j.biortech.2012.02.033
Stansell GR, Gray VM, Sym SD (2011) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24(4):791–801. https://doi.org/10.1007/s10811-011-9696-x
Zhang Z, Yan B, Luo Q (2003) Stability in different condition of zeaxanthin from corn. China Food Additives (z1): 241–246, 233
Liu W, Wu W, Selomulya C, Chen XD (2011) A single step assembly of uniform microparticles for controlled release applications. Soft Matter 7(7):3323. https://doi.org/10.1039/c0sm01371d
Wu WD, Liu W, Gengenbach T, Woo MW, Selomulya C, Chen XD, Weeks M (2014) Towards spray drying of high solids dairy liquid: effects of feed solid content on particle structure and functionality. J Food Eng 123:130–135. https://doi.org/10.1016/j.jfoodeng.2013.05.013
Wang S, Jiang X, Wang N, Lijun Yu, Zhen LI, He P (2009) Study on pyrolysis and combustion characteristics of seaweed biomass. J Power Eng 2009:596–601
Chen C, Qi Q, Huang D, Zeng T, Bu X, Huang Y, Huang H (2021) Effect of additive mixture on microwave-assisted catalysis pyrolysis of microalgae. Energy 229 https://doi.org/10.1016/j.energy.2021.120752
Chen C, Ma X, He Y (2012) Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresour Technol 117:264–273. https://doi.org/10.1016/j.biortech.2012.04.077
Arif M, Li Y, El-Dalatony MM, Zhang C, Li X, Salama E-S (2021) A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: an approach for biofuel generation and nutrients removal. Renewable Energy 163:1973–1982. https://doi.org/10.1016/j.renene.2020.10.066
Zou S, Yulong Wu, Yang M, Zhang J, Li C, Tong J (2007) Characteristics and dynamics of pyrolysis process microalgae. J Combust Sci Technol 13:330–334
Tahmasebi A, Kassim MA, Yu J, Bhattacharya S (2013) Thermogravimetric study of the combustion of Tetraselmis suecica microalgae and its blend with a Victorian brown coal in O2/N2 and O2/CO2 atmospheres. Bioresour Technol 150:15–27. https://doi.org/10.1016/j.biortech.2013.09.113
Chen C, Ma X, Liu K (2011) Thermogravimetric analysis of microalgae combustion under different oxygen supply concentrations. Appl Energy 88(9):3189–3196. https://doi.org/10.1016/j.apenergy.2011.03.003
Sanchez-Silva L, Lopez-Gonzalez D, Garcia-Minguillan AM, Valverde JL (2013) Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae. Bioresour Technol 130:321–331. https://doi.org/10.1016/j.biortech.2012.12.002
Tang Y, Ma X, Lai Z (2011) Thermogravimetric analysis of the combustion of microalgae and microalgae blended with waste in N2/O2 and CO2/O2 atmospheres. Bioresour Technol 102(2):1879–1885. https://doi.org/10.1016/j.biortech.2010.07.088
Richardson Jf, Harker JH, Backhurst JR (2002) Particle technology and separation processes, Oxford: Butterworth-Heinemann:1183
Morais Junior WG, Gorgich M, Corrêa PS, Martins AA, Mata TM, Caetano NS (2020) Microalgae for biotechnological applications: cultivation, harvesting and biomass processing. Aquaculture 528:735562. https://doi.org/10.1016/j.aquaculture.2020.735562
Aghbashlo M, Mandegari M, Tabatabaei M, Farzad S, Mojarab Soufiyan M, Görgens JF (2018) Exergy analysis of a lignocellulosic-based biorefinery annexed to a sugarcane mill for simultaneous lactic acid and electricity production. Energy 149:623–638. https://doi.org/10.1016/j.energy.2018.02.063
Soltanian S, Aghbashlo M, Almasi F, Hosseinzadeh-Bandbafha H, Nizami A-S, Ok YS, Lam SS, Tabatabaei M (2020) A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers Manage 212:112792. https://doi.org/10.1016/j.enconman.2020.112792
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51909258), the National Program on Basic Research Project for Governmental International Scientific and Technological Innovation Cooperation of China (2018YFE0110600), and the Key Laboratory of Algal Biology, Institute of Hydrobiology, Chinese Academy of Sciences. Special thanks to Dr. Minsung Park for his valuable suggestions on the manuscript’s revision.
Author information
Authors and Affiliations
Contributions
Haiyang Zhang conducted all the related analytical work and drafted the manuscript. Ting Gong, Jing Li, and Bo Pan conducted the spray drying experiment. Xuezhi Zhang and Ming Duan provided suggestions on the manuscript preparation and finalized the revised manuscript. Qiang Hu provided critical comments on the manuscript revision.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All authors agree with the content and all give explicit consent to submit the paper.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Gong, T., Li, J. et al. Study on the Effect of Spray Drying Process on the Quality of Microalgal Biomass: a Comprehensive Biocomposition Analysis of Spray-Dried S. acuminatus Biomass. Bioenerg. Res. 15, 320–333 (2022). https://doi.org/10.1007/s12155-021-10343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10343-8