Abstract
We investigated physiological responses of Ulva australis to metals and their implications for biomonitoring and management tool. To determine the capacity of Ulva to accumulate metals over the short-term, we undertook an in situ experiment where we transplanted thalli to sites with different levels of metal pollution. After 12 days, arsenic, copper, lead, and zinc accumulation was observed. Zinc was significantly greater (p = 0.001) at the most polluted site and was highly correlated (r = 0.87) with seawater total Zn concentration. We also assessed whether metal exposure can compromise U. australis performance by evaluating physiological responses and changes in thalli ultrastructure. We observed an increase in electron-dense bodies in the cell walls and vacuoles, which clearly indicates metal accumulation. However, there was no change in physiological performance (i.e. growth rate, Fv/Fm, rETRmax, or in photosynthetic pigments content) between the control and transplanted thalli (p > 0.05). Bioaccumulation capacity of U. australis was assessed by deploying thalli at a highly polluted site for 45 days, where zinc in Ulva markedly increased over time and was highly correlated with the environmental concentrations (total Zn in seawater, r = 0.85). The metal uptake rate increased steadily over time, confirming that Ulva is clearly capable of bioaccumulation. However, visual examination of the thalli suggested degradation over time, which might limit deployment time (20 days). Clearly, U. australis has potential as a biomonitor/management tool, particularly for zinc, but the results suggest it may be a useful tool for removing metals from the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is a worldwide concern, with a broad range of anthropogenic activities contributing metals to our environment, resulting in long-lasting impacts on aquatic ecosystems (Bird et al. 1998). As the human population has increased exponentially, the impact of contaminants on the environment has also increased (Kumar et al. 2011). Heavy metals are toxic, non-degradable contaminants, which can accumulate in living organisms (Fu and Wang 2011). Metal contamination can arise from both natural sources and human activities (Hurd et al. 2014). Estuaries are areas where contaminants accumulate (Guisti 2001), mainly because there are attractive places for human settlement. The Derwent Estuary in Tasmania, Australia, is heavily polluted by metals, largely as a result of the legacy of past industry. The estuary has been monitored for more than 15 years (Whitehead et al. 2010; Coughanowr et al. 2015), and this clearly shows that despite the marked reductions in metal inputs, some areas remain highly contaminated, particularly in the middle estuary, whilst the lower estuary has much lower levels (Whitehead et al. 2010).
Where metal pollution is a concern, there is the potential for bioaccumulation, and this can have adverse implications for both the ecosystem and human health. Biomonitoring is a technique that provides information on the contaminants that my affect ecosystems (Phillips and Rainbow 1994; Zhou et al. 2008). There are two ways that this can be achieved, the first is the ‘active’ approach that relies on native organisms to monitor environmental conditions; the second is the ‘passive’ approach which relies on transplanted organisms placed in the selected study area for a fixed period of time (Lacroix et al. 2015; Ronci et al. 2016). Active biomonitoring (ABM) is the approach most commonly used to evaluate metal impacts in the environment and is the focus of most environmental monitoring programs. However, a combination of the ABM and passive biomonitoring (PBM) can provide a better understanding of the impacts on the broader ecology (Brown et al. 2012) as this approach would provide an appreciation of both the short-term (PMB) and longer-term impacts (ABM). Where pollution exists, it may be desirable to find a way to remediate the affected areas and reduce detrimental impacts to the aquatic environment. Bioremediation is a process that utilises living organisms which have the ability to accumulate or degrade chemical contaminants into less toxic forms to clean up polluted environments (Vidali 2001; Juwarkar et al. 2010; Kumar et al. 2011). Heavy metal removal is a cost-effective alternative where bioremediation has been demonstrated in a range of organisms such as bacteria, fungi, plants and algae (Vieria and Volesky 2000; Juwarkar et al. 2010).
Many seaweed species (Phyla Ochrophyta (brown), Rhodophyta (red) and Chlorophyta (green)), have been shown to have the potential to accumulate metals in ABM (Luoma et al. 1982; Ho 1990; Brown et al. 1999; Zbikowski et al. 2007; Boubonari et al. 2008; Ryan et al. 2012), but there is little information on the potential of algae to be used for PMB. There have been many studies looking at the types of seaweeds that would be most suitable for bioremediation of nutrient-enrichment, with many of these focussed on the interactions of aquaculture and Integrated Multi-trophic Aquaculture (IMTA) (Troell et al. 1997, 1999; Chopin et al. 1999; Zhou et al. 2006; Buschmann et al. 2009; Grote 2016), but there is less information about the specific applications of seaweeds for bioremediation of metal-polluted environments.
Some studies have evaluated heavy metal effects in different species of seaweed, looking at physiological responses and ultrastructural alterations under laboratory or controlled conditions (Pellegrini et al. 1991; Baumann et al. 2009; Zakeri and Abu Bakar 2012; Gouveia et al. 2013; Felix et al. 2014; Santos et al. 2014). However, although laboratory experiments can provide useful information, there is still need to understand how such species might perform under real-world, field conditions. Field experimentation, particularly information obtained from transplant experiments, should complement laboratory experiments (Rainbow 1995) and provide a much better overall understanding of bioaccumulation and remediation potential. Although there are some studies that have looked at in situ metal uptake or metal release in seaweeds (Myklestad and Eide 1978; Ho 1984, 1990; Malea et al. 1995; Malea & Haritonidis 2000; Boubonari et al. 2008; Ryan et al. 2012), others have examined in vitro how heavy metal pollution affects the physiological performance and ultrastructure of species such as Ulva sp., Ulva laetevirens and Ulva intestinalis (Schiavon et al. 2012; Vecchia et al. 2012; Andrade et al. 2004). A field study of Ulva lactuca and Sargassum stenophyllum found them to be relatively pollution-tolerant but showed that in relation to an increasing urban pollution gradient, the physiological performance of S. stenophyllum declined while U. lactuca improved along an urban pollution gradient (Scherner et al. 2012). In general, the cosmopolitan ulvacean species are good indicators of pollution (Rainbow and Phillips 1993). We selected Ulva australis for the focus of this study as recent research has shown that it takes up metals and is widely distributed throughout of the Derwent Estuary (D. Farias, unpublished data).
There is evidence that Ulva can tolerate metal pollution and that under controlled conditions may be useful for metal remediation, but it is still not clear how effective these algae might be as in situ remediation tools in highly metal-polluted systems. Under highly polluted conditions, the remediation potential of seaweeds may actually be limited by their ability to survive the complex mix of anthropogenic stressors that are present at these locations not causing mortality (Volesky 1990; Pinto et al. 2003). In this study, we assess in situ the biomonitoring and bioaccumulation potential of U. australis to determine whether it might be a suitable management tool in a highly metal-impacted environment. The aims of this study were to use deployed thalli to evaluate whether metal uptake can compromise the physiology of U. australis, to determine to what extent U. australis can accumulate metals in contaminated areas and assess the management implications for using U. australis as a biomonitor.
Methods
Study sites
The study sites were located to reflect a range of metal-impacted levels. An unpolluted control site was located 73 km southeast of Hobart city (42°52′S/147°19′E) on Bruny Island (BI), well beyond the influence of any metal contamination from the Derwent Estuary (Fig. 1 and Table 1). Two sites with historical “metal pollution” were selected from the Derwent Estuary (Coughanowr et al. 2015), to receive transplanted seaweeds collected from control site. Transplant site 1 (TS1) is a recreational area located in the middle-lower estuary and transplant site 2 (TS2) is an enclosed bay, situated in the middle estuary (Fig. 1). TS2 is mainly affected by contaminants derived from urbanisation, recreational activities and industrial discharge and is characterised by high levels of metal such as cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) identified in seawater and sediment. This region also has the highest levels of ammonia + ammonium and nitrate-nitrite in the estuary (Whitehead et al. 2010; Coughanowr et al. 2015).
Metal content in U. australis and physiological and ultrastructural performance
To evaluate metal accumulation capacity and whether metals can compromise U. australis performance, we used passive biomonitoring approach to analyse physiological and ultrastructural responses.
Thalli were collected from the control site, on 19 August 2015, and transplanted back into the same site and to sites TS1 and TS2. Forty-five individuals of 3.1 ± 1.5 g were haphazardly collected from the shoreline at low tide and transported in an insulated container with seawater to be transplanted elsewhere at the control site and at TS1 and TS2 the same day.
Ulva thalli were weighed and after thalli placed in each of five plastic mesh (0.6 cm mesh pore) baskets that were 18 cm height × 13 cm diameter (n = 5). Each of the five baskets was attached to a 1.5 m long × 2.5 cm diameter steel pole: the baskets containing Ulva plus the pole comprised one experimental unit (Fig. 2). Three experimental units (n = 3) were pushed into the sediment by snorkelling ensuring the top of the baskets were deployed at 2-m depth at high tide. The poles at each site were positioned to ensure they were 5 m away from one another. Limited replication (n = 3) was tested due to practical deployment conditions on the field. After 12 days, the Ulva thalli at each site were collected and metal content, growth rate, photosynthetic performance and concentration of photosynthetic pigments were assessed (see below). The ultrastructure of each thallus was examined (see below) and any differences noted. Additionally, ‘ambient’ Ulva plants (i.e. those that had grown naturally at each site) were collected from control site, TS1 and TS2, from the shoreline during low tide for metal comparison with the metal content in transplanted thalli.
Metal content in thalli
Metal content in U. australis was measured at the start and end of the experiment on both the transplanted thalli and ambient thalli. Ulva samples were transported to the laboratory in an insulated container, and then rinsed to remove epiphytes and sand following the methods of Gledhill et al. (1998). To avoid contamination, all plastic ware were washed with alkaline detergent Decon 90.2% solution and 5% HNO3 acid bath for at least 24 h. All material was then rinsed with Milli-Q water (18 MΩ).
Initial wet weight (W i ) was recorded prior to freezing at −24 °C for up to a week until analysis. Frozen samples were then freeze-dried for 48 h until constant weight and the final weight (W f ) recorded. Samples were then ground in a coffee grinder to <2-mm particle size. Ground freeze-dried samples were sent to Analytical Services Tasmania (AST), a National Association of Testing Authorities, Australia (NATA) certified laboratory, for analysis of As, Cu, Pb and Zn using an inductively coupled plasma atomic emission spectroscopy (ICP-AES). A certified reference material (CRM), dogfish muscle CRM DORM-2, was used to test the accuracy and precision of the analysis. The metal detection limit of this analysis was 0.1 mg kg−1 dry matter basis (DMB) for As, Cu, Pb and Zn. Any evidence of damage to the thalli was also noted as a potential response to the experiment.
Growth rate
Growth rate (Gr) of U. australis was calculated by comparing the initial and final wet weight, following Yong et al. (2013). Gr (% day−1) = ((Wt/Wi)1/t − 1) × 100, where W t = final wet weight (g), W i = initial wet weight (g) and t = time (days).
Photosynthetic performance
Chlorophyll a fluorescence in U. australis was measured using a pulse-amplitude modulated (PAM) diving fluorometer (Walz, Germany). The PAM was configured at Gain 4 according to Scherner et al. (2013). Before every pulse measurement, seaweeds were dark-adapted for 20 min using Walz underwater clips. Measurements were performed in situ on each replicate (n = 3) transplant sample before the experiment was started and at the end of the experiment and on the ambient Ulva thalli from every study site (n = 3). Photosynthetic parameters measured were maximum quantum yield of photosystem II (PSII) = Fv/Fm and maximum relative electron transport rate (rETRmax), which is the maximal relative rate of electron transport activity (μmol electrons m−2 s−1) (Longstaff et al. 2002; Schreiber et al. 2011). rETRmax was calculated from the rapid light curves (RLCs) as described in Ralph and Gademann (2005) using the exponential curve model of Platt et al. (1980). All parameters were calculated by the PAM processor version 1.0 (PAM-Processor 2015) using R (R Core Team 2013).
Photosynthetic pigments
Chlorophyll a (Chl a) and chlorophyll b (Chl b) contents were analysed by collecting 0.5 g of Ulva at the start and end of the experiment. Samples were frozen at −20 °C until analysis. For pigment extraction, thalli were placed in a 15-mL polypropylene tube with 3 mL of dimethyl sulfoxide 99.9% (DMSO; Sigma-Aldrich) for 1 h in an oven at 40 °C. Then, 200 μL of solution was added to a 6.4-mm diameter 96-well microplate (IWAKI) and absorbance was read using a micro-plate reader (Biotek synergy HT) to detect Chl a (663 nm) and Chl b (645 nm). Pigments were quantified according to Wellburn (1994) and the results of photosynthetic pigments expressed in μg g−1 wet weight.
Ultrastructure
Samples for ultrastructure analysis were collected at the end of the transplant experiment. Individual Ulva were sampled to collect 1-mm length sections. These were placed in a vial and fixed in 2 mL of 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH = 7.2) and 2% paraformaldehyde overnight. Samples were post-fixed with 1% osmium tetroxide and 0.05 M sodium cacodylate for 8 h followed by dehydration in an ethanol gradient series. Fixation time and reagent concentrations were modified from those previously described by Bouzon et al. (2011). Finally, samples were embedded in Spurr’s resin (Low viscosity, ProSciTech) and 60-nm sections cut with an ultramicrotome (Leica EM UC7) before viewing in a transmission electron microscope (JEOL JEM-1011 TEM). Block cutting and TEM analysis were undertaken at the Central Laboratory of Electron Microscopy, Federal University of Santa Catarina, Florianópolis, Brazil.
Environmental data
The Derwent Estuary Program (DEP) provided details on the total Zn (μg L−1) and nutrient (i.e. ammonium, nitrate and total phosphorus) concentrations in surface seawater (0.1 m depth) at TS1 and TS2 at the time of sampling. In addition, surface seawater samples, at 0.1 m depth (250 mL), were collected for total metal analysis from the control site at the start of the experiment (Table 1), and nutrient data (i.e. ammonium, nitrate and phosphate) were provided by the Storm Bay project (FRDC project 2014/031) for the control site. Total Zn metal analysis and nutrients in water were analysed by AST. The water detection limit for Zn was 1 μg L−1.
Bioaccumulation assessment
To address the bioaccumulation capacity of U. australis, we conducted an in situ experiment in a highly metal-polluted site. The metal accumulation capacity in a long-term deployment and bioaccumulation rate were assessed as follows.
To determine metal uptake rate and metal bioaccumulation, individual thalli of U. australis (14.5 ± 1.2 g) were deployed on 6 January 2016 in individual baskets as described above. In this case, four mesh baskets each containing one weighted Ulva individual were attached to each pole, giving 6 experimental units, which were deployed at TS2. Samples were monitored for growth and metal accumulation (see above) at the start of the experiment (t0), and at regular intervals (t1 = 15 days, t2 = 30 days and t3 = 45 days) thereafter, with one basket being removed from each experimental unit each time. Any evidence of damage to the plants was also noted as a potential response to the treatment.
To evaluate the association between metal accumulated in thalli and metals available in the environment, surface water samples were collected with a 50-mL syringe and a filtered through a Sartorius Stedim 0.45-μm filter (Minisart), into 50-mL plastic centrifuge tubes, and kept at −24 °C until analysis. Seawater samples were analysed by AST using an ICP-AES. Metal concentrations in seawater are expressed as μg L−1. Data loggers (HOBO pendant) were attached to three of the experimental units (n = 3) to provide details of any differences in the temperature (°C) and light (μmol photons m−2 s−1) throughout the 45 days of experiment.
Zinc bioaccumulation in U. australis is expressed as the concentration factor (CF), which is the ratio of the metal (Zn) content in seaweed (C o ) and the metal concentration detected in seawater (C sw ), CF = Co mg kg−1/Csw μg L−1 (Conti and Cecchetti 2003). Additionally, the metal accumulation rate (Mr) provides an estimate of the metal (Zn) content in the thalli at experimental time (At f ) less the metal content in the thalli at the beginning (At i ) divided by time (t): Mr = (Atf − Ati)/t.
Statistical analysis
A one-way ANOVA (95% confidence interval) was used to evaluate differences in Ulva metal content, Gr, photosynthesis performance and photosynthetic pigments content between transplanted thalli. Levene’s test was used prior to analysis to assess normality and homogeneity of the data; no transformation was required. Analysis was carried out with SPSS IBM Statistics version 22.
Photosynthesis performance, changes in photosynthetic pigment contents and changes in metal concentration of ambient versus transplanted plants were assessed using a two-way ANOVA (95% confidence interval, factors: site and treatment). Again, Levene’s test was used to assess normality and homogeneity of variance, with variance heterogeneity corrected using weighted least square regression (WLS). A Tukey post hoc test was conducted where significant differences were observed, in order to further clarify the difference between ambient and transplanted thalli. Analysis was conducted in R studio statistical software (R Core Team 2013).
The relationship between Zn accumulated in U. australis and total Zn in surface waters was assessed using a Pearson Correlation test, conducted in R studio statistical software (R Core Team 2013).
Results
Metal content in U. australis and physiological and ultrastructural performance
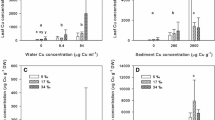
At the end of the 12-day experiment, As, Cu, Pb and Zn were detected in the transplanted U. australis at each of the three study sites, with Zn content being consistently higher than other metals (Fig. 3). However, As, Cu and Pb were not accumulated to any significant level in Ulva and so were not considered further in this analysis. For Zn, there were significant differences between control and transplanted sites (p = 0.001, F = 11.908, df = 2) with a strong interaction between site (contaminant level) and treatment (transplanted plants) (p = 0.018, F = 12.148, df = 3) (Fig. 4). Post hoc comparison revealed significantly higher levels of Zn accumulated in thalli transplanted to TS2 compared to control plants (p = 0.001), Zn content was greater in transplanted thalli at TS2 than at TS1 (p = 0.001), and Zn was greater in transplanted thalli at TS1 in relation to the control (p = 0.015).
Metal content (mg kg−1 dry matter biomass, DMB) in U. australis, arsenic (As), copper (Cu), lead (Pb) and zinc (Zn) after 12 days of transplantation. Control site, transplanted site 1 (TS1) and transplanted site 2 (TS2). Values expressed on mean (±SE, n = 3). Letters above the bars indicate differences between control, TS1 and TS2 shown as a, b, c and d (transplanted plants only). Same letters denote no significant differences
Zinc (Zn) content (mg kg−1 dry matter biomass, DMB) in U. australis at control site, transplanted site 1 (TS1) and transplanted site 2 (TS2). Metal levels expressed as mean concentration (±SE, n = 3). Letters above the bars indicate how the transplanted thalli compare to ambient plants within each sites shown as a, b, c and d, and differences between control, TS1 and TS2; shown as x, y and z (transplanted plants only). Same letters denote no significant differences
Ulva australis collected at the control sites had approximately 12.6 mg kg−1 of Zn, compared with ambient plants that were grown at TS1 and TS2, which had 30.5 and 113.7 mg kg−1 DMB, respectively (Fig. 4). After 12 days, the levels of Zn in transplanted thalli at the control site were not markedly different (p > 0.05) from that observed in the control ambient plants (i.e. in 12 days, plants accumulated 81.5% of the initial content or 10.3 mg kg−1). However, at TS1, the Zn content in the transplanted U. australis had increased to 107.3% of ambient levels or 32.8 mg kg−1. Interestingly, transplanted Ulva at the most polluted site (TS2) took up only 44.8% of the ambient loads (50.9 mg kg−1) (Fig. 4).
The concentration factor (CF) results show that Zn bioaccumulation was measurable over 12 days of deployment; CF was lower at control and semi-polluted sites (TS1) than at TS2, the more polluted site (Table 2). There was a high correlation between the total Zn concentration in surface seawater and Zn accumulated in Ulva (r = 0.87, p = 0.001, df = 7).
Physiological performance
Growth rate, maximum quantum of yield (Fv/Fm), the maximum relative electron transport rate (rETRmax) and photosynthetic pigment content did not appear to be affected by the different metal contents at the transplant locations. No significant differences (p > 0.05) in growth rate, Fv/Fm, rETRmax (Table 3) or photosynthetic pigments (Chl a and Chl b) between control and transplanted thalli at either of the contaminated sites were observed (Table 3). Similarly, there was no significant difference (p > 0.05) in growth rate, Fv/Fm, rETRmax or photosynthetic pigments when transplanted plants were compared with ambient plants (Table 3).
Ultrastructure
The cytology of the control cells shows that the internal organelles are well organised, and the key cellular components, such as chloroplast, starch grains, lipid bodies, Golgi body and vacuoles, are clearly visible (Fig. 5a). Chloroplasts are delimited by vacuoles and the thylakoids are parallel enclosing starch granules (Fig. 5b), a mitochondrion is visible near the chloroplast (Fig. 5b, c), and the cell wall shows organised cellulose microfibrils sitting parallel with each other (Fig. 5c, d). A few mitochondria were visible associated with the chloroplast and Golgi (Fig. 5e).
Transmission electron microscopy (TEM) micrographs of U. australis ultrastructure from control thalli. a Cell general view with internal components well organised; b cell wall, starch granules and chloroplast; c cell wall, vacuole and thylakoids of chloroplast parallel organised; d cellulose fibrils of cell wall and e mitochondria on chloroplast. C chloroplast, CW cell wall, G Golgi, L lipid droplets, M mitochondria, S starch granules, V vacuole and *internal Golgi material
However, 12 days after being transplanted to a semi-polluted site (TS1), there were marked changes in the cell cytology. Cells showed evidence of electron-dense material in the vacuole and an increase in the number of mitochondria in relation to the control (Fig. 6a–d). Thylakoids of the chloroplast appear to be organised in groups and slightly disarranged enclosing a starch grain (Fig. 6b, c). Cellulose fibrils of the wall are irregularly positioned and electron-dense material deposited that was not observed in the control (Fig. 6c, d). Thalli transplanted to the high-polluted site (TS2) showed a higher level of damage within the cell. The cytoplasm is retracted and modified vacuoles show that metals have affected plasmolysis; the amount of starch grains is also noticeably reduced (Fig. 7a) in relation to the control. In a number of cells, electron-dense material was observed inside the vacuoles (Fig. 7a, b) and similarly on the cell wall (Fig. 7c). Although the thylakoids were not strongly affected, an increase in mitochondria number suggests increased of mitochondrial activity (Fig. 7d).
Transmission electron microscopy (TEM) micrographs of U. australis ultrastructure transplanted to a semi-polluted site (TS1). a Electron-dense bodies deposited on vacuole; b thylakoids of the chloroplast slightly altered; c electron-dense bodies deposited on the cell wall; d cell wall cellulose fibrils slightly unorganised. C chloroplast, CW cell wall, M mitochondria, S starch granules, V vacuole
Transmission electron microscopy (TEM) micrographs of U. australis ultrastructure transplanted to a high-polluted site (TS2). a Cell general view demonstrating irregularity on the cytoplasm delimitation; b in a number of cells electron-dense materials was observed inside of vacuoles (arrows); c cell wall fibrils and electron-dense bodies; d chloroplast is not visible affected and e increment of mitochondria on chloroplast. Repetitive results were identified in all cell and samples analysed making the data robust. C chloroplast, CW cell wall, M mitochondria, S starch granules, V vacuole
Bioaccumulation assessment
There was clear evidence of an increase in Zn content in U. australis over the time (Table 1), with a significant increase in the Zn content of the transplanted thalli (p = 0.001, F = 22.632, df = 3). Although there was no significant difference between Zn content in control and transplanted thalli after 15 days (p > 0.05), the levels were significantly greater at the transplanted sites after 30 days, and levels continued to increase up to 45 days (p = 0.001). There was a strong correlation between total Zn concentration in seawater and Zn content in the Ulva (r = 0.85, p = 0.001, dg = 22). Bioaccumulation was evident after 15 days at the highly polluted site (TS2).
The CF differed depending on length of exposure, increasing as metal exposure time increased. The metal accumulation rate (Mr) showed a similar pattern, also increasing over time (Table 2), from 13.3 mg kg−1 day−1 after the first 15 days increasing to 17.9 and 24.3 mg kg−1 day−1 after 30 and 45 days, respectively. Zn content started at 18.8 ± 1.5 mg kg−1 (t 0 ) and reached the highest concentrations, 863 mg kg−1, in the transplanted thalli after 45 days (Table 1). The transplanted thalli appeared visibly degraded after 15 days, and the damage in the thalli continued to increase over the time, being markedly deteriorated after 30 and 45 days (Fig. 8).
Discussion
This study provides evidence of metal accumulation in U. australis, confirming that in situ deployment of this species can be an effective means to monitor metal pollution but also suggesting that Ulva deployment may have potential as a means to remove metals under certain conditions.
Passive biomonitoring (PBM) assessment showed that transplanted U. australis thalli accumulate metals to levels similar to those detected in ambient plants, with the final Zn content reflecting background contamination levels. Metal content in algae clearly increased as metal levels in the environment increased, which is consistent with previous observations (Mehta and Gaur 2005). In order to utilise seaweeds effectively as a bioindicator, it is important to know how long it takes to achieve a level of contamination that would be equivalent to that observed in seaweed under ambient conditions. In this case, equilibrium with ambient metal content was achieved relatively in a short period of time: 12 days. After 12 days, there was a linear correlation between the level of metals accumulated in the transplanted thalli and the metal available in the environment. It has been suggested that a linear relation between these parameters is an indication of a good bioindicator (Rainbow and Phillips 1993; Hurd et al. 2014). Long-term deployment, 45 days, showed that U. australis could accumulate Zn at very high concentrations, and that accumulation increased linearly over time. The mean Zn concentration at 45 days (731.4 mg kg−1) was 58 times higher than that observed at the control site. This was considerably higher than levels previously reported for U. lactuca 66 mg kg−1 from polluted sites (Ho 1990), and 10 mg kg−1 in Ulva sp. from unpolluted areas (Ryan et al. 2012). These results are consistent with those previously reported for Zn in transplanted Ascophyllum nodosum and Fucus vesiculosus, where Ho (1984) in a seminal study found that Zn had increased 19 and 95%, respectively, after 2 months compared to the concentration detected in ambient plants.
Zn content in Ulva assessed over 45 days was markedly higher than that in plants deployed for only 12 days, suggesting that a longer exposure time enhanced bioaccumulation. Bioaccumulation is the active process by which an organism uptakes metals; in this process, the organisms can resist/tolerate particular toxicants (Oliveira et al. 2011). However, we observed that the longer the plants were deployed, the greater the thallus deterioration was observed. Whilst this could be just natural deterioration related to the seasonal life-cycle of Ulva (Hurd et al. 2014), it could also be related to experimental conditions (e.g. experimental baskets). Consequently, we would suggest limiting the deployment time for U. australis no longer than 20 days, as this timeframe allowed plants to accumulate high concentrations of Zn (∼300 mg kg−1) without visual deterioration. This strategy would enable to uptake high amount of metals and quickly “clean-up” of toxicants in the environment.
Metal accumulation was greatest at TS2, which is one of the most polluted areas, both in terms of metals and nutrients, in the Derwent Estuary (Coughanowr et al. 2015). Several studies have suggested that ambient concentrations of nutrients such as nitrate, ammonium and phosphate can influence metal accumulation (Lee and Wang 2001), with an increasing metal uptake capacity when nitrogen is widely available (Pinto et al. 2003). In a related species, U. fasciata, it has been shown that under controlled conditions, the rate of Cd accumulation increased with increasing nitrate concentration (Lee and Wang 2001). Therefore, it might be suggested that high levels of nutrients (i.e. ammonium and nitrite) and total inorganic nitrogen (360 μg L−1) available at TS2 (Prince of Wales Bay) (Coughanowr et al. 2015) may have facilitated the metal uptake, providing the optimum environmental conditions for U. australis to accumulate metals.
Salinity has been also shown to affect metal uptake, increasing when salinity decreases. A previous study showed that metal concentration in U. australis sampled as a part of ABM was higher at less saline areas (D. Farias unpublished data). This may be a function of the fresh water having higher concentrations of metals than seawater (Hurd et al. 2014). The PBM in the current study showed that major ultrastructural changes were apparent at lower salinities. The effect of salinity on uptake has been shown in a laboratory experiment (Felix et al. 2014), who showed that Cd absorption in the red algae Pterocladiella capillacea increased when salinity decreased resulting in damage to the ultrastructure and negatively affect the physiological performance. Salinity has been recommended as a relevant parameter that should be considered when using seaweeds in estuarine biomonitoring programs (Munda 1984; Mamboya et al. 2009). The results suggest that the combined effects of low salinity, high nutrients levels and high metal loads at site TS2 would make this site an optimal location for biomonitoring, both ABM and PBM, as well as good location for evaluating the in situ efficiency of U. australis as a potential bioremediation tool.
Internal cellular changes showed the effects of metal accumulation. Whilst cellular changes were observed in transplanted Ulva plants from both sites, TS1 and TS2, there was a greater level of change (i.e. incorporation of electron-dense bodies and the increment of mitochondria and vacuoles) in thalli from TS2 site. The presence of electron-dense bodies clearly indicate metal deposited within the cell, a feature previously observed in laboratory experiments (D. Farias unpublished data) and confirming the role of the cell wall in metal accumulation in U. australis. Previous laboratory experiments had demonstrated metal deposits in Hypnea musciformis exposure to Cd (Bouzon et al. 2011), in P. capillacea (Felix et al. 2014) and in U. flexuosa (formerly Enteromorpha flexuosa) exposed at 50 μg L−1 of Cu (Andrade et al. 2004). The increase in the number of vacuoles could be explained by the increment in the Golgi activity. The Golgi is an active organelle that forms new vesicles (Lanubile et al. 1997), and cytoplasm vesiculation has been related to the association between cell wall and metals ions in U. laetevirens exposed to Cd as a metal tolerance mechanism (Vecchia et al. 2012). This alteration has been observed in the microalga Chlorella vulgaris exposed to Cd, where it was also proposed to be a mechanism to improve metal tolerance and maintain low cytosolic ion concentrations (El-Naggar and Sheikh 2014). There were more vacuoles observed in the transplanted thalli from TS2, suggesting that there was a higher level of cellular processes involved in managing metal accumulation. Interestingly, despite the marked changes observed in both metal accumulation and ultrastructure, there was no evidence of any significant effects on the growth rate, maximal photochemical yield (Fv/Fm), maximum relative electron transport rate (rETRmax) or pigment content of transplanted U. australis thalli. This could suggest acclimation of transplanted plants.
Most of the investigations of the physiological effects of metal contamination on seaweed have been undertaken under controlled conditions. Unfortunately, the results of these lab-based experiments are highly variable and may not necessarily reflect the complexity of natural situation (Hurd et al. 2014). For instance, the concentrations of Zn and Cu can affect the Fv/fm in Ulvacean (Andrade et al. 2004; Han et al. 2008; Baumann et al. 2009) and similarly, mercury (Hg) has been shown to severely affect the Fv/Fm of Caulerpa racemosa, C. lentillifera and U. reticulata (Zakeri and Abu Bakar 2012). While Pb increased the electron transport rate ETR in S. cymosum, Cu inhibited ETR after 7 days of exposure (Costa et al. 2015). The current study was specifically developed to evaluate metal accumulation in situ, under the complex range of conditions that really occur in nature, as it was demonstrated in Padina gymnospora and S. stenophyllum (Amado Filho et al. 1999) and therefore is better placed to characterise the applicability of U. australis as a monitoring and management tool under actual field conditions. The results show that U. australis can be transplanted to metal-polluted areas for a short period of time without affecting the photosynthesis performance.
In a recent study, Zakeri and Abu Bakar (2012) suggested the maximum quantum yield (Fv/Fm) as the most effective parameter to evaluate metal toxicity in algae. Using this approach Scherner et al. (2012) showed in situ that the Fv/Fm in U. lactuca improved 26 days after transplantation from clean to polluted areas, whilst Fv/Fm declined in the brown seaweed S. stenophyllum subject to the same conditions. This clearly demonstrated that there are differences in tolerance between species exposed to contamination. However, in this research, we showed that the photosynthetic performance is not only a relevant method to evaluate in situ metal effects; the assessment of the ultrastructure is a valuable and novel tool that can clearly demonstrate metal accumulation effects in seaweeds, and one which would add valuable to in situ assessment.
Whilst the results of this study clearly show the potential value of U. australis as a bioindicator of metal pollution and the effectiveness of this species in metal accumulation, the levels obtained at the more polluted sites do raise some concerns with respect to the potential for adverse environmental interactions. Concentrations of Zn accumulated in U. australis at the most polluted site (TS2) were high (above 700 mg kg−1 after 45 days) and as such may provide a mechanism for transfer of metals into the food web (Young 1975; Huang et al. 2008). There are no specific guidelines for acceptable levels of metals in seaweeds, either in terms of seafood safety or environmental acceptability. However, it is suggested that levels of Zn in sediments should be below 410 mg kg−1 to protect environmental values within aquatic ecosystems (ANZECC 2000). Whilst these values cannot be directly compared, the fact that the levels obtained in seaweeds are almost double that recommended for sediments might suggest that there is a risk here and further research might be needed to better characterise this before employing Ulva in the natural environment. The levels of Zn which are considered toxic for human consumption in crustaceans (<40 mg kg−1), molluscs (130–290 mg kg−1) and fish (<15 mg kg−1) vary markedly (FSANZ 2006) but in all cases are considerably lower than those observed in U. australis at the most contaminated site after 45 days. As explained above, these levels cannot be directly compared and clearly if Ulva is used for remediation would never be used for human consumption. But, the very high levels observed in U. australis at the most contaminated site might suggest that there could be an indirect risk of toxicity and/ or trophic accumulation.
In conclusion, passive biomonitoring showed that in 12 days, U. australis uptake metal rates that reflected background loads, with higher uptake at the most polluted sites, showing the potential of this species as a bioindicator of metal pollution. Metal accumulation did not affect U. australis physiology as growth rate, photosynthesis performance (Fv/Fm and rETRmax) or photosynthetic pigment content. However, there was evidence of internal cellular changes (incorporation of electron-dense bodies) that could be related to metal load.
The bioaccumulation assessment, 45 days, demonstrated that accumulation increased over the time. However, there was deterioration of the thalli over time and as a result, it is suggested that 20 days is the most appropriate time for deployment U. australis. This timeframe would allow the plant to accumulate high levels of metals in a heavy metal-impacted environment whilst still remaining healthy and viable. These recommendations will enable U. australis to be more effectively used as a biomonitor to provide better understanding for managing metal pollution.
References
Amado Filho GM, Andrade LR, Karez CS, Farina M, Pfeiffer WC (1999) Brown algae species as biomonitors of Zn and Cd at Sepetiba Bay, Rio de Janeiro, Brazil. Mar Environ Res 48:212–224
Andrade LR, Farina M, Amado Filho GM (2004) Effects of copper on Enteromorpha flexuosa (Chlorophyta) in vitro. Ecotox Environ Safe 58:117–125
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality, vol 1. The guidelines. Australian and New Zealand Environment and Conservation Council and the Agriculture and Resource Management Council of Australia and New Zealand
Baumann HA, Morrison L, Stengel DB (2009) Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotox Environ Safe 72:1063–1075
Bird K, Bourdine K, Busch W (1998) Marine algae as a tool for bioremediation of marine ecosystems. In: Altman A (ed) Agricultural Biotechnology. CRC Press, Boca Raton, pp 601–613
Boubonari T, Malea P, Kevrekidis T (2008) The green seaweed Ulva rigida as a bioindicator of metals (Zn, Cu, Pb and Cd) in a low-salinity coastal environment. Bot Mar 51:472–484
Bouzon ZL, Schmidt EC, Almeida AC, Yokoya NS, Oliveira MC, Chow F (2011) Cytochemical characterization and ultrastructural organization in calluses of the agarophyte Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Micron 42:80–86
Brown MT, Hodgkinson WM, Hurd CL (1999) Spatial and temporal variations in the copper and zinc concentrations of two green seaweeds from Otago Habour, New Zealand. Mar Environ Res 47:175–184
Brown MT, Newman JE, Han T (2012) Inter-population comparisons of copper resistance and accumulation in the red seaweed, Gracilariopsis longissima. Ecotoxicology 21:591–600
Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henríquez L (2009) Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manage 52:243–249
Chopin T, Yarish C, Wilkes R, Belyea E, Lu S, Mathieson A (1999) Developing Porphyra/salmon integrated aquaculture for bioremediation and diversification of the aquaculture industry. J Appl Phycol 11:463–472
Conti ME, Cecchetti G (2003) A biomonitoring study: trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ Res 93:99–112
Costa GB, de Felix MR, Simioni C, Ramlov F, Oliveira ER, Pereira DT, Maraschin M, Chow F, Horta PA, Lalau CM, da Costa CH, Matias WG, Bouzon ZL, Schmidt EC (2015) Effects of copper and lead exposure on the ecophysiology of the brown seaweed Sargassum cymosum. Protoplasma 253:111–125
Coughanowr C, Whitehead S, Whitehead J, Einoder L, Taylor U (2015) State of Derwent: a review of environmental data from 2009 to 2014. pp 250
El-Naggar AH, Sheikh HM (2014) Response of the green microalga Chlorella vulgaris to the oxidative stress caused by some heavy metals. Life Sci J 11:1249–1257
Felix MR, Osorio LK, Ouriques LC, Farias-Soares FL, Steiner N, Kreusch M, Pereira DT, Simioni C, Costa GB, Horta PA, Chow F, Ramlov F, Maraschin M, Bouzon ZL, Schmidt EC (2014) The effect of cadmium under different salinity conditions on the cellular architecture and metabolism in the red alga Pterocladiella capillacea (Rhodophyta, Gelidiales). Microsc Microanal 20:1411–1421
FSANZ (2006) Safe seafood Australia, a guide to the Australian primary production and processing standard for seafood, 2nd edn. Food Standards Australia New Zealand, Canberra, p 130
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Gledhill M, Brown MT, Nimmo M, Moate R, Hill SJ (1998) Comparision of techniques for the removal of particulate material from seaweed tissue. Mar Environ Res 45:295–307
Gouveia C, Kreusch M, Schmidt EC, Felix MR, Osorio LK, Pereira DT, dos Santos R, Ouriques LC, de Paula MR, Latini A, Ramlov F, Carvalho TJ, Chow F, Maraschin M, Bouzon ZL (2013) The effects of lead and copper on the cellular architecture and metabolism of the red alga Gracilaria domingensis. Microsc Microanal 19:513–524
Grote B (2016) Bioremediation of aquaculture wastewater: evaluating the prospects of the red alga Palmaria palmata (Rhodophyta) for nitrogen uptake. J Appl Phycol 28:3075–3082
Guisti L (2001) Heavy metal contamination of brown seaweed and sediments from the UK coastline between the wear river and the tees river. Environ Int 26:275–186
Han T, Kang SH, Park JS, Lee HK, Brown MT (2008) Physiological responses of Ulva pertusa and U. armoricana to copper exposure. Aquat Toxicol 86:176–184
Ho Y (1984) Zn and Cu concentrations in Ascophullum nodosum and Fucus vesiculosus (Phaeophyta, Fucales) after transplantation to an estuary contaminated with mine wastes. Conserv Recycling 7:329–337
Ho Y (1990) Ulva lactuca as bioindicator of metal contamination in intertidal waters in Hong Kong. Hydrobiologia 203:73–81
Huang X, Ke C, Wang W-X (2008) Bioaccumulation of silver, cadmium and mercury in the abalone Haliotis diversicolor from water and food sources. Aquaculture 283:194–202
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, Cambridge, p 562
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biol 9:215–288
Kumar A, Bisht BS, Joshi VD, Dhewa T (2011) Bioremediation of polluted environment: a management tool. Int J Env Sci 1:1079–1093
Lacroix C, Richard G, Seguineau C, Guyomarch J, Moraga D, Auffret M (2015) Active and passive biomonitoring suggest metabolic adaptation in blue mussels (Mytilus spp.) chronically exposed to a moderate contamination in Brest harbor (France). Aquat Toxicol 162:126–137
Lanubile R, Piro G, Dalessandro G (1997) Effects of Brefeldin a on the synthesis and transport of cell wall polysaccharides and proteins in pea root seedling. J Exp Bot 48:1925–1933
Lee WY, Wang WX (2001) Metal accumulation in the green macroalga Ulva fasciata: effect of nitrate, ammonium and phosphate. Sci Total Environ 278:11–22
Longstaff BJ, Kildea T, Runcie JW, Cheshire A, Dennison W, Hurd CL, Kana T, Raven JA, Larkum AWD (2002) An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynth Res 74:281–293
Luoma SN, Bryan GW, W. J. L (1982) Scavenging of heavy metals from particulates by brown seaweed. Mar Pollut Bull 13:394–396
Malea P, Haritonidis S (2000) Use of the green alga Ulva rigida C. Agardh as an indicator species to reassess metal pollution in the Thermaikos Gulf, Greece, after 13 years. J Appl Phycol 12:169–176
Malea P, Haritonidis S, Kevrekidis T (1995) Metal content of some green and brown seaweeds from Antikyra Gulf (Greece). Hydrobiologia 310:19–31
Mamboya F, Lyimo TJ, Landberg T, Björk M (2009) Influence of combined changes in salinity and copper modulation on growth and copper uptake in the tropical green macroalga Ulva reticulata. Estuar Coast Shelf Sci 84:326–330
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotech 25:113–152
Munda IM (1984) Salinity dependent accumulation of Zn, Co and Mn in Scytosiphon lomentaria (Lyngb.) link and Enteromorpha intestinalis (L.) link from the Adriatic Sea. Bot Mar 27:371–376
Myklestad S, Eide I (1978) Exchange of heavy metals in Ascophyllum nodosum (L.) Le Jol. in situ by means of transplanting experiments. Environ Pollut 16:277–284
Oliveira RC, Palmieri MC, Garcia OJ (2011) Biosorption of metals: state of the art, general features, and potential applications for environmental and technological processes. In: Shaukat SS (ed) Progress in Biomas and Bionergy Production. InTech, Riejeka p 445.
PAM-Processor (2015) https://github.com/RobTheOceanographer/pam_in_R/releases. Accessed June 2015
Pellegrini L, Pellegrini M, Delivopoulos S, Berail G (1991) The effects of cadmium on the fine structure of the brown alga Cystoseira barbata forma repens Zinova et Kalugina. Brit Phycol J 26:1–8
Phillips D, Rainbow P (1994) Biomonitoring of trace aquatic contaminants. Chapman & Hall, London, p 371
Pinto E, Sigaud-Kutner T, Leitão M, Okamoto O, Morse D, Colepicolo P (2003) Heavy metal-induce oxidative stress in algae. J Phycol 39:1008–1018
Platt T, Harrison WG, Irwin B, Horne EP, Gallegos CL (1980) Photosynthesis and photoadaptation of marine phytoplankton in the Arctic. Deep Sea Res 29:1159–1170
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/
Rainbow PS (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192
Rainbow PS, Phillips DJH (1993) Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26:593–601
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ronci L, Meccoli L, Iannilli V, Menegoni P, De Matthaeis E, Setini A (2016) Comparison between active and passive biomonitoring strategies for the assessment of genotoxicity and metal bioaccumulation in Echinogammarus veneris (Crustacea: Amphipoda). Ital J Zool 83:162–172
Ryan S, McLoughlin P, O’Donovan O (2012) A comprehensive study of metal distribution in three main classes of seaweed. Environ Pollut 167:171–177
Santos RW, Schmidt EC, Felix MR, Polo LK, Kreusch M, Pereira DT, Costa GB, Simioni C, Chow F, Ramlov F, Maraschin M, Bouzon ZL (2014) Bioabsorption of cadmium, copper and lead by the red macroalga Gelidium floridanum: physiological responses and ultrastructure features. Ecotox Environ Safe 105:80–89
Scherner F, Bonomi Barufi J, Horta PA (2012) Photosynthetic response of two seaweed species along an urban pollution gradient: evidence of selection of pollution-tolerant species. Mar Pollut Bull 64:2380–2390
Scherner F, Ventura R, Barufi JB, Horta PA (2013) Salinity critical threshold values for photosynthesis of two cosmopolitan seaweed species: providing baselines for potential shifts on seaweed assemblages. Mar Environ Res 91:14–25
Schiavon M, Moro I, Pilon-Smits EA, Matozzo V, Malagoli M, Dalla Vecchia F (2012) Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. Aquat Toxicol 122-123:222–231
Schreiber U, Klughammer C, Kolbowski J (2011) High-end chlorophyll fluorescence analysis with the MULTI-COLOR-PAM. I. Various light qualities and their applications. PAM Appl Notes 1:1–21
Troell M, Halling C, Nilsson A, Buschmann AH, Kautsky N, Kautsky L (1997) Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 156:45–61
Troell M, Ronnback P, Halling C, Kaustsky N, Buschmann A (1999) Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive mariculture. J Appl Phycol 11:89–97
Vecchia FD, Marzocchi M, Maistro S, Moro I (2012) Morpho-physiological effects of cadmium on two Ulva species. Algol Stud 138:13–26
Vidali M (2001) Bioremediation. An overview. Pure Appl Chem 73:1163–1172
Vieria R, Volesky B (2000) Biosorption: a solution to pollution? Int Microbiol 3:17–24
Volesky B (1990) Biosorption of heavy metals. CRC Press, Boca Raton
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Whitehead J, Coughanowr C, Agius J, Chrispijn J, Taylor U, Wells F (2010) State of the Derwent Estuary 2009: a review of pollution sources, loads and environmental quality data from 2003 to 2009. Department of Primary Industry, Parks, Water and Environment, Hobart, Tasmania, Australia. pp 189
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Young ML (1975) The transfer of 65Zn and 59Fe along a Fucus serratus (L.) → Littorina obtusata (L.) food chain. J Mar Biol Assoc UK 55:583–610
Zakeri HA, Abu Bakar L (2012) Copper-, lead- and mercury-induced changes in maximum quantum yield, chlorophyll a content and relative growth of three Malaysian green macroalgae. Malaysian J Fundament Appl Sci 9:16–21
Zbikowski R, Szefer P, Latala A (2007) Comparison of green algae Cladophora sp. and Enteromorpha sp. as potential biomonitors of chemical elements in the southern Baltic. Sci Total Environ 387:320–332
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of North China. Aquaculture 252:264–276
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150
Acknowledgements
The authors would like to acknowledge the Derwent Estuary Program (DEP) and Storm Bay project (FRDC project 2014/031) for providing the environmental data to the Central Laboratory of Electron Microscopy, Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil (LCME-UFSC) for the use of their transmission electron microscope and to the Prince of Wales Bay Marina (POW) for their facilities. Special thanks are given to Lisette Robertson for her valuable support in the laboratory and to Travis Baulch and Luis Henriquez for their support on the field. This research was funded by The Holsworth Wildlife Research Endowment—Equity Trustees Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farias, D.R., Hurd, C.L., Eriksen, R.S. et al. In situ assessment of Ulva australis as a monitoring and management tool for metal pollution. J Appl Phycol 29, 2489–2502 (2017). https://doi.org/10.1007/s10811-017-1073-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1073-y