Abstract
Eelgrass (Zostera marina L.) is an important organism in coastal marine waters and is highly likely to encounter exposure to multiple stressors, both anthropogenic contaminants and natural stressors. Here, we exposed eelgrass to a range of Cu concentrations and salinities, and also varied exposure route between sediment and water. Measured endpoints were Cu accumulation in root and leaves, relative growth rate, leaf mortality, chlorophyll concentration, and maximum photosynthetic quantum yield. Cu accumulation from the sediment was translocated to all parts of the plant, while Cu taken up from the water showed a tendency to remain in leaves. Effects on relative growth rate and leaf mortality were found only following uptake of Cu from the sediment. We tested effects of different salinities, acting as multiple stressors, together with Cu, but found only weak effects with little interaction with Cu. Experiments with anthropogenic contaminants that marine plants are mainly exposed to through the sediment should be done using sediment exposure, as the common practice of using only water exposure will lead to underestimation of harmful effects. Future studies should take all relevant factors into consideration, as anthropogenic inputs and natural factors are prone to fluctuations due to e.g., climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The marine angiosperm eelgrass (Zostera marina L.) is an important organism in coastal marine waters in northern Europe as well as in USA. It functions as a foundation species, defining its own ecosystem type—seagrass ecosystems (Burkholder et al., 2007). Eelgrass beds stabilize the coastal sediments, thus reducing re-suspension and the associated internal nutrient loading to the water column (van der Heide et al., 2011). In addition, eelgrass beds provide shelter and substrate for juvenile fish and shellfish, which is of ecological as well as economic importance (Heck et al., 2003). As was the case in most parts of the eelgrass distribution range (Orth et al., 2006; Waycott et al., 2009), the Danish eelgrass communities were significantly reduced in area coverage between 1960 and 1990, where almost 90% of the eelgrass covered area was lost (Frederiksen et al., 2004). It is generally accepted that the loss of eelgrass was mainly due to eutrophication, resulting in increased turbidity in the coastal waters, especially due to phytoplankton growth (Nielsen et al., 2002a, b). Recent years have seen the oligotrophication of Danish waters (Riemann et al., 2016), but eelgrass has only responded weakly if at all to this (Riemann et al., 2016). The reasons behind this hysteresis in eelgrass recolonization are assumed to include changes in ecosystem interactions and sediment characteristics that are only reversible on long time scales if at all (Valdemarsen et al., 2010, 2014). However, it is highly likely that effects of multiple stressors, including anthropogenic stressors such as various contaminants, as well as natural stressors, such as variations in salinity and temperature, may contribute to the lack of eelgrass recolonization.
Little is known about the combined effects of contaminants (including heavy metals, TBT (Tributyltin), PAHs (Polycyclic aromatic hydrocarbons) and other anthropogenic stressors on seagrasses. The published literature is restricted to a relatively small number of primarily physiological studies (Macinnis-Ng & Ralph, 2002; Macinnis-Ng & Ralph, 2003, 2004a), showing negative, but not dramatic effects. Such physiological results may not translate directly to larger scales of biological organization which means that studies of contaminant effects with direct ecological relevance to seagrass individuals, populations as well as communities are virtually absent.
Similar to nutrient loadings, the loading of certain contaminants to coastal environments have been reduced in recent years (HELCOM, 2010). However, more persistent chemicals require many years (or decades) after a potential ban to disappear from the environment, and at the same time new chemicals constantly emerge as environmental contaminants. Metal-based antifouling agents (AFA) have been used in increasing amounts since the complete EU ban of TBT in 2008 and are thus of particular interests, since the continued leaching of these into relevant habitats is to be expected. Metal-based AFAs, such as zinc pyrithione (ZPT) and copper pyrithione (CPT) as well as copper in other forms (mainly copper thiocyanate (CuSCN) or copper oxide (Cu2O)) may pose a risk to non-target aquatic organisms, including eelgrass.
Both metal–pyrithione complexes tend to have short half-lives (Turley et al., 2000) and will undergo photolysis within minutes following exposure to sunlight. However, the half-lives depend on the physical–chemical properties of the medium (i.e., sediment and overlying water), and even when the complexes are broken down, their metal components remain in the environment. Bioavailability and toxicity of metals are known to be crucially dependent on chemical form, which again is determined by the physical and chemical properties of the medium (Namiesnik & Rabajczyk, 2010). It is well known from terrestrial plants that heavy metals are taken up through the roots and translocated to other plant parts together with nutrients such as nitrogen and phosphorus (Meharg & MacNair, 1992, Epstein & Bloom, 2005), where their main effects are to disturb the cofactor binding to proteins and DNA and to cause the production of reactive oxygen species (Apel & Hirt, 2004). In aquatic plants, as well as in terrestrial plants, nutrients are primarily taken up through the roots (Denny, 1972), indicating that this uptake route is probably also important for contaminants. In the Mediterranean seagrass Posidonia oceanica, an acropetal allocation pattern (from roots/rhizomes to leaves) has been found (Sanz-Lazaro et al., 2012). Acropetal allocation is consistent with existing knowledge of general translocation patterns of inorganic nutrients in eelgrass, as well as in other clonal plants (Alpert, 1991, 1996). Despite this, ecotoxicological work with aquatic plants is usually done with non-rooted species (Ceratophyllum; Lemna) or with plants rooted in artificial substrate and only exposed to contaminants through the water (Macinnis-Ng & Ralph, 2003, 2004a; Ambo-Rappe et al., 2011), so that little is known about the relative mechanisms or effects of root versus leaf uptake of heavy metals or other contaminants. Though copper is an essential plant micronutrient, it may inhibit photosynthesis, growth, and zinc uptake as well as alter antioxidant defense mechanisms and increase lipid peroxidation in aquatic plants under environmentally relevant concentrations that exceed concentrations optimal for plant growth (Monferran et al., 2009; Thomas et al., 2013).
The effects of natural stressors, such as varying temperatures and salinities, has been studied in some depth, and it has been shown that eelgrass growth and survival is negatively affected by decreasing salinity and increasing temperature (Nejrup & Pedersen, 2008; Salo & Pedersen, 2014; Salo et al., 2014), such as would be the result of predicted climate change in the Baltic area, including the Danish coasts. However, the effects of these natural stressors have not been studied in connection with effects of anthropogenic contaminants, so the joint effects, including any synergies of natural and anthropogenic stressors are largely unknown. Neither is it known how efforts to protect and re-establish eelgrass communities are affected by the joint effects of eutrophication and other anthropogenic and natural stressors, since this has not been included in studies of the hysteresis in eelgrass re-establishment.
In the present study, we investigate the joint effects of copper and salinity on eelgrass. While the toxic effects of copper on plants are well established, copper is used in this study as a model compound of known toxicity to evaluate the joint effects of an anthropogenic contaminant and the well-known natural stressor, salinity.
Materials and methods
Collection and cultivation of plant material
Plants were collected from Roskilde Fjord (55°54.053 N, 12°2.643 E), Denmark, at wading depth in August. Temperature was 14°C and salinity was 17‰ at the site at the time of collection. Mean water temperature at this location generally varies between 1°C (February) and 19°C (August), and salinity between 16 and 21‰, depending on freshwater run-off, so that the salinity is lowest in the winter months and highest in the late summer/early autumn. Entire turfs of plants were dug up with intact root/rhizome systems in sediment. The turfs were transferred to plastic tubs and kept covered with ambient seawater during transfer to the laboratory which was done within 30 min. In the laboratory, the turfs were transferred to a larger tank with ambient seawater, placed in a temperature-controlled room at 15°C. The plants were maintained in this tank for 14 days prior to experimentation. The plants were illuminated with an irradiance of 200 µmol m−2 s−1 in a 16/8 h. light/dark cycle.
Collection and treatment of sediment
Sediment was collected at the same location as the plants. The sediment was sieved on-site to remove larger particles, macrofauna, etc. After sieving, particles with grain size <1 mm were retained. The sieved sediment was sandy and had a water content of 18%. In the laboratory, ca. 8 kg DW sediment was spiked with CuCl2·2H2O (Merck, Darmstadt, Germany) to a nominal concentration of 3000 µg Cu g−1 DW sediment. For sediment spiking, a stock solution with a concentration of 25.2 g Cu l−1 was prepared by dissolving 67.65 g CuCl2·2H2O in 1 l demineralized water. Stock sediment was prepared by directly adding the stock solution to sieved sediment in a volume of 119 ml kg−1 DW sediment. After adding the stock solution, the sediment was carefully mixed and placed on an orbital shaker (80 rpm) for 14 days to equilibrate. After equilibration, the sediment was again carefully mixed, and the copper concentrations of three subsamples were measured by AAS after freeze-drying to be 2800 µg Cu g−1 DW sediment. Of this stock sediment, 400 g was mixed with 3600 g control sediment to a resulting concentration of 280 µg Cu g−1. The remaining sieved sediment was used for controls treatments (i.e., no added Cu). The different Cu treatments were chosen to cover Cu sediment concentrations ranging from a worst-case scenario (2800 µg g−1), a concentration typical of that measured in harbor sediments around the world (280 µg g−1), and a typical background copper level (none added, but measured to 1.5 µg g−1) (Anonymous, 1999; Bloom & Ayling, 1977; Madsen et al., 1999).
Preparation of water
Seawater was prepared at three different salinities, 5, 17, and 34‰, by diluting full-strength seawater (34‰) with tap water. These salinities were chosen to span the full range of salinities at which eelgrass is found. The lowest salinity which supports eelgrass growth is 5‰ (Salo et al., 2014), and eelgrass is growing in full-strength seawater at 34‰ in the Mediterranean Sea (Rigollet et al., 1998). The ambient salinity in Roskilde Fjord at the collection site is 17‰. For each salinity, three different water Cu treatments were prepared: No Cu-addition (control), 8.4 µg Cu ml−1 and 84 µg Cu ml−1. Cu was added as CuCl2·2H2O. The water Cu concentrations were chosen to equal sediment pore water concentrations of the experimental sediment Cu treatments, calculated based on assumptions of equilibrium partitioning (Vanderkooij et al., 1991) and using a median K sw value of 50 l g−1 found for a range of Dutch surface waters.
Experimental procedure
After allowing 14 days for equilibration of sediment Cu concentrations and plant recovery, individual eelgrass shoots (ramets) were carefully cut from the turfs and sediment was rinsed from their roots and rhizomes, taking care not to damage the plants. Care was taken only to use intact shoots where no leaves, roots, or rhizomes were lost during this procedure. These shoots were then planted in experimental sediment in 1 l plastic bags with background (control), low (280 µg Cu g−1), or high (2800 µg Cu g−1) Cu concentration. The bags contained only sediment. After planting the shoots, the plastic bags were sealed to prevent Cu exchange between the sediment and the overlying water. To measure the relative leaf growth rate of the shoots, we used a leaf-marking technique (Sand-Jensen, 1975), using the leaf sheath of the third leaf on the shoot as a reference point. After blotting the three youngest leaves on the shoot with absorbent paper, they were marked with a transverse line, using a non-toxic permanent marker pen, 3 cm above the leaf sheath of the third-youngest leaf. After marking, the shoots were transferred to Plexiglas tubes with an inner diameter of 6 cm. One shoot was placed in each tube, so that the experimental unit is a tube containing a single shoot. The tubes were when filled with experimental seawater of different salinities and either control (no added Cu-addition), low (8.4 µg Cu ml−1), or high (84 µg Cu ml−1) Cu concentrations. Water was filled to a height of approximately 23 cm, (approximately 650 ml), ensuring that the plant was submerged. The experiment was not fully factorial, so for each of the three salinities, plants were either exposed to one of two sediment Cu concentrations or to one of two water Cu-exposures—not to Cu in sediment and in the water at the same time. There was one control set of plants (no Cu to water or sediment) for each salinity. An overview of combinations of treatments is given in Table 1. Individual tubes were placed in a basin with a water level low enough to ensure that there was no possibility of carry-over of water from one tube to the others in a thermostatted room at 15°C under an irradiance of 200 µmol m−2 s−1 in a 16/8 h. light/dark cycle. The plants were kept under these conditions for an experimental period of 14 days, after which relative growth rates and chlorophyll fluorescence parameters were measured immediately. A duration of 14 days was chosen to allow enough time for stressors to effect endpoints, without risking to actually kill the plants before the end of the experiment. Measurements of relative growth rate in Z. marina is usually done over a few weeks (Nejrup & Pedersen, 2008), while the effects on maximum quantum yield are usually determined after some hours (Macinnis-Ng & Ralph, 2003). Following these measurements, plant material was frozen (−20°C) for later analyses of leaf chlorophyll concentrations as well as Cu concentrations. These analyses were completed within 1 month of terminating the experiment.
Measurement of relative leaf growth rates and leaf mortality
As stated above, we used a leaf-marking technique (Sand-Jensen, 1975) to measure the relative leaf growth rate (RGR) of the eelgrass shoots. The growth of leaf 1–3 was determined on the basis of the displacement of the marked line relative to the reference point. The growth of leaf 4 and older leaves is considered insignificant (Sand-Jensen, 1975). Numbers of new leaves were recorded, and the length of their visible parts measured. After termination of the experiment, leaves of each shoot were cut off, freeze-dried, and weighed. The leaf length growth was converted to dry matter increase from these individual leaf length–dry weight relationships for each shoot.
To enable the calculation of leaf mortality, the number of leaves older than leaf 3 was counted at the time of marking. Leaf mortality was then calculated as the fraction of number of leaves older than leaf 3 missing after 14 days to total number of leaves at the time of marking.
Chlorophyll fluorescence parameters
Chlorophyll a fluorescence was measured using Pulse Amplitude Modulated (PAM) fluorometry (Maxwell & Johnson, 2000). The level of stress was evaluated from values of maximum quantum yield (F v/F m) of PSII (Maxwell & Johnson, 2000). Maximum quantum yield was measured on the three youngest, fully developed, leaves on each shoot. Measurements were performed at the same part of each leaf to reduce variability, and were performed after 15 min dark acclimation of the shoots, and were done with a Walz Imaging-PAM (Walz, Effentrich, Germany), using default settings.
Chlorophyll concentration
Approximately, 5 mg of freeze-dried and homogenized leaf material was extracted in ethanol overnight at room temperature. The supernatant was carefully extracted with a pipette, and the absorption of the chlorophyll extract was measured spectrophotometrically at 665 and 750 nm. Chlorophyll concentration was calculated using equations and absorption coefficients derived from the literature (Jespersen & Christoffersen, 1987) according to the following equation:
where A 665 and A 750 are absorption coefficients at 665 and 750 nm, respectively; v is the volume of ethanol used for extraction; and M is the amount of leaf material extracted.
Cu analysis of plant tissue and sediment
Post-exposure plant tissues were divided into leaves and roots/rhizomes, and then freeze-dried (Christ Alpha 1–2, Osterode, Germany) overnight at 50°C. A weighed sub-sample (10–20 mg) was then subjected to a microwave digestion (LS-1200 MEGA, Milestone, Sorisole, Italy) procedure ISO15587-2. Briefly, samples were digested in 70% HNO3 at sequential microwave powers (250, 400, 650, and 250 W) for 6 min each. Samples were then passed through pre-washed filters (once with HNO3 and three times with Milli-Q water) into volumetric flasks resulting in digests of known volume. Plant tissue digests were measured by graphite furnace mode on the atomic absorption spectrometry (AAS; SpectrAA 220, Varian Ltd., Walton-on-Thames, UK). Procedural blank samples (HNO3 digests only) and certified reference material (lobster hepatopancreas (LUTS-1), National Research Council of Canada) were routinely measured alongside sample runs for quality control. In order to expose eelgrass to the nominal concentrations of spiked sediments, the initially spiked stock was measured by flame mode on the same AAS instrument. Sediment samples (0.5 g) were similarly freeze-dried and subjected to the same microwave digestion procedure as described for plant tissue. All equipment involved in the analysis of Cu was acid washed before use.
Statistical analysis
As mentioned above, the experimental design was not fully factorial, as the plants were only subjected to Cu through water or through sediment—not both. Because of this, statistical data analysis is carried out separately for each set of Cu treatments—water exposure or sediment exposure. Within each exposure route, response variables were subjected to two-way ANOVA with Cu concentration and salinity as fixed factors. Tukey’s test was subsequently used to compare individual means across significantly different treatment levels. Data were tested for homogeneity of variance (Levine’s test) and normal distribution (Kolmogorov–Smirnoff goodness-of-fit test) before being analyzed by ANOVA. When necessary, data were log-transformed to ensure that conditions for ANOVA analysis were met. Either the untransformed or transformed data fulfilled the distributional assumptions for the ANOVA analysis, with the exception of the leaf F v/F m data which still had issues with variance homogeneity, despite data transformation. We chose to conduct the same two-way ANOVA analysis on these data to make comparisons to the analyses of the other parameters easier, but any significant differences indicated by these ANOVA results should be interpreted with caution. All tests were carried out using SYSTAT v. 13 with α = 0.05.
Results
The full results of ANOVA testing are provided in the supplementary material (Tables S1 and S2). In this section, we only give the P-values for the various tests to indicate significance level.
Cu accumulation
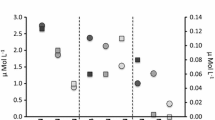
Clear differences in accumulation patterns were seen as a consequence of exposure route. When exposed to Cu in the water phase, the eelgrass shoots were almost exclusively accumulating Cu in the leaves, while very little Cu was found in roots and rhizomes (Fig. 1A, C). Following water exposure, Cu accumulation in the root and rhizome was consistently low, <200 µg Cu g−1 DW, and was independent of both salinity and water Cu concentration (Fig. 1C; ANOVA, P > 0.05 in both cases). Leaf concentrations, in contrast, varied from 40 µg Cu g−1 DW, when water Cu concentration was 0 µg Cu g−1 DW, to 2000 µg Cu g−1 DW, when water Cu concentration was 84 µg Cu g−1 DW (Fig. 1A). There was a highly significant effect of water Cu concentration (Fig. 1A; ANOVA, P < 0.0001), and the post hoc test showed that both Cu water concentrations resulted in a higher leaf Cu accumulation than was the case in the control treatment, while there was no significant difference in Cu accumulation between these two elevated Cu water concentrations. A weakly significant effect of salinity on leaf Cu accumulation was also seen (Fig. 1A; ANOVA, P = 0.044), so that Cu accumulation was higher at the highest salinity than at the lowest salinity, while Cu accumulation at ambient (medium) salinity was not significantly different from either of the other two salinities.
Tissue Cu concentrations (mean values (µg Cu g−1 DW) ± 1 SD, n = 3) as a result of plant exposure to Cu through different routes. A, C show water exposure and B, D sediment exposure, Cu concentrations in leaves (A, B) and roots/rhizomes (C, D) are shown. Open bars Salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Lowercase letters (a–c) indicate statistically significant differences between Cu treatments (A, B, D). Only treatments where statistically significant differences are found are indicated with letters. Lowercase letters x–y (A) indicate statistically significant effects of salinity treatments. There were no statistically significant interaction effects, so these letters are only shown for the first Cu treatment, but should be assumed to be the same for the other two Cu treatments as well
When Cu was added to the sediment, it was clearly taken up into the roots and rhizomes, but also translocated to the leaves (Fig. 1B, D). Root and rhizome concentrations were highest, up to 8000 µg Cu g−1 DW, while leaf Cu concentrations showed a maximum of 6000 µg Cu g−1 DW at the highest sediment Cu exposure (Fig. 1B, D). Both root/rhizome and leaf Cu concentrations increased significantly with increasing Cu concentration in the sediment (Fig. 1B, D; ANOVA, P < 0.0001 in both cases), While there was no significant overall effect of salinity on Cu accumulation in either case (ANOVA, P > 0.05), there was a significant Sal × Cu interaction (P = 0.033) for Cu concentrations in roots and rhizomes, where a lower Cu accumulation was observed for the highest salinity at the lower Cu level. At the highest Cu level, there were no significant differences in Cu accumulation, however.
Leaf growth rates
Leaf growth rates were significantly affected by Cu exposure through roots and rhizomes, but interestingly not by Cu exposure through the leaves themselves (Fig. 2A, B). In the latter case, leaf RGR varied between 0.008 and 0.011 g g−1 d−1 with no significant effects of either water Cu concentration or salinity (Fig. 2A; ANOVA, P > 0.05 in both cases). In contrast, when the plants were exposed to Cu through the sediment, leaf RGR decreased significantly with increasing sediment Cu concentrations (Fig. 2B; ANOVA, P = 0.001), from around 0.010 g g−1 d−1 in the control treatment to 0.001–0.006 g g−1 d−1 at the highest sediment Cu concentration (Fig. 2B). No effect of salinity was seen (ANOVA, P > 0.05).
Relative leaf growth rate (g g−1 d−1) in shoots exposed to Cu through the water (A) or the sediment (B). Bars and error bars show mean values ± 1 SD. Open bars salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Lowercase letters (a, b) indicate statistically significant differences between Cu treatments (B). Only treatments where statistically significant differences are found are indicated with letters
Leaf number and mortality
The total number of leaves on the eelgrass shoots was significantly reduced at the highest Cu concentration, whether exposed through the water or through the sediment. When the exposure took place through the sediment the number of leaves was reduced from 9 to 10 per shoot to 5–7 per shoot (Fig. 3B; ANOVA, P = 0.0030). The significant differences were observed between the control treatment and the highest sediment Cu concentration. At the intermediate sediment Cu concentration, the number of leaves was intermediate, but not significantly different from the number at either the control treatment or the highest sediment Cu concentration. When exposed to Cu through the water phase, the number of leaves were reduced to 6–8 per shoot (Fig. 3A; ANOVA, P = 0.0046). The reduction in the number of leaves was not related to water Cu concentration, with plants in intermediate and high Cu water concentrations showing no significant difference in leaf numbers. There was no effect of salinity on leaf number in any treatment (ANOVA, P > 0.05 in all cases).
Number of leaves per eelgrass shoot in shoots exposed to Cu through the water (A) or the sediment (B). Bars and error bars show mean values ± 1 SD. Please note that some error bars are missing due to lack of variability. Open bars salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Lowercase letters (a, b) indicate statistically significant differences between Cu treatments
Leaf mortality, measured as the fraction of dead leaves, increased significantly with sediment Cu concentration (Fig. 4B; ANOVA, P < 0.00001), but was only marginally, and insignificantly, increased with water Cu concentration (Fig. 4A; ANOVA, P = 0.056). Again, no effect of salinity was seen (Fig. 4; ANOVA, P > 0.05 in all cases).
Fraction of dead leaves (leaf mortality) in eelgrass in shoots exposed to Cu through the water (A) or the sediment (B). Bars and error bars show mean values ± 1 SD. Open bars salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Lowercase letters (a–c) indicate statistically significant differences between Cu treatments (B). Only treatments where statistically significant differences are found are indicated with letters
Leaf chlorophyll content
When the eelgrass shoots were exposed to Cu through the water phase, a statistically significant effect of salinity was seen, so that the leaf chlorophyll content was higher the higher the salinity (Fig. 5A; ANOVA, P = 0.003). This effect was seen at all Cu concentrations and was independent of the Cu concentration. No effects of water Cu concentration on leaf chlorophyll concentration were seen (Fig. 5A; ANOVA, P > 0.05). When the eelgrass shoots were exposed to Cu through the sediment, a different pattern was seen. The ANOVA revealed no statistically significant individual effects of either Cu or salinity (Fig. 5B; ANOVA, P > 0.05 in both cases), but there is a statistically significant interaction between Cu and salinity (Fig. 5B; ANOVA, P = 0.041). This is because the control treatment, as described above, showed an increase in chlorophyll concentration with increasing salinity, while the opposite pattern, a decrease in chlorophyll concentration with salinity was seen at the highest sediment Cu exposure.
Leaf chlorophyll concentration (µg g−1 DW) in shoots exposed to Cu through the water (A) or the sediment (B). Bars and error bars show mean values ± 1 SD. Open bars salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Lowercase letters x–z (A) indicate statistically significant effects of salinity treatments. There were no statistically significant interaction effects, so these letters are only shown for the first Cu treatment, but should be assumed to be the same for the other two Cu treatments as well
Maximum quantum yield (F v/F m)
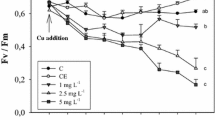
Maximum quantum yields are shown in Fig. 6. It was not possible to find a data transformation that fulfilled the requirements for ANOVA testing, so it is necessary to be cautious in the interpretation of the statistical analyses of these data. However, even when taking this into account, the data indicate that a leaf was either dead, as evidenced by its black or brown coloration and no measurable chlorophyll fluorescence, or it was living and fully functional or at least only slightly photo-inhibited, as evidence by F v/F m values of 0.60–0.75 (Fig. 6). Only in very few cases were F v/F m values below this range found in living leaves (Fig. 6). In no cases could variations in F v/F m values be related to treatments. The number of dead and thus missing leaves seem to increase with increasing Cu exposure, especially when plants were exposed to Cu through the sediment (Fig. 6). This confirms the general pattern emerging that leaves were fully functional photosynthetically until they died.
Maximum quantum yield (F v/F m) and number of missing (dead) leaves in shoots exposed to Cu through the water (A) or the sediment (B). Maximum quantum yield is shown by the wide bars, where bars and error bars show mean values ± 1 SD. Open bars salinity 5‰, hatched bars. Salinity 17‰, cross-hatched bars salinity 34‰. Thin black bars indicate the mean number of missing leaves per shoot
Discussion
Our data clearly show that Cu that is taken up in the leaves of the eelgrass plants is minimally translocated to other parts of the plant (specifically roots and rhizomes), whereas Cu taken up through roots and rhizomes is readily translocated to the rest of the plant. In line with our results, inorganic nutrients are generally taken up in seagrasses through roots and rhizomes and translocated both to leaves on the same shoot as well as to other shoots (ramets) in the same clone (Marbà et al., 2002). An exception from the general pattern of translocation of inorganic nutrients from roots to leaves in eelgrass is the re-absorption of inorganic nutrients in the roots and rhizomes that takes place at the end of the growth season as nutrients are re-mobilized from the leaves and moved to roots and rhizomes and stored there during the winter (Pedersen & Borum, 1993). While we do not see a similar pattern here for Cu, probably due to the fact that we are working with actively growing shoots in a summer situation, our study adds to the limited knowledge on trace metal translocation in seagrasses.
We do not see an effect of Cu on leaf growth rate and leaf mortality when the eelgrass plants are exposed to Cu in water through the leaves, but only when exposure takes place from sediment through roots and rhizomes. This is probably because Cu is only translocated to the basal meristems of the shoots when taken up through the below-ground parts. In the meristem, Cu is expected to affect cell division and elongation (Corellou et al., 2000), as it has also been documented for macroalgae (Nielsen et al., 2003; Nielsen & Nielsen, 2005). This suggests that Cu could also be affecting root and rhizome growth, something not within the scope of present study, but worthy of future investigation.
In macroalgae, clear effects of Cu on chlorophyll fluorescence parameters are well documented (Nielsen & Nielsen, 2008). The same clear effects are not seen in eelgrass in this study, where the leaves have a tendency to either being fully functional with only very weak chlorophyll fluorescence signals of stress, or they are dead with no chlorophyll content at all. This indicates that chlorophyll fluorescence can only serve as an early indicator of Cu stress in eelgrass to a very limited extent. Interestingly, this is in contrast to what is generally found in both macroalgae (Nielsen & Nielsen, 2005) and in other seagrasses, where clear effects of copper on chlorophyll fluorescence parameters have been observed (Macinnis-Ng & Ralph, 2002). The lack of effects on maximum quantum yields is all the more surprising, given that effects are seen after only a few hours of exposure to contaminants in other experiments (Macinnis-Ng & Ralph, 2003). Similarly, the effect of Cu on chlorophyll fluorescence have been reported in species of terrestrial higher plants (Schroder et al., 1994) and it is well known that Cu has harmful effects on the photosynthetic apparatus, especially the D1 protein–chlorophyll complex (Kupper et al., 2002).
Surprisingly, salinity affects the endpoints measured here to only a very limited extent, although salinity in other studies has been shown to have strong effects on the same parameters (Salo & Pedersen, 2014; Villazan et al., 2015). Salinity in the location in which the plants were sampled is quite constant, as described in Materials and Methods, so it is not likely that the plants were already acclimated to fluctuating salinities. However, a strong positive effect of salinity on leaf chlorophyll concentration is found in plants exposed to Cu through the water. In these plants, there is also a weak, but statistically significant, tendency for a higher Cu accumulation in the leaves at higher salinities. This could indicate that a higher nutrient uptake occurs in the shoots at higher salinities (Villazan et al., 2015), with an associated higher Cu uptake through a common-ion effect (Ashraf et al., 2015). A similar effect of salinity was not seen in the plants that were exposed to Cu through roots and rhizomes; however, there was some indication that a high salinity lowered root Cu uptake at the lowest Cu exposure, but not at the highest. This pattern can possibly be explained by Cu disturbing the normal functioning of roots and nutrient uptake in these plants (Yang et al., 2015).
The strong effect that Cu had on plant performance in this study when plants were exposed through roots and rhizomes, indicates that contaminant effects on seagrasses in general are highly likely underestimated as most experiments have been done using only leaf exposure. This could mean that although there is compelling evidence that the lack of recolonization of eelgrass in Danish waters following oligotrophication (Riemann et al., 2016) is mainly caused by changes in sediment characteristics (Mascaró et al., 2009) and ecosystem changes (Canal-Verges et al., 2010), contaminants may play a hitherto overlooked contributing role alone or together with other stressors. This warrants further research that takes population effects of sub-lethal contaminant concentrations as well as the effects of multiple stressors into consideration.
Conclusion
In the present study, we document that Cu exposure route (sediment or water) is an important factor in determining the ecotoxicological effects experienced by eelgrass. Exposure to roots and rhizomes has a much stronger, more harmful, effect to the plants than leaf exposure. This is significant because in natural ecosystems, the largest Cu pools (and many other contaminants) are found in the sediment (HELCOM, 2010) so plants in a natural setting will be especially exposed through this route. This finding is also significant because most experiments testing seagrasses response to contaminants are done using water-only leaf exposure and often with the plants growing in terrestrial soil, rather than in natural sediments (Ambo-Rappe et al., 2011; Macinnis-Ng & Ralph, 2002, 2004b). This is because it is easy to be handled logistically and because it allows much easier control of exposure concentrations of contaminants, but it has profound consequences for interpretation of results. Such experiments must be assumed to underestimate the contaminant effects on seagrasses, probably contributing to the general tendency to regard contaminant effects on seagrasses as negligible. Our study shows that contaminant effects, especially from sediments, are worthy of investigation, both singly and in combination with more recognized stressors, such as temperature, light, and salinity, even though we found only weak effects of salinity in this study. Thus as anthropogenic inputs increase and natural conditions are more prone to fluctuation, future research into ecosystem health should take all these factors into account.
References
Alpert, P., 1991. Nitrogen sharing among ramets increases clonal growth in Fragaria chiloensis. Ecology 72: 69–80.
Alpert, P., 1996. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. Journal of Ecology 84: 395–406.
Ambo-Rappe, R., D. L. Lajus & M. J. Schreider, 2011. Heavy metal impact on growth and leaf asymmetry of seagrass, Halophila ovalis. Journal of Environmental Chemistry and Ecotoxicology 3: 149–159.
Anonymous, 1999. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Copper Canadian Environmental Quality Guidelines. Canadian Council of Ministers of the Environment, Winnipeg.
Apel, K. & H. Hirt, 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399.
Ashraf, U., A. S. Kanu, Z. W. Mo, S. Hussain, S. A. Anjum, I. Khan, R. N. Abbas & X. R. Tang, 2015. Lead toxicity in rice: effects, mechanisms, and mitigation strategies-a mini review. Environmental Science and Pollution Research 22: 18318–18332.
Bloom, H. & G. M. Ayling, 1977. Heavy-metals in Derwent Estuary. Environmental Geology 2: 3–22.
Burkholder, J. M., D. A. Tomasko & B. W. Touchette, 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350: 46–72.
Canal-Verges, P., M. Vedel, T. Valdemarsen, E. Kristensen & M. R. Flindt, 2010. Resuspension created by bedload transport of macroalgae: implications for ecosystem functioning. Hydrobiologia 649(1): 69–76.
Corellou, F., S. R. Bisgrove, D. L. Kropf, L. Meijer, B. Kloareg & F. Y. Bouget, 2000. A S/M DNA replication checkpoint prevents nuclear and cytoplasmic events of cell division including centrosomal axis alignment and inhibits activation of cyclin-dependent kinase-like proteins in fucoid zygotes. Development 127: 1651–1660.
Denny, P., 1972. Sites of nutrient absorption in aquatic macrophytes. Journal of Ecology 60: 819–829.
Epstein, E. & A. J. Bloom, 2005. Mineral Nutrition of Plants. Principles and Perspectives. Sinauer, Sunderland.
Frederiksen, M. S., D. Krause-Jensen, M. Holmer & J. S. Laursen, 2004. Long-term changes in area distribution of eelgrass (Zostera marina) in Danish coastal waters. Aquatic Botany 78: 167–181.
Heck, K. L., G. Hays & R. J. Orth, 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology-Progress Series 253: 123–136.
HELCOM, 2010. Hazardous substances in the Baltic Sea – An integrated thematic assessment of harzardous substances in the Baltic Sea Baltic Sea Environment Proceedings no 120B. Baltic Marine Environment Protection Commission – Helsinki Commission, Helsinki.
Jespersen, A.-M. & K. Christoffersen, 1987. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Archiv für Hydrobiologie 109(3): 445–454.
Kupper, H., I. Setlik, M. Spiller, F. C. Kupper & O. Prasil, 2002. Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. Journal of Phycology 38: 429–441.
Macinnis-Ng, C. M. O. & P. J. Ralph, 2002. Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni. Marine Pollution Bulletin 45: 100–106.
Macinnis-Ng, C. M. O. & P. J. Ralph, 2003. In situ impact of petrochemicals on the photosynthesis of the seagrass Zostera capricorni. Marine Pollution Bulletin 46: 1395–1407.
Macinnis-Ng, C. M. O. & P. J. Ralph, 2004a. In situ impact of multiple pulses of metal and herbicide on the seagrass, Zostera capricorni. Aquatic Toxicology 67: 227–237.
Macinnis-Ng, C. M. O. & P. J. Ralph, 2004b. Variations in sensitivity to copper and zinc among three isolated populations of the seagrass, Zostera capricorni. Journal of Experimental Marine Biology and Ecology 302: 63–83.
Madsen, T., L. Samsøe-Petersen, K. Gustavson & D. Rasmussen, 1999. Økotoksikologisk vurdering af antibegroninghindrende biocider og biocidfrie bundmalinger. Miljøprojekt. vol nr. 507. Danish Environmental Protection Agency, Copenhagen.
Marbà, N., M. A. Hemminga, M. A. Mateo, C. M. Duarte, Y. E. M. Mass, J. Terrados & E. Gacia, 2002. Carbon and nitrogen translocations between seagrass ramets. Marine Ecology-Progress Series 226: 287–300.
Mascaró, O., T. Valdemarsen, M. Holmer, M. Pérez & J. Romero, 2009. Experimental manipulation of sediment organic content and water column aeration reduces Zostera marina (eelgrass) growth and survival. Journal of Experimental Marine Biology and Ecology 373: 26–34.
Maxwell, K. & G. N. Johnson, 2000. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51: 659–668.
Meharg, A. A. & M. R. MacNair, 1992. Genetic correlation between arsenate tolerance and the rate of influx of arsenate and phosphate in Holcus lanatus L. Heredity 69: 336–341.
Monferran, M. V., J. A. S. Agudo, M. L. Pignata & D. A. Wunderlin, 2009. Copper-induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environmental Pollution 157: 2570–2576.
Namiesnik, J. & A. Rabajczyk, 2010. The speciation and physico-chemical forms of metals in surface waters and sediments. Chemical Speciation and Bioavailability 22: 1–24.
Nejrup, L. B. & M. F. Pedersen, 2008. Effects of salinity and water temperature on the ecological performance of Zostera marina. Aquatic Botany 88: 239–246.
Nielsen, H. D. & S. L. Nielsen, 2005. Photosynthetic responses to Cu2+ exposure are independent of light acclimation and uncoupled from growth inhibition in Fucus serratus (Phaeophyceae). Marine Pollution Bulletin 51: 715–721.
Nielsen, H. D. & S. L. Nielsen, 2008. Evaluation of imaging and conventional PAM as a measure of photosynthesis in thin- and thick-leaved marine macroalgae. Aquatic Biology 3: 121–131.
Nielsen, S. L., K. Sand-Jensen, J. Borum & O. Geertz-Hansen, 2002a. Depth colonization of eelgrass (Zostera marina) and macroalgae as determined by water transparency in Danish coastal waters. Estuaries 25: 1025–1032.
Nielsen, S. L., K. Sand-Jensen, J. Borum & O. Geertz-Hansen, 2002b. Phytoplankton, nutrients, and transparency in Danish coastal waters. Estuaries 25: 930–937.
Nielsen, H. D., M. T. Brown & C. Brownlee, 2003. Cellular responses of developing Fucus serratus embryos exposed to elevated concentrations of Cu2+. Plant, Cell and Environment 26: 1737–1747.
Orth, R. J., T. J. B. Carruthers, W. C. Dennison, C. M. Duarte, J. W. Fourqurean, K. L. Heck, A. R. Hughes, G. A. Kendrick, W. J. Kenworthy, S. Olyarnik, F. T. Short, M. Waycott & S. L. Williams, 2006. A global crisis for seagrass ecosystems. Bioscience 56: 987–996.
Pedersen, M. F. & J. Borum, 1993. An annual nitrogen budget for a seagrass Zostera marina population. Marine Ecology Progress Series 101: 169–177.
Riemann, B., J. Carstensen, K. Dahl, H. Fossing, J. Hansen, H. Jakobsen, A. Josefson, D. Krause-Jensen, S. Markager, P. Stæhr, K. Timmermann, J. Windolf & J. Andersen, 2016. Recovery of Danish coastal ecosystems after reductions in nutrient loading: a holistic ecosystem approach. Estuaries and Coasts 39: 82–97.
Rigollet, V., T. Laugier, M. L. de Casabianca, A. Sfriso & A. Marcomini, 1998. Seasonal biomass and nutrient dynamics of Zostera marina L. in two Mediterranean lagoons: Thau (France) and Venice (Italy). Botanica Marina 41(2):167–179.
Salo, T. & M. F. Pedersen, 2014. Synergistic effects of altered salinity and temperature on estuarine eelgrass (Zostera marina) seedlings and clonal shoots. Journal of Experimental Marine Biology and Ecology 457: 143–150.
Salo, T., M. F. Pedersen & C. Bostrom, 2014. Population specific salinity tolerance in eelgrass (Zostera marina). Journal of Experimental Marine Biology and Ecology 461: 425–429.
Sand-Jensen, K., 1975. Biomass, net production and growth dynamics in an eelgrass (Zostera marina L.) population in Vellerup Vig, Denmark. Ophelia 14: 185–201.
Sanz-Lazaro, C., P. Malea, E. T. Apostolaki, I. Kalantzi, A. Marin & I. Karakassis, 2012. The role of the seagrass Posidonia oceanica in the cycling of trace elements. Biogeosciences 9: 2497–2507.
Schroder, W. P., J. B. Arellano, T. Bittner, M. Baron, H. J. Eckert & G. Renger, 1994. Flash-induced absorption-spectroscopy studies of copper interaction with photosystem-II in higher-plants. Journal of Biological Chemistry 269: 32865–32870.
Thomas, G., H. J. Stark, G. Wellenreuther, B. C. Dickinson & H. Kupper, 2013. Effects of nanomolar copper on water plants-Comparison of biochemical and biophysical mechanisms of deficiency and sublethal toxicity under environmentally relevant conditions. Aquatic Toxicology 140: 27–36.
Turley, P. A., R. J. Fenn & J. C. Ritter, 2000. Pyrithiones as antifoulants: environmental chemistry and preliminary risk assessment. Biofouling 15: 175–182.
Valdemarsen, T., P. Canal-Vergés, E. Kristensen, M. Holmer, M. D. Kristiansen & M. R. Flindt, 2010. Vulnerability of Zostera marina seedlings to physical stress. Marine Ecology Progress Series 418: 119–130.
Valdemarsen, T., C. O. Quintana, E. Kristensen & M. R. Flindt, 2014. Recovery of organic-enriched sediments through microbial degradation: implications for eutrophic estuaries. Marine Ecology Progress Series 503: 41–58.
van der Heide, T., E. H. van Nes, M. M. van Katwijk, H. Olff & A. J. P. Smolders, 2011. Positive feedbacks in seagrass ecosystems – evidence from large-scale empirical data. PLoS One 6(1): e16504.
Vanderkooij, L. A., D. Vandemeent, C. J. Vanleeuwen & W. A. Bruggeman, 1991. Deriving quality criteria for water and sediment from the results of aquatic toxicity tests and product standards – application of the equilibrium partitioning method. Water Research 25: 697–705.
Villazan, B., T. Salo, F. G. Brun, J. J. Vergara & M. F. Pedersen, 2015. High ammonium availability amplifies the adverse effect of low salinity on eelgrass Zostera marina. Marine Ecology Progress Series 536: 149–162.
Waycott, M., C. M. Duarte, T. J. B. Carruthers, R. J. Orth, W. C. Dennison, S. Olyarnik, A. Calladine, J. W. Fourqurean, K. L. Heck, A. R. Hughes, G. A. Kendrick, W. J. Kenworthy, F. T. Short & S. L. Williams, 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106: 12377–12381.
Yang, Q., L. H. Wang, Q. Zhou & X. H. Huang, 2015. Toxic effects of heavy metal terbium ion on the composition and functions of cell membrane in horseradish roots. Ecotoxicology and Environmental Safety 111: 48–58.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: André Padial
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nielsen, S.L., Banta, G.T., Khan, F.R. et al. Copper in the sediment: a major stressor for eelgrass, Zostera marina L.. Hydrobiologia 788, 143–155 (2017). https://doi.org/10.1007/s10750-016-2994-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2994-1