Abstract
The bioremediation capacity of the red macroalga Palmaria palmata was assessed by two experiments. First, uptake rates of P. palmata cultured in four treatments with varying levels and ratios of the N sources ammonium (NH4 +) and nitrate (NO3 −) (18/15, 0/30, 30/45, 50/65 μM) were measured over a 3-h period to evaluate N source preference. Secondly, P. palmata were cultured in five treatments with varying levels and ratios of ammonium and nitrate (300/12, 0/312, 500/12, 0/512, 250/262 μM) for 3 weeks to evaluate specific growth rates, protein content, and ammonia toxicity. Palmaria palmata had a higher affinity for NH4 + than for NO3 − as N source. However, in the single N source trials, NO3 − uptake was higher than that of NH4 +. The maximum specific growth rate of 11.99 % day−1 was observed in the 0/512 μM ammonium/nitrate treatment after 3 weeks, whereas the minimum specific growth rate of 2.21 % day−1 was observed in the 500/12 μM ammonium/nitrate treatment after 3 weeks. NO3 − supported higher growth rates, whereas NH4 + increased tissue N, and therefore protein content. Total protein content of the algal tissue was significantly higher in P. palmata of the NH4 + treatments, reaching up to 20.6 % DW, than of those from the NO3 -treatments. Palmaria palmata showed signs of poisoning after 3 weeks in the highest NH4 + treatment. This study indicates that P. palmata is a suitable species for ecological engineering in integrated multitrophic aquaculture systems as it shows a relatively high growth performance, high nutrient uptake rates, and elevated protein content under NH4 + supply.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, nearly 50 % of the total finfish and invertebrate production and 96 % of the total seaweed production are generated by the growing aquaculture sector (Chopin 2014, FAO 2014). While the industry is expected to grow, there is also the need for eco-intensification of aquaculture. The cultivation of seaweed together with fed species in integrated multi-trophic aquaculture (IMTA) is improving the overall ecological efficiency, sustainability, and economics of the business (Neori and Nobre 2012). Thus, the prospects of seaweed culture for bioremediation and its cost-efficient production in IMTA systems turn it into an important tool in environmental management (Troell et al. 2003). Consequently, seaweed cultivation should be intensified and more seaweed species of temperate regions need to be studied regarding their capacity for bioremediation.
Total ammonia nitrogen (TAN) (unionized ammonia (NH3) and ionized ammonia (NH4 +)) is a main excretory product of the N metabolism of fish and is toxic to most fish species at concentrations above 1.5 mg NH3-N L−1 (Dosdat et al. 1996, Hagopian and Riley 1998). Empirical studies showed differences between macroalgae species in the biofiltration of dissolved nitrogen from aquaculture wastewater (Hernandez et al. 2002). There is some evidence that these varying efficiencies for N uptake are a result of a complex interplay between nutrient availability and the position in the zonation of an ecosystem (Sánchez de Pedro et al. 2013). Suitable seaweed candidate species for IMTA systems should have high growth rates, high nutrient uptake efficiencies, and practical methods of cultivation (Neori et al. 2004). Several studies have demonstrated the ability and effectiveness of seaweed as biofilter integrated in fed finfish/shrimp culture (Neori et al. 1991, 1996; Nelson et al. 2001, Schuenhoff et al. 2003). Seaweed biofilters were even found to be more effective than the traditional method of bacterial bio-filtration (Cahill et al. 2010). Most studies on seaweed from temperate regions as biofilters in IMTA used the green algae Ulva spp. and the red algae Gracilaria spp., which are well-established aquaculture species and whose nutrient uptake abilities are high compared to most other seaweeds (e.g., Martinez-Aragon et al. 2002; Neori et al. 2000, 2004; Msuya and Neori 2008; Abreu et al. 2013). Furthermore, species of the genus Porphyra (Porphyra and Pyropia; Sutherland et al. 2011) are expected to be suitable candidates for IMTA, as they have high nutrient uptake and growth rates (Kang et al 2014). Both genera of red seaweeds, Gracilaria and Porphyra, are characterized by a high surface area to volume ratio, due to their fine branched or rather thin appearance. However, in the case of eco-intensification of offshore aquaculture operations, the macroalgae species additionally needs to be robust enough to withstand a high energy environment (Buck and Buchholz 2005). Therefore, the more robust red seaweed dulse, Palmaria palmata (Linnaeus) Weber & Mohr, is a possible candidate as seaweed biofilter for North Atlantic IMTA systems in high energy environments.
Dulse is an edible red alga growing in the intertidal or shallow subtidal of the North Atlantic, and its culture methods are well established (Le Gall et al. 2004; Pang and Lüning 2004, 2006). Knowledge of the bio-mitigation capacity of dulse will have practical implications for intended eco-intensification of mass cultivation in IMTA systems at exposed marine sites. However, when comparing the results of the few existing studies on dulse, the outcomes regarding N source preference and N uptake efficiency are to some extent inconsistent (Morgan and Simpson 1981; Martinez and Rico 2004; Corey et al. 2013). The influence of N source on N storage and therefore protein content was not studied in dulse. Furthermore, P. palmata was described as an ammonium (NH4 +)-sensitive species (Morgan and Simpson 1981), which could reduce its biofilter suitability for IMTA systems. Hence, the purpose of this study was to evaluate the bioremediation capacity of dulse to clarify its suitability for IMTA operations.
Therefore, adjusted nutrient concentrations were used in two experiments to fill the gap in knowledge on P. palmata in order to validate its prospects for IMTA cultivation. In the short-term experiment, N source preference and N uptake efficiencies were investigated, whereas the hebdomadal nutrient uptake experiment, running for 3 weeks, examined the influence of N source on growth rates, protein content, and the NH4 + sensitivity.
Materials and methods
Prior to the experiments, clean, apical tips of P. palmata blades were cut into 1-cm lengths and placed into transparent glass bottles filled with 5 L of filtered, autoclaved seawater containing 100 mL Provasoli nutrient solution (34 psu, pH 8, Provasoli 1968). The seaweed blades were acclimated by culturing for up to 3 weeks in tumbling suspension by continuous aeration in a 10 °C temperature controlled room on a 16:8 light/dark (L/D) cycle under approximately 60 μmol photons m−2 s−1 of irradiance. All glassware used was rinsed with 10 % HCl followed by autoclaving prior to use. Postacclimation tissue samples were collected in triplicate and frozen at −20 °C for further analysis.
Design of experiments
For the two experiments, the first one a short experiment and the second a hebdomadal experiment, two to three blades of P. palmata, 10 to 14 cm in length and equivalent to ca. 2.0 g fresh weight per 1 L transparent glass beaker, were kept in filtered (0.2 μm), autoclaved North Sea water and aerated with pressurized air. Provasoli nutrient solution without nitrogen (N) was added in 10 mL portions to each of the 20 1 L beakers, containing sufficient phosphate, vitamins, and micronutrients. Temperature was maintained at 10 °C under saturating light intensity of 125 μmol photons m−2 s−1 (Martinez and Rico 2004), at a photoperiod of 16:8 (L/D, Sagert and Schubert 2000). Light was supplied by 36 W, 965 Biolux fluorescent lamps (Osram, Germany). Irradiance was measured using a light meter (Li-Cor Li-189 with a flat Li-Cor Quantum sensor, USA).
In the short experiment, the seaweed blades were cultured with varying levels of ammonium chloride (NH4Cl) and sodium nitrate (NaNO3) in four different treatments (Table 1), each conducted in triplicate. For each treatment, a control beaker without seaweed was maintained for the duration of the experiment. Nutrient uptake was monitored, following stocking of algal blades to the beakers, after 15, 30, 60, 120, and 180 min by taking water samples of 15 mL with sterile syringes, which were filtered (sterile 0.2 μm) into sterile centrifuge tubes and stored at −80 °C until further analyses. The concentrations of ammonium, nitrate, nitrite, and phosphate of samples were measured with an Alliance Instruments Evolution III continuous flow autoanalyzer (Salzburg, Austria).
In the hebdomadal experiment, the seaweed blades were cultured with five levels of the nutrients NH4Cl and NaNO3 (Table 1), each conducted in triplicate for 3 weeks. For each treatment, a control beaker without seaweed was maintained for the duration of the experiment, enabling measurement of possible ammonium loss by volatilization or bacteria. Semi-weekly nutrients and Provasoli solution without N were exchanged, and once a week, the seaweed biomass was dried on the surface with paper towels and weighed (fresh weight (FW)). Specific growth rate (SGR [% day−1]) was determined weekly by weighing the samples to the nearest 0.001 g within 2 min, and it was calculated using the following formula:
where FW a and FW b are the fresh weight (g) at days t a and t b , respectively. Tissue samples were taken by reducing the seaweed biomass to the initial density of ca. 2.0 g L−1 at each weighing. Tissue samples were frozen at −20 °C until further analyses. The dry weight (DW)/fresh weight (FW) ratio of 7.9 was determined by drying tissue samples to constant weight at 60 °C.
Dried tissues were ground to powder using pestle and mortar, weighed into tin cups and then analyzed for total nitrogen (N) and carbon (C) using a EURO EA Elemental Analyzer (EURO VECTOR Instruments, Milan, Italy). Total protein content of P. palmata was calculated according to Lourenço et al. (2002). The calculation of protein content of red algae using a nitrogen-to-protein conversion factor of 4.59 has proven to be a strong proxy for whole biomass protein quantification (Lourenço et al. 2002). Analyzing total elemental nitrogen is based on high-temperature combustion and is less liable to interferences.
Nitrogen removal (g N g−1 DW day−1) of dulse was calculated according to Kim et al. (2007):
where B t and B 0 are the biomass (g) at day t (final) and day 0 (initial), respectively.
In both experiments, phosphate (P) was not limiting for P. palmata nitrogen uptake or growth as it was around 16–18 μM mL−1 in the beginning of the different trials and it was not depleted until the water exchange or the end of the experiments (data not shown). In the hebdomadal experiment, N/P ratios were 18:1 and 30:1, representing concentrations near the Redfield ratio and the average optimal ratio for seaweed growth (Atkinson and Smith 1983). Nutrient concentration of the control beakers without seaweed did not change significantly for the duration of the experiments.
Statistical analysis
Data were analyzed for normality, homogeneity of variance, and the difference between treatments and weeks were analyzed by using ANOVA followed by a Tukey’s HSD post hoc analysis (p = 0.05). Statistical analyses were performed using STATISTICA 9 (StatSoft, USA).
Results
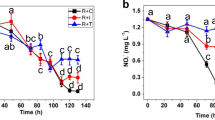
In the short experiment, mean nutrient uptake by P. palmata was 93.7 % in the treatment enriched with 30 μM NO3 −, while in the 18 μM NH4 +, only 69.9 % were taken up after 180 min (Fig. 1a). When both nutrients were supplied in higher concentrations of 30 μM NH4 + and 45 μM NO3 − and 50 μM NH4 + and 65 μM NO3 −, mean uptake after 180 min was higher for NH4 + than for NO3 − (Fig. 1b). For the 30 μM NH4 + and 45 μM NO3 − nutrient treatment, 71.9 and 53 %, respectively, were taken up by the red algae. In the treatment with the highest concentration of 50 μM NH4 + and 65 μM NO3 −, uptake was 75.2 % NH4 + and 34.2 % NO3 −. Furthermore, uptake of nutrients within the first 15 min of the experiment was 3 to 4.5 times higher for NH4 + than for NO3 − (Fig. 1b).
In the hebdomadal experiment, N source and concentration had a significant effect on growth rate of P. palmata (p < 0.05). In general, P. palmata from the nitrate treatments showed significantly higher mean SGRs than those from the ammonium treatments after the 3-week experiment (p < 0.05; Fig. 2). However, the observed variances in mean SGR of the red alga were not significantly different during the first week of the experiment. Dulse in the treatment with 250/262 μM ammonium/nitrate grew at a similar mean SGR as in the pure ammonium treatments during the three weeks. Highest mean SGR of 11.99 % day−1 was observed in the 0/512 μM ammonium/nitrate treatment after 3 weeks, whereas the lowest mean SGR of 2.21 % day−1 was observed in the 500/12 μM ammonium/nitrate treatment after 3 weeks (Fig. 2). Within the same treatments, there was a significant increase in mean SGR at 0/512 μM ammonium/nitrate (p < 0.05) and a significant decrease in mean SGR from week 2 to week 3 at 500/0 μM ammonium/nitrate (p < 0.05). Furthermore, the fronds of P. palmata in the latter treatment showed signs of bleaching after 3 weeks.
Nitrate availability also had a significant effect (p < 0.05) on tissue N of P. palmata and therefore on protein content (Table 2). Total protein content of the algal tissue was significantly higher in P. palmata of the ammonium treatments than of those from the nitrate treatments (p < 0.05) (Table 2). This could be observed especially in weeks 2 and 3 of the experiment. The C/N ratios of the ammonium and nitrate treatments were significantly different during all 3 weeks of the experiment (Table 2), being lower in those with added ammonium. The highest mean C/N ratio of 15.98 was observed in the tissue of P. palmata at 0/312 ammonium/nitrate after the first week of the experiment, whereas the lowest mean C/N ratio of 6.51 was detected in seaweed tissue at 500/12 ammonium/nitrate after 3 weeks (Table 2). Tissue C % was not affected by nutrient supply. In general, mean protein content of P. palmata increased significantly in 300/12, 500/12 and 250/262 μM ammonium/nitrate from week 1 to week 3 (p < 0.05). The C/N ratio significantly decreased in these nutrient treatments during the 3-week experiment (p < 0.05).
Mean N removal rate was between 0.29 and 0.89 mg N g−1 DW day−1 during experiment A (Fig. 3). In general, N removal rate increased from week 1 to week 2 in all treatments, except at 250/262 μM NH4 +/NO3 −, where it remained around 0.63 mg N g−1 DW day−1 for 3 weeks. In week 3, only the NO3 − treatments showed a slight increase in N removal rate. The only significant decrease in N removal rate was observed at 500/12 μM NH4 +/NO3 − in week 3 (p < 0.05; Fig. 3).
Discussion
This study focused on the suitability of P. palmata for IMTA, in which the main N source is NH4 +. Palmaria palmata showed clearly a higher affinity for NH4 + than for NO3 − as N source, when both nutrients were available. However, in the single N source trials, nitrate uptake was higher than that of NH4 +, indicating a suppression of NO3 − uptake in presence of NH4 +. Inhibition of NO3 − uptake by NH4 + was observed in some macroalgae in the three major seaweed divisions, Chlorophyta, Rhodophyta, and Phaeophyta (Hanisak and Harlin 1978; D’Elia and DeBoer 1978; Haines and Wheeler 1978). NH4 + is already a reduced type of N and theoretically, less energy is needed for its incorporation into amino acids and proteins than for NO3 − (Syrett 1962). However, Thomas and Harrison (1987) concluded that the level of suppression of NO3 − uptake by NH4 + varies depending on the extent of NO3 − depletion in the algal tissue. However, N tissue deficiency seems not to be a major factor in nutrient preference of P. palmata, as low initial N content (N, 1.8 ± 0.11 % DW Martinez and Rico 2004; 1.89 ± 0.24 % DW, this study) in the tissue lead to a similar affinity for both N sources (21.5 μM NH4 +, 65.2 μM NO3 −) in the study of Martinez and Rico (2004), whereas a greater affinity for NH4 + was found in this study. The suppression of NO3 − uptake by NH4 + in P. palmata is supported by the study of Morgan and Simpson (1981) using much higher nutrient concentrations (0.5–2 mM), whereas Corey et al. (2013) reported that low concentrations of 30 μM NH4 + enhanced NO3 − uptake (270 μM NO3 − added). In this study, low concentrations of NH4 + were used in order to trace a threshold level of enhancement or inhibition of NO3 − uptake. This threshold level could not be located and the results of this study suggest that the suppression of NO3 − uptake is rather an indirect result of NH4 + uptake. Presumably, the relationship between the uptake rates of the two N sources is more complex than previously thought.
Despite the higher affinity for NH4 +, uptake of NO3 − supported higher growth rates of P. palmata in this study. Similar results were found by Morgan and Simpson (1981) and Demetropolous and Langdon (2004), where P. palmata and P. mollis were more productive under NO3 − supply than with NH4 + in the long term at higher nutrient concentrations than used in this study. Macrophytes are able to store surplus nutrients in their tissue (Thomas and Harrison 1985). If internal nutrients are depleted, growth rates usually decline. Generally, the C/N ratio of macroalgae lies between 5 and 40 (Niell 1976; Hanisak 1979). C/N ratios of 10–15 equal a status of sufficient nutrient supply, whereas a lower ratio indicates N storage, and a higher ratio reflects N limitation (Fredriksen and Rueness 1989). Corey et al. (2013) found only minor differences in growth rates of P. palmata depending on N source. The initial C/N ratio of the red alga was 7.3 (Corey et al. 2013), already indicating a N surplus, which might have led to these result. The initial C/N ratio of P. palmata in this study indicated a slight N limitation of fronds at the beginning of the experiment. In the NH4 + trials, C/N ratio reached values below 10 after the second week, demonstrating the storage of excess N in the tissue. However, in the NO3 − trials, C/N ratios stayed above 10 even after the third week, despite increasing values of N % DW, indicating no storage of N, but its support of the higher productivity.
In this study, due to the storage of N, protein content of P. palmata increased up to 20–20.6 % DW in the NH4 + trials after 3 weeks. Protein content in cultured P. palmata was found to reach up to 35–36 % DW under higher nutrient supply than in this study (Morgan et al. 1980, Demetropoulus and Langdon 2004). In wild P. palmata from the French Atlantic coast, protein levels between 9 % DW in summer and 25 % DW in winter were calculated (Galland-Irmouli et al. 1999). In the previous studies, the protein content was calculated with the Kjeldahl method (tissue N × 6.25). However, the relative portion of nonprotein N is greater in red algae compared to other plants and consequently a lower nitrogen-to-protein conversion factor (4.59) was calculated for red macroalgae (Lourenço et al. 2002) and used in this study. Using the Kjeldahl method, the highest protein content of P. palmata would have been 27.4 % DW after 3 weeks supplied with high NH4 +. In contrast, in the NO3 − trials, P. palmata tissue contained less protein but had higher SGRs than in the NH4 + trials. Supporting this finding, high NO3 − supply to P. mollis in tank culture resulted in increased SGRs instead of high protein content, as tissue N was not statistically different over the range of NO3 − additions at different light levels (Demetropoulus and Langdon 2004). Therefore, N source and N tissue history have an effect on growth rate and biochemical composition in the red alga P. palmata. Coculture of P. palmata with fed organisms in an IMTA system would lead to higher protein content in the red alga due to high NH4 + supply. Depending on the intended application, the culture method of P. palmata can be modified in terms of main N source, either focusing on growth and biomass production or on quality of tissue, such as protein content.
However, excessive supply of NH4 + led to first signs of NH4 + sensitivity in dulse, which were visible at 500 μM NH4 + exposure for 3 weeks, not only directly through bleached tissue but also indirectly by significantly reduced SGRs. Morgan and Simpson (1981) found signs of toxication during long-term exposure at 500 μM NH4 + four times a week with flushing of three to four tank volumes day−1. In P. mollis, signs of toxication were visible after 2-week exposure to concentrations of 7059 μM NH4NO3 four times a week and flushing of one tank volume day−1 (Demetropolous and Langdon 2004). The concentrations used in this study of 300 and 500 μM NH4 + correspond to 5.4 and 9 mg L−1 TAN, respectively, which would relate to a concentration of 1.03 and 1.71 mg L−1 NH3 +, respectively, at pH 7 and 10 °C (Goddard 1996). These are unfavorable concentrations in recirculating aquaculture systems (RAS), as NH3 + influences growth and performance of fish at a concentration of 0.02–0.1 mg L−1 and at 0.09 to 3.35 mg L−1 NH3 + becomes toxic to fish depending on the species, fish size, exposure time, and other environmental factors (Handy and Poxton 1993; Person-Le Ruyet et al. 1995). However, TAN released by intensive aquaculture varies substantially depending on species, fish size, stocking density, feed and feeding rate used, type of aquaculture system and temperature (Handy and Poxton 1993). The TAN concentrations in intensive RAS hatcheries are usually below 110 μM for the more susceptible young fish stages (Howell and Baynes 2004), but can reach for Atlantic halibut up to 2225 μM day−1, depending on feeding regime, temperature, and fish size (Kim et al. 2013). However, there would only be a punctual high nutrient supply after fish feeding and this would only be relevant, where aquaculture effluents are discharged directly into seaweed tanks. In RAS, nutrient concentrations would be kept lower due to protection of fish health and growth, whereas in open water, such high concentrations would not be reached due to dilution.
In conclusion, this study provides important data of P. palmata performance, nutrient uptake rates, and protein content, presenting its prospects for IMTA and ecological engineering. Palmaria palmata is a promising extractive candidate for temperate water IMTA due to its N uptake efficiency and its relatively high productivity. In a medium-scale RAS, the excess N of 1 kg turbot supported the growth of more than 6.5 kg of dulse (Grote and Buck, in review). These results do not automatically extrapolate to commercial-scale operations because the nutrient uptake can be nonlinear, influenced by many different variables, such as temperature. A modeling approach as the first step to understand these interrelations, enabled the calculation of dimension of the seaweed culture needed in relation to the culture size of the fed organism (Grote and Buck, in review). However, more research is needed to provide more information on bioremediation of P. palmata in open cultures on commercial scale with varying environmental conditions in order to show the economic and technical viability of such operations. Therefore, studies in large-scale RAS and offshore systems are required to clearly demonstrate the feasibility of P. palmata mass culture in commercial- scale IMTA systems.
References
Abreu MH, Pereira R, Yarish C, Buschmann AH, Sousa-Pinto I (2013) IMTA with Gracilaria vermiculophylla: productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
Atkinson MJ, Smith SV (1983) C:N:P ratios of benthic marine plants. Limnol Oceanogr 28:568–574
Buck BH, Bucholz CM (2005) Response of offshore cultivated Laminaria saccharina to hydrodynamic forcing in the North Sea. Aquaculture 250:674–691
Cahill PL, Hurd CL, Lokman M (2010) Keeping the water clean—seaweed biofiltration outperforms traditional bacterial biofilms in recirculating aquaculture. Aquaculture 306:153–159
Chopin T (2014) Seaweeds: top mariculture crop, ecosystem service provider. Global Aquaculture Advocate 17:54–56
Corey P, Kim JK, Duston J, Garbary DJ, Prithiviraj B (2013) Bioremediation potential of Palmaria palmata and Chondrus crispus (Basin Head): effect of nitrate and ammonium ratio as nitrogen source on nutrient removal. J Appl Phycol 25:1349–1358
D’Elia CF, DeBoer JA (1978) Nutritional studies of two red algae. II. Kinetics of ammonium and nitrate uptake. J Phycol 14:266–272
Demetropoulos CL, Langdon CJ (2004) Enhanced production of Pacific dulse (Palmaria mollis) for co-culture with abalone in a land-based system: nitrogen, phosphorus, and trace metal nutrition. Aquaculture 235:433–455
Dosdat A, Servais F, Métailler R, Huelvan C, Desbruyères E (1996) Comparison of nitrogenous losses in five teleost fish species. Aquaculture 141:107–127
FAO (2014) The state of world fisheries and aquaculture 2014. Food and Agriculture Organization of the United Nations. Fisheries and Aquaculture Dept, Rome
Fredriksen S, Rueness J (1989) Culture Studies of Gelidium latifolium (Grev.) Born et Thur. (Rhodophyta) from Norway. Growth and nitrogen storage in response to varying photon flux density, temperature and nitrogen availability. Bot Mar 32:539–546
Galland-Irmouli AV, Fleurence J, Lamghari R, Lucon M, Rouxel C, Barbaroux O, Bronowicki J-P, Villaume C, Guéant J-L (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Goddard S (1996) Feed management in intensive aquaculture. Chapman & Hall, New York, p 194pp
Hagopian DS, Riley JG (1998) A closer look at the bacteriology of nitrification. Aquacult Eng 18:223–244
Haines KC, Wheeler PA (1978) Ammonium and nitrate uptake by the marine macrophytes Hypnea musciformis (Rhodophyta) and Macrocystis pyrifera (Phaeophyta). J Phycol 14:319–324
Handy RD, Poxton MG (1993) Nitrogen pollution in mariculture: toxicity and excretion of nitrogenous compounds by marine fish. Rev Fish Biol Fisher 3:205–241
Hanisak MD (1979) Nitrogen limitation of Codium fragile spp. tomentosoldes as determined by tissue analysis. Mar Biol 50:333–337
Hanisak MD, Harlin MM (1978) Uptake of inorganic nitrogen by Codium fragile subsp. tomentosoides (Chlorophyta). J Phycol 14:450–454
Hernández I, Martínez-Aragón JF, Tovar A, Pérez-Lloréns JL, Vergara JJ (2002) Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste waters 2. Ammonium. J Appl Phycol 14:375–384
Howell BR, Baynes SM (2004) Abiotic factor. In: Moksness E, Kjørsvik E, Olsen Y (eds) Culture of cold-water marine fish., pp 7–27
Kang JH, Kim S, Joon-Baek L, Chung IK, Park SR (2014) Nitrogen biofiltration capacities and photosynthetic activity of Pyropia yezoensis Ueda (Bangiales, Rhodophyta): groundwork to validate its potential in integrated multi-trophic aquaculture (IMTA). J Appl Phycol 26:947–955
Kim JK, Kraemer GP, Neefus CD, Chung IK, Yarish C (2007) Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J Appl Phycol 19:431–440
Kim JK, Duston J, Corey P, Garary DJ (2013) Marine finfish effluent bioremediation: Effects on stocking density and temperature on nitrogen removal capacity of Chondrus crispus and Palmaria palmata (Rhodophyta). Aquaculture 414–415:210–216
Le Gall L, Pien S, Rusig AM (2004) Cultivation of Palmaria palmata (Palmariales, Rhodophyta) from isolated spores in semi- controlled conditions. Aquaculture 229:181–191
Lourenço SO, Barbarino E, De-Paula JC, Da S, Pereira LO, Lanfer Marquez UM (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res 50:233–241
Martinez B, Rico JM (2004) Inorganic nitrogen and phosphorous uptake kinetics in Palmaria palmata and Chondrus crispus (Rhodophyta). J Phycol 40:642–650
Martinez-Aragon JF, Hernandez I, Perez-Llorens JL, Vazquez R, Vergara JJ (2002) Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicenntrarchus labrax) waste waters: 1. Phosphate. J Appl Phycol 14:365–374
Morgan KC, Simpson FJ (1981) Cultivation of Palmaria (Rhodymenia) palmata: effect of high concentrations of nitrate and ammonium on growth and nitrogen uptake. Aquat Bot 11:167–171
Morgan KC, Shacklock PF, Simpson FJ (1980) Some aspects of the culture of Palmaria palmata in greenhouse conditions. Bot Mar 23:765–770
Msuya FE, Neori A (2008) Effect of water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J Appl Phycol 20:1021–1031
Nelson GS, Glenn EP, Conn J, Moore D, Walsh T, Akutagawa M (2001) Cultivation of Gracilaria parvispora (Rhodophyta) in shrimp-farm effluent ditches and floating cages in Hawaii: a two-phase polyculture system. Aquaculture 192:239–248
Neori A, Nobre AM (2012) Relationship between trophic level and economics in aquaculture. Aquac Econ Manage 16:40–67
Neori A, Cohen I, Gordin H (1991) Ulva lactuca biofilters for marine fishpond effluents. II. Growth rate, yield and C:N ratio. Bot Mar 34:483–489
Neori A, Krom MD, Ellner SP, Boyd CE, Popper D, Rabinovitch R, Davison PJ, Dvir O, Zuber D, Ucko M, Angel D, Gordin H (1996) Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 141:183–199
Neori A, Shpigel M, Ben-Ezra D (2000) A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 186:279–291
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Niell FX (1976) C:N ratio in some marine macrophytes and its possible ecological significance. Bot Mar 19:347–350
Pang S, Lüning K (2004) Tank cultivation of the red alga Palmaria palmata: effects of intermittent light on growth rate, yield and growth kinetics. J Appl Phycol 16:93–99
Pang S, Lüning K (2006) Tank cultivation of the red alga Palmaria palmata: year-round induction of tetrasporangia, tetraspore release in darkness and mass cultivation of vegetative thalli. Aquaculture 252:20–30
Person-Le Ruyet J, Chartois H, Quéméner L (1995) Comparative acute ammonia toxicity in marine fish and plasma ammonia response. Aquaculture 136:1–194
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and Collections of Algae. Proc. U.S.–Japan Conf.. Hakone. Sept. 1966. Jpn. Soc. Plant Phycol., pp 63–75
Sagert S, Schubert H (2000) Acclimation of Palmaria palmata (Rhodophyta) to light intensity: comparison between artificial and natural light field. J Phycol 36:1119–1128
Sánchez de Pedro R, Niell FX, Carmona R (2013) Differential nutrient uptake by two segregated red algae in an estuarine intertidal zone. Phycologia 52:461–471
Schuenhoff A, Shpigel M, Lupatsch I, Ashkenazi A, Msuya FE, Neori A (2003) A semirecirculating, integrated system for the culture of fish and seaweed. Aquaculture 221:167–181
Sutherland JE, Lindstrom SC, Nelson WA, Brodie J, Lynch MDJ, Hwang MS, Choi H-G, Miyata M, Kikuchi N, Oliveira MC, Farr T, Neefus C, Mols-Mortensen A, Milstein D, Müller KM (2011) A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). J Phycol 47:1131–1151
Syrett PJ (1962) Nitrogen assimilation. In: Lewin RA (ed) Physiology and biochemistry of algae. Academic Press, New York, pp 171–188
Thomas TE, Harrison PJ (1985) Effects of nitrogen supply on nitrogen uptake, accumulation and assimilation in Porphyra perforata (Rhodophyta). Mar Biol 85:269–278
Thomas TE, Harrison PJ (1987) Rapid ammonium uptake and nitrogen interactions in five intertidal seaweeds grown under field conditions. J Exp Mar Biol Ecol 107:1–8
Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yarish C (2003) Integrated mariculture: asking the right questions. Aquaculture 226:69–90
Acknowledgments
The German Federal Office for Agriculture and Food (Bundesanstalt für Landwirtschaft und Ernährung, BLE) funded this study, which is a part of the project Offshore Site Selection (OSS) (313-06.01-28-1-73.010-10). Many thanks go to Sabine Strieben, Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research (AWI), for her dedicated help and to Prof. Dr. C. Wiencke and the working group “Seaweed Biology,” AWI, for scientific and logistic assistance, especially Claudia Daniel and Andreas Wagner for technical support. I like to thank the two reviewers for improving the manuscript by critical comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grote, B. Bioremediation of aquaculture wastewater: evaluating the prospects of the red alga Palmaria palmata (Rhodophyta) for nitrogen uptake. J Appl Phycol 28, 3075–3082 (2016). https://doi.org/10.1007/s10811-016-0848-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0848-x