Abstract
Copper (Cu) resistance and accumulation of five populations of the red seaweed Gracilariopsis longissima collected from sites in south west England (Fal Estuary, Helford Estuary and Chesil Fleet) that differ in their degree of Cu contamination was assessed under controlled laboratory conditions, on two separate occasions (April and October). The effects of a range of Cu concentrations (0–250 μg l−1) on relative growth rates was the same for all populations with reductions observable at concentrations as low as 12 μg l−1 and cessation of growth at 250 μg l−1. There was no significant difference in the calculated EC50 values for the April and October samples, with means of 31.1 and 25.8 μg l−1, respectively. Over the range of concentrations used in this study, copper content increased linearly and the pattern of accumulation was the same for all populations at both time periods. From the linear regressions of the pooled data a concentration factor of 2.25 × 103 was calculated. These results imply that G. longissima has an innate tolerance to Cu and that populations have not evolved copper-tolerant ecotypes. In laboratory studies, accumulated Cu was released when transferred to ‘clean’ seawater with approximately 80% being lost after 8 days, with no significant difference between populations in their response. The results from a 30 days in situ transplantation experiment using two populations from the Fal Estuary provided further evidence for dynamic changes in Cu content in response to changes in Cu bioavailability. The findings in this study are discussed in the context of implications for seaweed biomonitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of soils, sediments and water by metals derived from human activities is a continuing problem in many parts of the world. Various metals, such as cadmium (Cd), mercury (Hg), lead (Pb), zinc (Zn) and copper (Cu), have been shown to elicit detrimental ecological effects. Whilst toxic to living organisms above certain threshold concentrations, some metals are essential micronutrients at lower concentrations. Copper is required for several important metabolic and physiological processes but at elevated concentrations it is considered one of the most toxic metals to photosynthetic organisms (Gledhill et al. 1997; Yruela 2005). The persistence of an environmental stress, such as metal pollution, can lead to directional selection of traits that benefit survival of individuals in the contaminated environment. In various species of angiosperm, natural populations have evolved resistance to metal pollution following exposure to elevated concentrations in their environment (Ernst et al. 1992; Macnair et al. 2000), while in others, including several aquatic species such as Typha latifolia and Phragmites australis, constitutive resistance, requiring no prior exposure to metals, has been observed (e.g. McNaughton et al. 1974; Ye et al. 2003; Deng et al. 2006). Populations of these species from metal-contaminated sites are no more resistant than their counterparts from non-contaminated sites, and they have the same internal distribution of metals. For marine macroalgae (seaweeds) such information is generally lacking as much of the research effort has been on inter-specific comparisons of responses to metal exposure and their accumulation potential for purposes of biomonitoring (e.g. Brown et al. 1998; Sawidis et al. 2001; Villares et al. 2005; Han et al. 2008). It is unclear whether distribution of these important primary producers relates to an ability to evolve metal resistance when growing in metal-contaminated waters and if species have the capacity to develop ecotypes expressing different degrees of resistance (Klerks and Weis 1987; Brown and Depledge 1998). From the very limited available literature there is evidence for both intra-specific variation in the degree of metal resistance (e.g. Russell and Morris 1970; Reed and Moffat 1983; Anderson et al. 1990; Nielsen et al. 2003), and constitutive resistance (e.g. Edwards 1972; Correa et al. 1996; Contreras et al. 2005). Clearly, further study is required to better elucidate the responses of populations of seaweeds growing at locations that differ in their levels of metal contamination, and to clarify mechanisms of resistance in these important marine primary producers. The results from such investigations would provide valuable insights into the evolution of resistance in seaweeds and would allow for a more rigorous evaluation of their suitability as biomonitors of metal pollution, as the implications of inter-population variation in metal resistance and accumulation are rarely considered.
The aims of this study were three fold. Resistance to Cu and its accumulation were assessed in five different populations of the red seaweed Gracilariopsis longissima (S. G. Gmelin) Steentoft, L. M. Irvine and Franham that were collected from sites across south west England subjected to different degrees of Cu contamination. Previously, we had shown that growth was the most sensitive physiological response in G. longissima to sub-lethal copper concentrations (Brown and Newman 2003), with reductions in relative growth rate (RGR) after 7 days exposure to environmentally relevant concentrations of Cu. Therefore, measurements of RGR and accumulation of Cu were taken following exposure to a range of Cu concentrations under laboratory conditions. Secondly, the ability to release Cu following transfer to seawater with no added metals was determined in the laboratory, as this is another important characteristic of a biomonitor. Finally, a reciprocal transplantation experiment was undertaken to ascertain the patterns of Cu accumulation and depuration in native and transplanted material in situ that would provide valuable information as to the potential of G longissima for biomonitoring.
Materials and methods
Collection of material and culture methods
Individual thalli of G. longissima were collected, from just below the low tide mark within 1 h of a spring low tide, on two separate occasions (April and October), from five submerged populations at sites in south west England: three from within the Fal Estuary (Mylor [50°17′84′′N; 05°05′46′′W], St. Just [50°18′30′′N; 5°01′71′′W] and Flushing [50°16′79′′N; 5°07′64′′W]), one from the adjacent Helford Estuary (50°09′88′′N; 5°13′63′′W) and one from Chesil Fleet (50°61′84′′N; 2°53′14′′W). The Fal estuary is heavily contaminated with metals such as Cu, Cd, Zn and arsenic (As) due to a long history of mining activities in the surrounding catchment area (Bryan and Langston 1992). The major input is from the Carnon River that flows via Restronguet Creek into the estuary system. Algae collected from the different locations within the Fal Estuary are subjected to a range of Cu concentrations in their natural environment (as high as 32–63.5 μg l−1 CuTotal; Restronguet > Mylor > St. Just > Flushing). Copper concentrations from the Helford Estuary and Chesil Fleet sites are at natural background levels of less than 1 μg l−1 CuTotal (Langston et al. 2003a, b).
On return to the laboratory, algae were washed in running filtered (0.45 μm cellulose nitrate membrane) seawater (salinity 33; pH 7.8–8.0; DOC ~100 μM) collected from Plymouth Sound and all visible epiphytes were carefully removed. For each population and sampling period, five replicate samples each comprising 10 individual apical tips (10 mm long) were prepared to determine their Cu contents (see below). The remainder of the material from each population was maintained in separate transparent, acid washed plastic tanks containing 10 l of aerated filtered seawater supplemented with 100 μM NaNO3 and 10 μM Na2HPO4·12H2O. These stock cultures were held at a constant temperature of 15°C and a photon irradiance of 30–40 μmol m−2 s−1 (supplied by Phillips cool white fluorescent tubes and measured by a Quantinet PAR meter) on a 12:12 light, dark cycle until required for experimentation, which was within 7 days of collection.
Laboratory experiments
Experiments were carried out in controlled environment chambers (Sanyo MLR-350/HT) at 15°C, 90% relative humidity, an irradiance of 40 μmol m−2 s−1 and a 12:12 light dark cycle following the procedures described in Brown and Newman (2003). From each population 10 mm, actively growing, apical tips were excised from a large number (>50) of individual thalli, at least 12 h before experimentation to ensure recovery from any trauma caused by cutting. Ten tips were haphazardly assigned to each of five replicate dishes per treatment. The duration of experiments was 7 days with material grown in acid washed polycarbonate Petri dishes containing 50 ml of culture media (nutrient supplemented filtered seawater) to which was added one of six nominal Cu (as CuSO4·7H2O) concentrations: control (no added Cu), 12.5, 25, 50, 100 and 250 μg l−1 Cu. The pH of the culture media was monitored daily (Denver Instruments benchtop probe) and was found to vary between 7.9 and 8.2. Growth in length was determined by a Quantinet Q570 image analysis system (see Brown and Newman 2003) and RGRs calculated according to the equation:
where L i and L f are the initial and final lengths (mm) of apical tips, respectively and d is the length of exposure in days.
At the termination of the experiments adhering water was removed from all material which was then frozen at −20°C and freeze–dried (Super Modulyo freeze drier, Girovac, North Walsham, UK) for 24 h. Approximately 0.1 g samples of the freeze–dried material were digested in 2 ml conc. HNO3 for 35 min in a microwave (CEM-2000; CEM Microwave Technology, Birmingham, UK), and then made up to 5 ml volume with Nano-pure distilled water. Changes in the concentration of Cu accumulated over the course of the experiments were determined by subtracting the initial Cu concentrations of the material collected from the field and these data plotted and analysed statistically.
In a separate experiment lasting 15 days, loss of Cu from G. longissima was investigated using material collected in April from the St. Just and Helford populations. There were two phases to the experiment, an ‘accumulation’ phase lasting 7 days during which apical tips (24 replicates with 10 tips per replicate) of both populations were exposed to either 0 or 100 μg l−1 Cu, and a subsequent ‘recovery’ phase of 8 days in seawater with no added Cu. Six replicate apical tips were harvested at the end of the ‘accumulation’ phase and on days 1, 5 and 8 of the ‘recovery’ phase and their Cu content determined. Two milliliter samples of media were taken prior to the start of the experiment, and from three replicate dishes of each treatment at the end of the accumulation phase, at the start and on days 1, 5 and 8 of the recovery phase to determine the total dissolved Cu concentrations. Samples from three replicate dishes with media containing 0 or 100 μg l−1 but without apical tips (blanks) were sampled after 7 days, providing information on any changes in total dissolved Cu concentrations not related to accumulation/loss by the seaweeds. Water samples collected during the accumulation/recovery experiment were acidified with 8 ml of 1 M HCl in preparation for Cu analysis. Digests, water samples and standards were analysed for total Cu by ICP-OES using a Varian 725-ES (Mulgrave, Australia). The instrument was calibrated using Cu standards prepared by serial dilution (2.0, 1.0, 0.5, 0.25 mg l−1 Cu) of plasma emission standards in 0.3 M HNO3 (Merck, Lutterworth, UK). Measured concentrations were within 10% of standards (1.9, 0.84, 5.03, 0.23 mg l−1 Cu, respectively). Operating protocols for the instrument are provided elsewhere (Maskarola et al. 2008).
In situ reciprocal transplantation experiment
Fifteen individual thalli of G. longissima were collected in March from Mylor and St. Just. After cleaning, five thalli of each population were stored frozen for Cu analysis. Each of the remaining 10 thalli was split (at the holdfast) into three ramets of approximately equal size and their fresh biomass weighed following removal of adhering water using a paper towel. One ramet per individual was attached to one of three nylon ropes (2 m long, 4 mm thick) by inserting between the strands of the rope, as described by Critchley (1993). On the following day the attached seaweeds were transported, in a darkened container with seawater, to the Fal Estuary where one rope was deployed at each of Mylor, St. Just and Restronguet Creek (N. B. there is no permanent population of G. longissima at this latter site). Ropes were anchored at both ends with concrete blocks, below the low tide mark within the zone of the native population (at Mylor and St. Just), and were retrieved after 30 days. The individual ramets were re-weighed and analysed for Cu, as described above. Water samples were collected from each of the three sites at the start and end of the transplantation experiment and analysed for total dissolved Cu.
Statistical analyses
Data were analysed (GLM ANOVA, t-tests, regression) using the statistical software package MINITAB 15. Tests for normality and homogeneity of variance (Sokal and Rolfe 1995) were carried out prior to parametric analyses and natural log transformations of the data performed if required. Differences between individual means were determined by post hoc Tukey’s test; significance was established at P < 0.05 for this procedure. NOEC (highest concentration with no significant difference from controls), LOEC (lowest concentration with a significant difference from control), EC50 (and EC20) values (effective concentration at which 50 and 20% inhibition occurs) were determined from the toxicity experiment using the linear interpolation method (Toxical 5.0, Tidepool Science, California, USA). The coefficient of variation (CV) was calculated to estimate the precision of the tests. For the transplantation experiment matched pair analyses was used for comparisons between ramets from the same individual.
Results
Laboratory experiments
Effects on growth (RGRs)
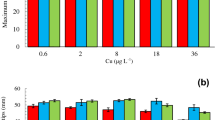
Copper affected the RGR of seaweeds collected from all five populations at both sampling times (Fig. 1a, b), with significant main effects (Population F4,240 = 10.57, P < 0.001; Cu treatment F5,240 = 3401.92, P < 0.001; Time F1,240 = 1630.57, P < 0.001), 2-way (Population × Cu treatment F20,240 = 10.13, P < 0.001; Time × Cu treatment F5,240 = 184.03, P < 0.001; except for Time × Population F4,240 = 1.29, P = 0.273) and 3-way interactions (Population × Cu treatment × Time F20,240 = 3.33, P < 0.001). The RGRs of seaweeds collected in October were significantly lower than those from April at all concentrations less than 100 μg l−1, including controls, but not at 100 and 250 μg l−1. For seaweeds collected in October, there were no significant differences between populations in their responses to individual Cu concentrations (Fig. 1a), whereas in April some differences between populations were apparent (Fig. 1b). Specifically, the significantly higher growth rates of St. Just and Flushing controls and of the St. Just and Mylor populations exposed to 25 μg l−1 were responsible for differences in the April sampling period. At both sampling times there was a general trend in all populations for significantly decreasing RGRs in a concentration dependent way; in October to a concentration of 50 μg l−1 and in April to 100 μg l−1. At the highest concentration tested (250 μg l−1) the growth of all populations from both sampling periods was reduced by approximately 90%, to almost zero. Since the behaviour of all populations to Cu exposure within a sampling period was the same or very similar, the data were pooled and a generalised dose–response curve was produced for each sampling period from which toxicological parameters were obtained (Table 1). The calculated NOEC and LOEC for both sampling times were <12 and 12 μg l−1, respectively and the EC50 (and EC20) values for April (31.1, 12.6 μg l−1) and October (25.8, 11.3 μg l−1) did not differ significantly. The CV for the data collected in April was 7.05 and 6.69% for EC50 and EC20, respectively and for data collected in October it was 4.46 and 6.55%, respectively.
Accumulation of Cu
Analysis of samples collected from field sites revealed significant difference in Cu concentration between populations and sampling periods (P < 0.001). Specifically, material sampled from Mylor Creek in both April (mean of 22.6 μg g−1 Cu) and October (mean of 18 μg g−1 Cu) had significantly higher concentrations of Cu than samples from all other populations which did not differ significantly from one another (mean of 5 μg g−1 Cu). The Cu concentrations of samples collected in April from sites within the Fal Estuary were significantly higher (P < 0.05) than their counterparts in October, reflecting greater bioavailability of the metal.
Analysis of changes in Cu concentrations in apical tips following 7 days exposure to Cu under controlled laboratory conditions indicated a significant increase with increasing external Cu concentration (F5,240 = 1946.65, P < 0.001), with no evidence for tissue saturation over the concentration range used in this study (Fig. 2). Further analysis revealed a lack of significance between populations and between sampling times (Population F4, 240 = 0.71, P = 0.583; Time F1,240 = 1.06, P = 0.305; Population × Cu treatment F20,240 = 0.25, P = 1.000; Time × Cu treatment F5,240 = 0.14, P = 0.982; Time × Population F4,240 = 1.22, P = 0.302; Population × Cu treatment × Time F20,240 = 0.47, P = 0.975). Therefore, the general relationship between Cu accumulated by G. longissima and the external Cu concentration was determined by pooling the data and computing a linear model defined by the equation:
where K D is the slope of the linear fit, forced through the origin, and is equivalent to the concentration factor. The result is a highly significant relationship (F1,297 = 49104.65, P < 0.001) with the formula:
Changes in Cu content (μg g−1 dw) of Gracilariopsis longissima following 7 days of exposure to a range of external Cu concentrations. Data obtained from the five populations sampled in April and October have been pooled. A linear regression line with the 95% prediction interval has been fitted to the data (n = 300)
Release of Cu
The pattern of growth reduction at 86.4 μg l−1 (measured in media on day zero of the accumulation phase; nominally 100 μg l−1) was similar to that recorded in the previous experiment. There were no significant difference in the response of the two populations to Cu; the mean RGR (0.8% day−1) of apical tips exposed to Cu was about 80% less than the controls (4.1% day−1). After 8 days recovery in the absence of external Cu there was no significant difference (P > 0.05) in the RGRs between controls and Cu treatment; the mean RGR for both populations was 3.8% day−1.
After 7 days of exposure the mean concentration of Cu accumulated by G. longissima was 215 μg g−1, with no significant (P > 0.05) difference between populations. Over the course of the recovery phase the Cu concentration in apical tips of both populations decreased significantly (P < 0.01) with time (Fig. 3). After 1 day, the accumulated Cu decreased by 20% and by day eight 82% had been lost.
The concentration of dissolved Cu in blanks ((86.4 ± 3.2 μg l−1) did not change with time whereas in the presence of apical tips the Cu concentration during the accumulation phase decreased significantly (P < 0.01) by c. 60% to 50.11 ± 2.8 μg l−1 on day seven. During the recovery phase, the decrease in accumulated Cu by the seaweeds (Fig. 3a) was accompanied by a significant increase in Cu in the media (Fig. 3b); the concentration of Cu increased from c. 0.24 μg l−1 at the start of the recovery period to 5.76 μg l−1 on day eight.
In situ reciprocal transplantation experiment
The concentrations of total dissolved Cu at the start of the transplantation experiment were: 13.27 μg l−1 for Restronguet Creek, 6.64 μg l−1 for Mylor and 1.65 μg l−1 for St. Just and at the end of the 30 days experimental period they were 18.45, 8.5 and 1.82 μg l−1, respectively. The RGRs (estimated from wet biomass) of individual ramets from the Mylor and St. Just populations were quite variable but no significant differences (P < 0.05) were found between the transplants and controls; the mean RGR was 0.70 ± 0.23% day−1. There was also no significant difference (P > 0.05) between the Mylor and St. Just ramets transplanted to Restronguet Creek, although the mean RGR (0.08 ± 0.04% day−1) at this site was significantly lower (P < 0.001) than at the other two sites. There was no evidence of ramets being grazed, and herbivores were not observed on ropes, which could have influenced the results.
The Cu concentration of ramets from Mylor returned to Mylor (control transplants) increased significantly (P = 0.019) from an initial mean of 25.5 to 41.8 μg g−1, whereas St. Just controls did not change significantly (P > 0.05; from 8.2 to 12.9 μg g−1) after 30 days (Fig. 4). Transplants from Mylor to St. Just had a mean Cu concentration of 31.7 μg g−1 which is significantly lower (P = 0.025) than the corresponding Mylor controls. The reciprocal transplants from St. Just to Mylor had a mean of 24.7 μg g−1 which is significantly higher (P = 0.002) than the St. Just controls. The ramets of both Mylor and St. Just individuals transplanted to Restronguet Creek accumulated the same (P > 0.05) mean concentrations of Cu (165 and 121 μg g−1, respectively), which were significantly higher (P ≤ 0.002) than all other transplanted material (Fig. 4).
Copper content (μg g−1 dw) of ramets of Gracilariopsis longissima transplanted from Mylor and St. Just to three sites (Mylor, St. Just and Restronguet Creek) in the Fal estuary for 30 days. Contents at the start of the transplantation experiment are also shown. Error bars represent the standard deviation of the mean (n = 5 or 10)
Discussion
Despite differences in the history of metal contamination at the field sites from which seaweeds were collected, the five populations of G. longissima included in this study all responded in the same way to Cu exposure, under laboratory conditions. A decrease in growth rate, compared with that of controls, was first observed at the lowest concentration tested (12.5 μg l−1), with reductions in a concentration dependent manner, thereafter. These results are consistent with those obtained in our previous study on the G. longissima population from St. Just (Brown and Newman 2003). Furthermore, the consistency of the calculated EC50 (and EC20) values over the two sampling periods indicates a general lack of seasonal variation in response to Cu. However, it is interesting to note that 50 μg l−1 was more toxic to material collected in October than April, which could be related to seasonal changes in the intracellular fraction of the total concentration of Cu accumulated and/or activity of the detoxification processes (Vasconcelos and Leal 2001; Pinto et al. 2003). To identify possible reasons, the mechanism(s) of resistance in G. longissima need to be elucidated, which are likely to involve a combination of exclusion and tolerance of the metal, as found in several other seaweed species (e.g. Gledhill et al. 1999; Garcia-Ríos et al. 2007; Pawlik-Skowrońska et al. 2007). The results for Cu accumulation also failed to identify any significant inter-population differences or seasonal effects, over the concentration range used. The relationship between Cu content and exposure concentration was linear, with a generalised concentration factor of 2.25 × 103, which falls within the typical range for seaweeds of 103–105 that varies widely between and within species (e.g. Seeliger and Edwards 1979; Malea et al. 1994).
The lack of any distinct differences in the responses of the five populations of G. longissima is highly significant as it provides good evidence for innate resistance to Cu and no support for the evolution of Cu-resistant ecotypes within this species. Our results are consistent with some earlier studies including those on Callithamnion hookeri (Edwards 1972) and Ulva compressa (Correa et al. 1996). More recent evidence for constitutive resistance comes from a study with the brown seaweed Scytosiphon lomentaria that involved reciprocal transplantation of seaweeds between sites with different histories of metal pollution (Contreras et al. 2005). High levels of plasticity in antioxidant responses to Cu were found, with transplants assuming the characteristics of native populations within a period of 96 h. Contrary to our results, there is evidence of inter-population differences in metal resistance in other species, with degree of resistance related to exposure history, most notably in the brown seaweeds Ectocarpus spp. (e.g. Russell and Morris 1970; Hall 1981) and Fucus spp. (e.g. Bryan and Gibbs 1983; Nielsen et al. 2003), and also the green seaweed U. compressa, although there are conflicting results for this latter species (e.g. Reed and Moffat 1983; Correa et al. 1996). To better understand the evolution of metal-resistance and associated mechanisms of resistance, in the phylogenetically distinct groups of seaweeds there should be a greater focus on identifying inter-population responses in a wider range of species. Additionally, such information would allow for a more rigorous appraisal of the utility of seaweeds as biomonitors of metal pollution, as currently those advocating their use rarely take account of possible inter-population variation in metal resistance and accumulation.
The literature is replete with studies stating that seaweed species are quantitative biomonitors of metal contamination in near-shore waters (e.g. Bryan and Hummerstone 1973; Serfor-Armah et al. 2001; Boubonari et al. 2008). The justification provided to support such claims is that seaweeds conform to the essential requirements of the ‘ideal’ biomonitor, namely: they are sedentary, abundant, long lived, available for sampling throughout the year, sufficiently large to provide material for analysis and are net accumulators of the metal in question (Rainbow 1995). However, one characteristic not given due consideration, but stated in a review by Phillips (1977) to be absolutely essential if spurious conclusions are to be avoided is, ‘that all organisms in a survey exhibit the same correlation between their metal contents and those in the surrounding water, at all locations studied, under all conditions.’ (italics from original text; Phillips 1977, pg 290). This key determinant would not be met where species from a single sampling site have different concentration factors, reflecting differences in accumulation potential due, at least in part, to allometric characteristics (e.g. Langston and Spence 1995; Stengel et al. 2004). Nor would it be met if there is intra-specific variation in metal resistance that modifies physiological functions and alters the pattern of metal accumulation, as is the case in Ulva spp. and Fucus spp., both well regarded biomonitors. In species of both genera there is evidence for increased resistance in natural populations growing in metal contaminated sites, the mechanism for which is, in part, due to metal exclusion (Reed and Moffat 1983; Nielsen et al. 2003). The suitability of such species as quantitative biomonitors of metal pollution is, therefore, questionable.
Another important characteristic of a biomonitor is its ability to release metals, as well as accumulate them, particularly when dissolved concentrations fluctuate over time (Lobban and Harrison 1994). Results from the field and laboratory experiments show that apical tips of G. longissima responded rapidly to changes in dissolved metal Cu concentrations. Within 30 days the growth rates of tips transplanted from St. Just and Mylor to the more contaminated Restronguet Creek decreased significantly and Cu content increased, compared with controls. Under laboratory conditions, on the transfer of material from a toxic concentration of Cu to ‘clean’ seawater, growth rates recovered to those of controls within 8 days. Accompanying the increase in growth was a decline in accumulated Cu and a concomitant increase in the media. Growing tips can contribute to this apparent loss of Cu due to dilution effects. However, according to our calculations, if we assume no loss of Cu from apical tips during this period, and that tip length relates to biomass, as previously shown (Brown and Newman 2003), dilution due to growth would account for only a 30% reduction in accumulated Cu. Therefore, the observed 82% loss of accumulated Cu cannot be explained by dilution alone. The rate of Cu depuration was non-linear, initially rapid and slowing thereafter, a pattern matched by an increase in the media, that would suggest that much of the Cu released can be accounted for by its presence in the media. While we did not discriminate between extracellular and intracellular bound Cu, the pattern of release would be explained by an initial loss of Cu weakly bound to cell wall and extracellular polysaccharides followed by a slower release from the intra-cellular pools of Cu comprising of free ions, inorganically bound Cu or Cu bound to organic ligands (Gledhill et al. 1999; Vasconcelos and Leal 2001; Andrade et al. 2010).
Although the metal content of seaweeds has been analysed extensively in laboratory investigations and ‘passive’ biomonitoring studies, data on accumulation and depuration in situ are less common. Transplantation experiments can be used to provide such information (Phillips 1994); the term ‘active’ biomonitoring (ABM) has been coined for this approach since it involves the intentional introduction of well-defined and responsive organisms into field sites for a known period of time (Chaphekar 1991). ABM offers several significant advantages over that of passive biomonitoring including: the period of time that individuals are exposed for is defined and can be manipulated depending on the objectives of the study, comparisons can be made between native and introduced individuals, which might indicate possible differences in resistance, monitoring stations can be selected independent of the natural (non)occurrence of the species being used, and resolution of statistic tests can be optimised by using similar groups of organisms (Lovett Doust et al. 1994; Smolders et al. 2003). In the limited number of studies employing this method, some have examined only the accumulation of metals in seaweeds transplanted from unpolluted to polluted sites (e.g. Ho 1984), but where release of metals has also been investigated, the extent of both accumulation and depuration was shown to be dependent on the metal, length of study, season and age of tissue sampled (e.g. Myklestad et al. 1978; Eide et al. 1980). An even more informative procedure is to incorporate into the design reciprocal transplantation of individuals as each population’s response at the different sites can be evaluated (e.g. Eide et al. 1980; Amado Filho et al. 1999; Contreras et al. 2005). For example, in a recent study by Hédouin et al. (2008) on the brown seaweed Lobophora variegata, a period of 30 days was sufficient to achieve equivalence between transplants and the resident population. By the end of the experiment (71 days) concentrations in transplants were significantly higher than those in the controls, an observation which could suggest that the native population had developed a mechanism that regulates accumulation/release of metals through genetic or physiological adaptation (Klerks and Weiss 1987). In contrast to these findings the concentrations of metals in reciprocal transplants to an unpolluted site remained unchanged for 103 days, results which didn’t reflect the fast depuration rates observed in the laboratory (Metian et al. 2008). Unlike these findings, the results presented for G. longissima provide good evidence for dynamic changes in the Cu content of simultaneously transplanted individuals. Transplants to more contaminated sites (i.e. St. Just to Mylor and St. Just and Mylor to Restronguet) rapidly accumulated between two and nine times more Cu and reciprocal transplants (Mylor to St. Just) had approximately 25% less Cu than their corresponding controls. However, in neither of the reciprocal transplants did Cu concentrations attain those of native controls, although there was trend towards this. Further studies are required to establish the most appropriate length of time to achieve this, but a period longer than 30 days would seem to be necessary. An added benefit of using G. longissima is its capacity to reproduce vegetatively, which we exploited to use ramets of individuals as replicates. By splitting an individual into ramets it was possible to effectively transplant the same individual (genet) into the different sites, which permitted match-pair analyses and thereby enhanced statistical resolution. Furthermore, from using this approach we were able to observe variation in the accumulation/depuration of Cu and growth rates of individuals within a site. If inter-individual responses are an important source of variation within a population, this can be exploited to enhance our understanding of the evolution of metal resistance in this and other seaweed species (Bennett 1987). As there was no replication of individuals within each site, it is not possible to test this hypothesis. However, inter-individual variation is known to be an important source of variability in the related gracilaroid species Gracilara chilensis (Santelices and Varela 1995).
In conclusion, the results of this study provide evidence for constitutive Cu resistance in the red seaweed G. longissma. The innate resistance and an ability to accumulate and release Cu, lends support for its potential use as a biomonitor, notwithstanding the need to assess its responses to a wider range of metals. More specifically, the in situ reciprocal transplantation study, that exploited the species’ capacity for vegetative reproductive and used ramets as replicates in its design, highlights the relevance and usefulness of G. longissima for active biomonitoring. Given the limitations and problems associated with passive biomonitoring, and lack of rigour in many studies advocating the use of particular species of seaweeds, we recommend that species with constitutive resistance be identified and prioritised for further detailed investigation and their potential as active biomonitors evaluated, using a combination of laboratory and field-based studies.
References
Amado Filho GM, Andrade LR, Karez CS, Farina M, Pfeiffer WC (1999) Brown algae species as biomonitors of Zn and Cd at Sepetiba Bay, Rio de Janeiro, Brazil. Mar Environ Res 48:213–224
Anderson BS, Hunt JW, Turpen SL, Coulon AR, Martin M (1990) Copper toxicity to microscopic stages of giant kelp Macrocystis pyrifera: interpopulation comparisons and temporal variability. Mar Ecol Prog Ser 68:147–156
Andrade S, Pulido MJ, Correa JA (2010) The effects of organic ligands exuded by intertidal seaweeds on copper complexation. Chemosphere 78:397–401
Bennett AF (1987) Inter-individual variability: an under-utilized resource. In: Feder MM, Bennett AF, Burggren WW, Huey RB (eds) New directions in ecological physiology. Cambridge University Press, Cambridge, pp 147–166
Boubonari T, Malea P, Kevrekidis T (2008) The green seaweed Ulva rigida as a bioindicator of metals (Zn, Cu, Pb and Cd) in a low-salinity coastal environment. Bot Mar 51:472–484
Brown MT, Depledge MH (1998) Determinants of trace metal concentrations in marine organisms. In: Bebianno MJ, Langston WJ (eds) Metabolism of trace metals in aquatic organisms. Chapman & Hall, London, pp 185–217
Brown MT, Newman JE (2003) Physiological responses of Gracilariopsis longissima (S. G. Gmelin) Steentoft, L. M. Irvine and Farnham (Rhosophyceae) to sublethal copper concentrations. Aquat Toxicol 64:201–213
Brown MT, Hodgkinson WM, Hurd CL (1998) Spatial and temporal variations in the copper and zinc concentrations of two green seaweeds from Otago Harbour, New Zealand. Mar Environ Res 47:1–10
Bryan GW, Gibbs PE (1983) Heavy metals in the Fal Estuary, Cornwall: a study of long-term contamination by mining waste and its effects on estuarine organisms. Mar Biol Assoc Occas Publ 2, Plymouth, 112 pp.
Bryan GW, Hummerstone LG (1973) Brown seaweed as an indicator of heavy metals in estuaries in south-west England. J Mar Biol Assoc UK 53:705–720
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76:89–131
Chaphekar SB (1991) An overview on bioindicators. J Environ Biol 12:163–168
Contreras L, Moenne A, Correa JA (2005) Antioxidant responses in Scytosiphon lomentaria (Phaeophyceae) inhabiting copper-enriched coastal environments. J Phycol 41:1184–1195
Correa JA, Gonzalez P, Sanchez P, Munoz J, Orellana MC (1996) Copper–algae interactions: inheritance or adaptation? Environ Monit Assess 40:41–54
Critchley AT (1993) Gracilaria (Rhodophyta: Gracilariales): an economically important agarophyte. In: Ohno M, Critchley AT (eds) Seaweed cultivation and marine ranching. Japan International Cooperation Agency, Yokosuka, pp 89–112
Deng H, Ye ZH, Wong MH (2006) Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ Pollut 141:69–80
Edwards P (1972) Cultured red alga to measure pollution. Mar Pollut Bull 3:184–187
Eide I, Myklestad S, Melson S (1980) Long-term uptake and release of heavy metals by Ascophyllum nodosum (L.) Le Jol. (Phaeophyceae) in situ. Environ Pollut 23:19–28
Ernst WHO, Verkleij JAC, Schat H (1992) Metal tolerance in plants. Acta Bot Neerland 41:229–248
Garcia-Ríos V, Freile-Pelegrín Y, Robledo D, Mendoza-Cózatl D, Moreno-Sánchez R, Gold-Bouchot G (2007) Cell wall composition affects Cd2+ accumulation and intracellular thiol peptides in marine red algae. Aquat Toxicol 81:65–72
Gledhill M, Nimmo M, Hill SJ, Brown MT (1997) The toxicity of copper (II) species to marine algae, with particular reference to macroalgae. J Phycol 33:2–11
Gledhill M, Nimmo M, Hill SJ, Brown MT (1999) The release of copper-complexing ligands by the brown alga Fucus vesiculosus (Phaeophyceae) in response to increasing total copper levels. J Phycol 35:501–509
Hall A (1981) Copper accumulation in copper-tolerant and non-tolerant populations of the marine fouling alga, Ectocarpus siliculosus (Dillw.) Lyngbye. Bot Mar 24:223–228
Han T, Kang S-H, Park J-S, Lee H-K, Brown MT (2008) Physiological responses of Ulva pertusa and U. armoricana to copper exposure. Aquat Toxicol 86:176–184
Hédouin L, Bustamante P, Fichez R, Warnau M (2008) The tropical brown alga Lobophora variegata as a bioindicator of mining contamination in the New Caledonia lagoon: a field transplantation experiment. Mar Environ Res 66:438–444
Ho YB (1984) Zn and Cu concentrations in Ascophyllum nodosum and Fucus vesiculosus (Phaeophyta, Fucales) after transplantation to an estuary contaminated with mine waste. Conserv Recycl 7:329–337
Klerks PL, Weis JS (1987) Genetic adaptation to heavy metals in aquatic organisms: a review. Environ Pollut 45:173–205
Langston WJ, Spence SK (1995) Biological factors involved in metal concentrations observed in aquatic organisms. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. John Wiley & Sons Ltd., New York, pp 407–478
Langston WJ, Chesman BS, But GR, Hawkins SJ, Readman J, Worsfold P (2003a) Site characteristics of the south west marine sites: Fal and Helford cSAC. Mar Biol Assoc Occas Publ 8, Plymouth, pp 160
Langston WJ, Chesman BS, But GR, Hawkins SJ, Readman J, Worsfold P (2003b) Site characteristics of the south west marine sites: Chesil and the Fleet c SAC, SPA. Mar Biol Assoc Occas Publ 11, Plymouth, pp 154
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, New York, p 366
Lovett Doust J, Schmidt M, Lovett Doust L (1994) Biological assessment of aquatic pollution: a review, with emphasis on plants as biomonitors. Biol Rev 69:147–186
Macnair MR, Tilstone GH, Smith SE (2000) The genetics of metal tolerance and accumulation in higher plants. In: Terry N, Baňuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press, Boca Raton, pp 235–250
Malea P, Haritonidis S, Stratis I (1994) Bioaccumulation of metals by Rhodophyta species at Antikyra Gulf (Greece) near an aluminium factory. Bot Mar 37:505–513
Maskarola K, Turner A, Brown MT (2008) Influence of synthetic surfactants on the uptake of Pd, Cd, and Pb by the marine alga Ulva lactuca. Environ Pollut 156:897–904
McNaughton SJ, Folsom TC, Lee T, Park F, Price C, Roeder D, Schmitz J, Stockwell C (1974) Heavy metal tolerance in Typha latifolia without the evolution of tolerant races. Ecology 55:1163–1165
Metian M, Giron E, Borne V, Hédouin L, Teyssié J-L, Warnau M (2008) The brown alga Lobophora variegata, a bioindicator species for surveying metal contamination in tropical marine environments. J Exp Mar Biol Ecol 362:49–54
Myklestad S, Eide I, Melsom S (1978) Exchange of heavy metals in Ascophyllum nodosum (L.) Le Jol. in situ by means of transplanting experiments. Environ Pollut 16:277–284
Nielsen H, Brownlee C, Coelho SM, Brown MT (2003) Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. New Phytol 160:157–165
Pawlik-Skowrońska B, Pirszel J, Brown MT (2007) Concentrations of phytochelatins and glutatjione found in natural assemblages of seaweeds depend on species and metals concentrations of the habitat. Aquat Toxicol 83:190–199
Phillips DJH (1977) The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments—a review. Environ Pollut 13:281–317
Phillips DJH (1994) Macrophytes as biomonitors of trace metals. In: Kramer KJM (ed) Biomonitoring of coastal waters and estuaries. CRC Press, Boca Raton, pp 85–102
Pinto E, Sigaud-Kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Rainbow PS (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192
Reed RH, Moffat L (1983) Copper toxicity and copper tolerance in Enteromorpha compressa (L.) Grev. J Exp Mar Biol Ecol 69:85–103
Russell G, Morris O (1970) Copper tolerance in the marine fouling alga Ectocarpus siliculosus. Nature 228:288–289
Santelices B, Varela D (1995) Regenerative capacity of Gracilaria fragments: effect of size, reproductive state and position along the axis. J Appl Phycol 7:501–506
Sawidis T, Brown MT, Zachariadis G, Sratis I (2001) Trace metal concentrations in marine macroalgae from different biotopes in the Aegean Sea. Environ Int 27:43–47
Seeliger U, Edwards P (1979) Fate of biologically accumulated copper in growing and decomposing thalli of two benthic red marine algae. J Mar Biol Assoc UK 59:227–238
Serfor-Armah Y, Nyarko BJB, Osae EK, Carboo D, Anim-Sampong S, Seku F (2001) Rhodophyta seaweed species as bioindicators for monitoring toxic element pollutants in the marine ecosystem of Ghana. Water Air Soil Pollut 127:243–253
Smolders R, Berveots L, Wepener V, Blust R (2003) A conceptual framework for using mussels as biomonitors in whole effluent toxicity. Hum Ecol Risk Assess 9:741–760
Sokal RR, Rolfe FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W. H. Freeman, New York, p 887
Stengel DB, Macken A, Morrison L, Morley N (2004) Zinc concentrations in marine macroalgae and a lichen from western Ireland in relation to phylogenetic grouping, habitat and morphology. Mar Pollut Bull 48:902–909
Vasconcelos MTSD, Leal MFC (2001) Seasonal variability in the kinetics of Cu, Pb, Cd and Hg accumulation by macroalgae. Mar Chem 74:65–85
Villares R, Carral E, Puente X, Carballeira A (2005) Metal levels in estuarine macrophytes: differences among species. Estuaries 28:948–956
Ye ZH, Baker AJM, Wong MH, Willis AJ (2003) Copper tolerance, uptake and accumulation by Phragmites australis. Chemosphere 50:795–800
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Acknowledgment
We gratefully acknowledge financial support to JEN from Astra Zeneca (Brixham Environment Laboratory).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, M.T., Newman, J.E. & Han, T. Inter-population comparisons of copper resistance and accumulation in the red seaweed, Gracilariopsis longissima . Ecotoxicology 21, 591–600 (2012). https://doi.org/10.1007/s10646-011-0819-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0819-6