Abstract
Seaweed farming has been identified as one of the entry point projects (EPPs) in Malaysia since the government introduced the Economic Transformation Programme, which aims to increase seaweed production to 150,000 t annually by 2020. To achieve this goal, micropropagation and subsequent acclimatization of the micropropagated seaweeds to the open sea is one of the available options to solve the seedling shortage problem. Acclimatization is an important process in which micropropagated seaweeds adjust to gradual changes in environments such as temperature, humidity, photoperiod, and pH. Success acclimatization is an important key for the seaweed tissue culture industry to move forward, and therefore, the protocol of acclimatization of micropropagated Kappaphycus alvarezii has been extensively optimized in this study. Direct planting out of the micropropagated seaweeds to the open sea without going through the nursery acclimatization phase may cause shock to the seaweeds due to sudden changes in environmental conditions. In a 2-week acclimatization study, seedlings were found to achieve optimum growth when cultivated in seawater enriched with mixed-algae fertilizer, natural seaweed extract (NSE), under a regimen of daily medium change and culture density of 0.40 g L−1. The acclimatized K. alvarezii has achieved 83.33 ± 5.77 % of survival in the seaweed farm with normal physiology and no epiphyte coverage. This study has provided useful information for seaweed cultivators to enhance the survival rate of micropropagated K. alvarezii through nursery acclimatization prior to serve as seedlings for commercial seaweed cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The seaweed processing industry has an annual demand of 7.5–8.0 million t of biomass with most of this derived from farms (Reddy et al. 2008). Since the introduction of seaweed farming to Malaysia in 1978, this industry has gained significance as an economic driver, especially in Sabah. This is primarily due to the important roles of carrageenan in various industries (Sade et al. 2006; Hayashi et al. 2010). The seaweed species which are widely cultivated for carrageenan production in Malaysia are Kappaphycus alvarezii, Kappaphycus striatum, and Eucheuma denticulatum (McHugh 2003; Phang 2006; Sade et al. 2006).
The Malaysian government has introduced the Economic Transformation Programme and identified seaweed farming as one of the 131 entry point projects (EPPs) under the National Key Economic Areas (NKEAs), which has targeted an increase in the seaweed production to 150,000 t by 2020 (ETP Handbook 2012). As a result of this program, the seaweed industry is expected to generate 12,700 job opportunities and contribute up to RM 1.4 billion (~US$0.43 billion) to the gross national income by 2020. However, this target seems difficult to achieve with current cultivation practices. The current problems faced by local cultivators are unpredictable environmental changes, attacks by predators, epiphytes, and diseases, where most of the cultures will be lost, and lack of healthy seaweed seedlings for cultivation (Mendoza et al. 2002; Hurtado et al. 2006; Vairappan 2006; Vairappan et al. 2008; Hayashi et al. 2010).
Micropropagation and subsequent acclimatization of the micropropagated seaweeds to the open sea is an option to provide healthier and epiphyte-free seedlings, in order to overcome these problems while achieving the national goal of commercial cultivation (Bixler and Porse 2011). Previous studies have revealed that the application of commercial extracts from brown seaweeds (Ascophyllum nodosum) in cultivation of K. alvarezii has resulted in higher growth rate and reduced epiphyte load on cultures (Loureiro et al. 2010; Borlongan et al. 2011). Although, the studies of micropropagating Eucheuma and Kappaphycus were started in the early 1990s, followed by optimization of culture media, plant growth regulators, and even culture conditions, but the studies of acclimatization are still limited (Dawes and Koch 1991; Baweja et al. 2009; Yong et al. 2011, 2014).
Direct planting out of the micropropagated seaweeds to the open sea without going through the nursery acclimatization phase may cause shock and stress to the seaweeds due to sudden changes in environmental conditions which will reduce their resistance to diseases and epiphytes. In order to obtain the higher growth rate and survival rate, a nursery system is recommended to provide a buffer condition to the seaweed culture before their adaption to the open sea environment. Acclimatization is necessary for micropropagated plants to adapt for in vivo or ex vivo conditions according to Dunstan and Turner (1984). In this study, acclimatization protocol for micropropagated K. alvarezii was established through application of fertilizers (brown seaweed extracts). The best fertilizer among the tested fertilizers was determined and applied in the subsequent acclimatization studies viz. frequency of medium change and culture density. Controlled parameters during the nursery acclimatization, such as nutrients, salinity, and temperature, should be closer to the natural conditions to provide a transition period for seaweeds to adapt to the open sea environment.
Materials and methods
Preparation of micropropagated K. alvarezii for acclimatization
Kappaphycus alvarezii micropropagules were cultivated according to Yong et al. (2014) under the optimized culture conditions. Micropropagated seedling samples in the range of 20 ± 5 g were selected to conduct acclimatization study in an outdoor nursery during the rainy season. In each cycle, the K. alvarezii was cultured in a cubic-shaped white fiberglass water tank with the capacity of 220 L each for 14 days, with continuous aeration throughout the experimental period. Seawater was filtered with filter cloth and pumped into the culture tank prior to use for experimental seaweed cultivation. The salinity and water temperature of the seawater were in the range of 30 to 35 ppt and 25 to 40 °C, respectively. The weights of K. alvarezii were recorded weekly for daily growth rate (DGR) determination using the formula DGR = {[(Wt / W0) ^ (1 / t)] − 1} × 100 % as recommended by Yong et al. (2013). The determined daily growth rates were then further analyzed for significant differences by one-way ANOVA using SPSS software version 16 (SPSS Inc.).
Application of fertilizers (brown seaweed extracts)
A total of three fertilizers were selected based on their source of seaweed extract and availability in Malaysian market, namely Acadian Marine Plant Extract powder (AMPEP), Gofar600 (GF), and natural seaweed extract (NSE). AMPEP is extracted from A. nodosum (Hurtado et al. 2009), whereas GF and NSE are the mixture extracts of several brown seaweeds including A. nodosum, Sargassum, and Laminaria with different ratios of concentration according to the manufacturer (Gofar Agro Specialties). Four treatments were tested: (a) filtered seawater only (control), (b) filtered seawater enriched with 3 mg L−1 AMPEP, (c) filtered seawater enriched with 3 mg L−1 GF, and (d) filtered seawater enriched with 3 mg L−1 NSE. The concentration of the fertilizer was determined in the beginning of the experiments and during the renewal of medium. For each treatment, four replicates (n = 4) were tested, and the medium was renewed once every 3 days. The determined fertilizer was then applied for the next parameter optimization study.
Frequency of medium change
Four treatments were tested with filtered seawater enriched with NSE (selected based on previous experiment): (a) medium change daily, (b) medium change once every 3 days, (c) medium change once every 5 days, and (d) medium change once every 7 days. The determined frequency of medium change was then applied for the culture density test.
Culture density test
The experiment was conducted with filtered seawater enriched with NSE, together with daily medium change, which were selected based on the previous experiment. Four treatments were tested: (a) 0.40, (b) 0.55, (c) 0.70, and (d) 0.85 g seedlings L−1. The culture density was determined at the beginning of the test, and the ratio of seedlings to the medium was adjusted through the quantity of seedlings.
Field trials
With all three parameters applied, a batch of ten acclimatized and ten non-acclimatized (as control) K. alvarezii seedlings, weighing 45 ± 5 g each, were transplanted to seaweed farms located at Semporna, Sabah. Both acclimatized and non-acclimatized K. alvarezii seedlings were grown in the seaweed farm using the traditionally practiced long-line method, together with the farm-propagated K. alvarezii. After 4 weeks of farming, cultivated seaweeds were harvested and observed. The experiment was carried out in triplicate. The survival rate and average growth rate of the K. alvarezii were determined in order to validate the success of nursery acclimatization. Data obtained were subjected to further analysis of significant differences by one-way ANOVA using SPSS software version 16 (SPSS Inc.).
Results and discussion
Effects of fertilizer treatment
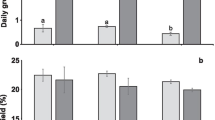
In the 2-week acclimatization study to select the best fertilizer among AMPEP, GF, and NSE, the result showed statistically significant differences in their daily growth rate (F (3,12) = 18.386, p < 0.05). The highest daily growth rate was achieved by treating the seaweed cultures with NSE (5.94 ± 0.21 % day−1), followed by GF, AMPEP, and control (5.19 ± 0.39, 4.55 ± 0.17, and 4.32 ± 0.48 % day−1, respectively) as shown in Fig. 1. Morphologically, there were no obvious differences observed between the K. alvarezii seedlings obtained from each treatment. Thus, NSE was selected for subsequent acclimatization studies.
Among the tested fertilizers, only AMPEP has been reported to be widely used in experimental cottonii cultivation (Hurtado et al. 2009; Hayashi et al. 2010), whereas no literature report was available on the application of GF and NSE in seaweed cultivation. These fertilizers were selected based on their contents, which were composed of brown seaweed extracts. Thus, the fertilizers are believed to contain more essential nutrients as compared with artificial nutrients (Baweja et al. 2009). As reported by Hurtado et al. (2009), extract of A. nodosum consists of macronutrients and micronutrients which are needed to improve the growth of K. alvarezii. In this study, the application of NSE in culture media showed better growth performance.
Previous studies on the extract of A. nodosum reported a positive influence on the growth and health of K. alvarezii (Hurtado et al. 2009; Loureiro et al. 2010, 2014; Borlongan et al. 2011). The use of A. nodosum extracts was first reported by Hurtado et al. (2009) with its application in tissue culture of Kappaphycus varieties. The study by Hurtado et al. (2009) showed that addition of A. nodosum extract into the culture medium promoted shoot formation of Kappaphycus varieties in shorter period. Besides, Kappaphycus cultures also showed higher growth rate and lower populations of epiphytes after soaking in the extract of A. nodosum solution (Loureiro et al. 2010; Borlongan et al. 2011). Furthermore, the extract of A. nodosum was also found to be efficient in promoting growth of K. alvarezii while reducing the cover of Neosiphonia sp. (Borlongan et al. 2011). The brown seaweed extract is also a potential elicitor of the natural defenses of K. alvarezii against pathogens as well as in ameliorating the negative impacts of long-term exposure to oxidative busts (Loureiro et al. 2012).
In the current study, K. alvarezii appeared to have better growth when cultivated in mixed-algae extracts namely GF and NSE. Generally, a greater variety of algae extracts will supply a wider range of nutrients to the crops. There are about 400 species of Sargassum found throughout all oceans, and Sargassum was first described nearly 200 years ago by Agardh in 1820 (Liu et al. 2012). About 200 bioactive compounds, such as meroterpenoids, phlorotannins, fucoidans, sterols, and glycolipids, have been identified from this genus. Its wide range of pharmacological properties suggests that Sargassum is a rich source of health maintaining and promoting agents (Liu et al. 2012). The uses of Sargassum are not limited to human consumption only, but as plant growth promoter as well (Williams and Feagin 2010; Kumari et al. 2011, 2013). Alginate from the Sargassum sinicola has been shown to promote the growth of the microalga Chlorella sorokiniana (Yabur et al. 2007).
Laminaria has also a long history as a soil conditioner in sandy soils with low organic matter content (Haslam and Hopkins 1996). The role of seaweed as soil conditioners is associated with biological mineralization of the seaweed and the interaction between soil particles and organic compounds derived either directly or indirectly from the seaweed (Stephenson 1968). Thorsen et al. (2010) also revealed the advantages of the use of Laminaria in agriculture as a nutrient provider, seed germination promoter, and rooting agent. Besides, Laminaria is rich in bioactive compounds which have been found to be pharmaceutically important as antimicrobial, antitumor, antioxidant, antiviral, anticoagulant, and other activities which might be useful in treating K. alvarezii to overcome infections (Wang et al. 2010; Peng et al. 2012; Saha et al. 2012; Kim et al. 2013).
The application of seaweed extract as fertilizer is cheaper and environmental friendly. Considering the wide range of nutrients and growth regulators supplied in the seaweed extracts, they can be fully applied and replace the artificial media which are chemically produced (Hurtado et al. 2009). When more types of seaweed extracts are mixed together in a fertilizer, more varieties of functioning elements are available to promote faster and healthier growth. Besides, problems such as eutrophication and harmful alga blooms, which are usually caused by the artificial fertilizer, may be avoided or eliminated by using the seaweed extracts as fertilizer.
Frequency of medium change
K. alvarezii cultivated with daily medium change achieved the highest growth rate (6.98 ± 0.12 %) and was significantly different from the other three treatments (F (3,12) = 8.440, p < 0.05) (Fig. 2). Morphologically, there were no obvious differences or abnormalities found on the seedlings in all treatments. Thus, for the next experiment, the addition of NSE with daily medium change was used to determine the optimum culture density.
The frequency of medium change is often related to the availability of nutrients in the medium and the period of the nutrients to be used up. As in the acclimatization stage, manual addition of nutrient supplements should be minimized to prepare the seaweed explants to adapt to the natural environment. Therefore, only fertilizer was added, instead of complete culture medium as used in micropropagation. The availability of nutrients can only be maintained by changing the medium, and the frequency of change decides the growth performance of the seaweed explants. Besides, daily medium change also maintains the salinity of the medium in an outdoor nursery to overcome desalination due to salt precipitation. However, the frequency of medium change has always been used as a fixed variable, rather than a manipulated variable in other published seaweed cultivation studies.
Acclimatization of reproductive cells of K. striatum prior to transfer to the field was carried out in a concrete tank with flow-through water system to maintain the nutrient concentration in the culture medium as reported by Luhan and Sollesta (2010). High frequencies of medium change also prepare the seaweed explants to adapt to the natural sea condition. However, cost of operation is another factor that needs to be considered in the nursery set up for commercial cultivation.
Culture density
In the 2-week acclimatization study, K. alvarezii explants showed the highest growth rate (7.14 ± 0.30 % day−1) at a culture density of 0.40 g L−1which was significantly different (F (3,24) = 50.227, p < 0.05) from the other density treatments (Fig. 3). However, there was no morphological difference observed or sign of disease in all treatments.
Similar to the study of medium change frequency, the study of culture density is closely related to the availability of nutrient content in the medium. As in higher density of cultivation, the nutrients might not be enough for all the explants in a given period, and the growth of the explants may be affected. Besides, light penetration may be reduced due to self-shading of the explants (Manríquez-Hernández 2013). With the decrease of nutrient availability and lower light penetration, the photosynthesis rate of the seaweeds is likely to decrease, and finally, high culture density may actually reduce the productivity of the cultivation (Bidwell et al. 1985).
Higher cultivation density also may reduce the water flow in the medium, and therefore, the seaweeds might suffer from nutrient depletion. According to Glenn and Doty (1992), water motion affects seaweed growth rates by decreasing the thickness of the unstirred layer of water around the thallus, thereby enhancing the diffusion rate of materials into and out of the thallus. As the culture density increases, the weaker is the diffusion for the entry and exit of materials into the center of the thallus, and the greater might be the water motion requirement, which will then lead to higher cost of operation in commercial cultivation.
Field trials
In the field, the average daily growth rate of the surviving acclimatized K. alvarezii was significantly higher (F (1,4) = 12.108, p < 0.05) than non-acclimatized K. alvarezii (3.91 ± 0.16 and 3.56 ± 0.07 % day−1, respectively). These results were greater than the result reported by Yassir (2012), who recorded 3.39 ± 0.18 % day−1 for farm-propagated K. alvarezii (Fig. 4). The survival rate of the acclimatized K. alvarezii was 83.33 ± 5.77 %, which was significantly higher (F (1,4) = 25.000, p < 0.05) than that of the non-acclimatized K. alvarezii (50.00 ± 10.00 %). We infer that the unrecovered acclimatized K. alvarezii seedling was either untied from the culture line due to strong waves or consumed by predators such as sea turtle, whereas unrecovered non-acclimatized K. alvarezii was believed to be affected by epiphytes or “ice-ice” disease. Furthermore, there were no epiphytes observed on the acclimatized K. alvarezii, whereas the non-acclimatized and farm-propagated K. alvarezii were covered with Neosiphonia sp. (Fig. 5).
Photos of a acclimatized, b non-acclimatized, and c farm-propagated K. alvarezii after 4 weeks of cultivation in a commercial seaweed farm in Semporna, Sabah. The epiphyte coverage on the thallus of the farm-propagated K. alvarezii was obviously higher than that of the non-acclimatized K. alvarezii. Besides, neither epiphytes nor abnormality was found in acclimatized K. alvarezii
According to Borlongan et al. (2011) and Loureiro et al. (2012), epiphyte infections of K. alvarezii could be reduced by exposing the explants to the extracts of A. nodosum, both in the laboratory or seaweed farm. Addition of extract of A. nodosum to K. alvarezii in vitro was found able to reduce stress, while promoting growth (Loureiro et al. 2014). In a commercial farm, the epiphytes are never fully removed. According to Vairappan et al. (2008), about 90 % of commercial farms in the major seaweed production countries are suffering from epiphyte infections, and there is still no effective solution to fully remove the epiphytes from these farms.
Epiphyte cover on the non-acclimatized seaweeds is believed to be due to stress caused by sudden change of environment and weakening of the resistance to the epiphytes. According to Vairappan (2006), epiphyte outbreaks and “ice-ice” disease are more common when the seaweeds were in stress conditions, especially during the dry and hot seasons. After being infected by the Neosiphonia sp., the productivity of the farms was decreased for about 20 %, and the carrageenan quality dropped as well (Vairappan et al. 2008). By applying the optimized culture conditions in an outdoor nursery, the production of acclimatized K. alvarezii was found to be more promising and attractive as compared with the commonly farmed seaweeds.
In conclusion, the acclimatization of micropropagated K. alvarezii using filtered seawater with the addition of NSE, daily medium change, and a culture density of 0.40 g L−1 in the outdoor nursery was found to promote faster and healthier growth of K. alvarezii. With reference to the farming result, the acclimatization was successful in ensuring the survival and epiphyte resistance of the micropropagated K. alvarezii. These findings are important for acclimatization of micropropagated seaweeds in the nursery prior to their use as seedlings in the seaweed farming industry.
References
Baweja P, Sahoo D, Garcia-Jiménez P, Robaina RR (2009) Seaweed tissue culture as applied to biotechnology: problems, achievements and prospects. Phycol Res 57:45–58
Bidwell RGS, McLachlan J, Lloyd NDH (1985) Tank cultivation of Irish moss, Chondruscrispus Stackh. Bot Mar 28:87–97
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Borlongan IAG, Tibubos KR, Yunque DAT, Hurtado AQ, Critchley AT (2011) Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J Appl Phycol 23:615–621
Dawes CJ, Koch EW (1991) Branch, micropropagule and tissue culture of the red algae Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J Appl Phycol 3:247–257
Dunstan DI, Turner KE (1984) The acclimatization of micropropagated plants. In: Vasil I (ed.) Laboratory procedures and their applications. Elsevier Science, p 123–129
Glenn EP, Doty MS (1992) Water motion affects the growth rates of Kappaphycus alvarezii and related red seaweeds. Aquaculture 108:233–246
ETP Handbook (2012) Chapter 15: transitioning from agriculture to agribusiness. In: Economic Transformation Programme: a road map for Malaysia. Performance Management and Delivery Unit (Pemandu), Malaysia, p 513–550
Haslam SFI, Hopkins DW (1996) Physical and biological effects of kelp (seaweed) added to soil. Appl Soil Ecol 3:257–261
Hayashi L, Hurtado AQ, Msuya FE, Bleicher-Lhonneur G, Critchley AT (2010) A review of Kappaphycus farming: prospects and constraints. Cell Origin Life Extrem 15:251–283
Hurtado AQ, Critchley AT, Trespoey A, Bleicher-Lhonneur G (2006) Occurrence of Polysiphonia epiphytes in Kappaphycus farms at Calaguas Is., Camarines Norte, Philippines. J Appl Phycol 18:301–306
Hurtado AQ, Yunque DA, Tibubos K, Critchley AT (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Kim YH, Kim JH, Jin HJ, Lee SY (2013) Antimicrobial activity of ethanol extracts of Laminaria japonica against oral microorganisms. Anaerobe 21:34–38
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633
Kumari R, Kaur I, Bhatnagar AK (2013) Enhancing soil health and productivity of Lycopersicon esculentum Mill. using Sargassum johnstonii Setchell & Gardner as a soil conditioner and fertilizer. J Appl Phycol 25:1225–1235
Liu L, Heinrich M, Myers S, Dworjanyn SA (2012) Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol 142:591–619
Loureiro RR, Reis RP, Critchley AT (2010) In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J Appl Phycol 22:101–104
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2012) Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): a “vaccine-like” effect on Kappaphycus alvarezii (Doty) Doty ex. P.C. Silva. J Appl Phycol 24:427–432
Loureiro RR, Reis RP, Marroig RG (2014) Effect of the commercial extract of the brown alga Ascophyllum nodosum Mont. on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva in situ submitted to lethal temperatures. J Appl Phycol 26:629–634
Luhan MRJ, Sollesta H (2010) Growing the reproductive cells (carpospores) of the seaweed, K. striatum, in the laboratory until outplanting in the field and maturation to tetrasporophyte. J Appl Phycol 22:579–585
Manríquez-Hernández JA (2013) Interaction of irradiance and stocking density on nutrient uptake by red macroalgae. Implications for bioremediation of fish farm effluents. M. Sc. Thesis. Dalhousie University
McHugh DJ (2003). A guide to the seaweed industry. FAO Fisheries Technical Paper 441
Mendoza WG, Montano NE, Ganzon-Fortes ET, Villanueva RD (2002) Chemical and gelling profile of ice-ice infected carrageenan from Kappaphycus striatum (Schmitz) Doty “Sacol” strain (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 14:409–418
Peng Z, Liu M, Fang Z, Zhang Q (2012) In vitro antioxidant effects and cytotoxicity of polysaccharides extracted from Laminaria japonica. Int J Biol Macromol 50:1254–1259
Phang SM (2006) Seaweed resources in Malaysia: current status and future prospects. Aquat Ecosyst Heal Manag 9:185–202
Reddy CRK, Jha B, Fujita Y, Ohno M (2008) Seaweed micropropagation techniques and their potentials: an overview. J Appl Phycol 20:609–617
Sade A, Ali I, Mohd Ariff MR (2006) The seaweed industry in Sabah, East Malaysia. J SE Asian Stud 11:97–107
Saha S, Navid MH, Bandyopadhyay SS, Schnitzler P, Ray B (2012) Sulfated polysaccharides from Laminaria angustata: structural features and in vitro antiviral activities. Carbohydr Polym 87:123–130
Stephenson WA (1968) Seaweed in agriculture and horticulture. Faber and Faber, London
Thorsen MK, Woodward S, McKenzie BM (2010) Kelp (Laminaria digitata) increases germination and affects rooting and plant vigour in crops and native plants from an arable grassland in the Outer Hebrides, Scotland. J Coast Conserv 14:239–247
Vairappan CS (2006) Seasonal occurrences of epiphytic algae on the commercially cultivated red alga Kappaphycus alvarezii (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 18:611–617
Vairappan CS, Chung CS, Hurtado AQ, Soya FE, Bleicher-Lhonneur G, Critchley A (2008) Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J Appl Phycol 20:477–483
Wang J, Zhang Q, Zhang Z, Song H, Li P (2010) Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol 46:6–12
Williams A, Feagin R (2010) Sargassum as a natural solution to enhance dune plant growth. Environ Manag 46:738–747
Yabur R, Bashan Y, Hernandez-Carmona G (2007) Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J Appl Phycol 19:43–53
Yassir SM (2012) Algae farming via mini estate system in Sabah. In Proc. of Bio-Borneo 2012. 24th–26th February 2012. Kuching, Sarawak
Yong WTL, Ting SH, Chin WL, Rodrigues KF, Anton A (2011) In vitro micropropagation of Eucheuma seaweeds. Int Proc Chem Biol Environ Eng 7:58–60
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Yong WTL, Ting SH, Yong YS, Thien VY, Wong SH, Chin WL, Rodrigues KF, Anton A (2014) Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol. doi:10.1007/s10811-013-0191-4
Acknowledgments
The authors wish to thank the PEMANDU, Malaysia, for funding the research under the Seaweed Research Grant of Project EPP3 (GPRL/SPS/2), and Gofar Agro Specialties for their sponsorship in the form of enrichment fertilizers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, Y.S., Yong, W.T.L., Thien, V.Y. et al. Acclimatization of micropropagated Kappaphycus alvarezii (Doty) Doty ex Silva (Rhodophyta, Solieriaceae) in outdoor nursery system. J Appl Phycol 27, 413–419 (2015). https://doi.org/10.1007/s10811-014-0289-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0289-3