Abstract
The increasing demands for seaweeds have promoted the designation of the seaweed industry as the third Entry Point Project (EPP) under the National Key Economic Area (NKEA) initiative of the Economic Transformation Programme (ETP) of the Malaysian government. The farming of carrageenophytes has emerged as a successful enterprise and provides a promising alternative livelihood option for low-income coastal communities in several countries. However, over time, the productivity of the red seaweed crop has declined in some regions due to sourcing of seedlings from single, selected genetic stocks considered to have initially higher yield potential but which resulted in strain fatigue or loss of vigour. To circumvent the crop productivity issues arising from clonal propagation, the raising of planting materials from the development and successful micro-propagation of Kappaphycus has been initiated in order to support the sustainability of selected, farmed carrageenophytes. Three species of Kappaphycus (K. alvarezii (to include 2 strains—brown and green) K. malesianus (aring-aring) and K. striatus) were used in the present study to optimize the use of Ascophyllum Marine Plant Extract Powder (AMPEP K+) which had previously been demonstrated to be effective as a culture medium ingredient, acting as a biostimulant, when applied with the addition of terrestrial plant growth regulators (PGRs). The optimum combination of 3 mg L−1 AMPEP K+ + PGRs was used in out-planting the microplantlets to a sea-based nursery. Salinity and turbidity were found to be positively correlated with growth rates in open water. The use of the brown seaweed-derived extract acting as a biostimulant and as the main ingredient of the cost-effective culture medium for the micro-propagation for all four strains of Kappaphycus tested was highly encouraging, so much so that the treatment has the potential to be promoted as a generic protocol for the economic and commercial mass production of new plantlets (asexual seedlings) which are urgently required for Malaysian seaweed farming to meet its fullest potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Commercial production by cultivation of Kappaphycus, a genus of red seaweed predominantly cultivated in the Southeast Asian region, i.e. the Philippines, Indonesia and Malaysia and to some extent Vietnam and Cambodia (Hayashi et al. 2017), will continue to increase because of growing demands in the global market as raw materials for food ingredients (Campbell and Hotchkiss 2017) in processed food, dairy products, water gels and also cosmetics, personal care items and pharmaceuticals.
Continuous vegetative propagation of Kappaphycus and Eucheuma, since their first introduction for commercial farming in 1970 (Doty 1973; Doty and Alvarez 1981), led to loss of vigour, susceptibility to ‘ice–ice’ and epi-endophytes, and consequently significantly decreased productivity and production. Several attempts to generate new plantlets from spores (Azanza and Aliaza 1999; Azanza and Ask 2003; Luhan and Sollesta 2010, to name a few) were not pursued further, mainly due to the marked seasonality and scarcity of fertile plants bearing spores from wild populations and also the painfully slow growth of sporelings required to develop into young plants (seedlings for out-planting) under laboratory conditions (Luhan, pers. comm.)
The year-round availability and accessibility of different strains of Kappaphycus for micropropagation purposes makes it more convenient, economic and reliable to adapt rather than having to seek fertile plants as a starting point. Several earlier studies showed that micropropagation of Kappaphycus and Eucheuma (various spp.) through tissue culture (Dawes and Koch 1991; Dawes et al. 1993; Hurtado and Cheney 2003; Reddy et al. 2003; Hurtado and Biter 2007; Hayashi et al. 2008; Sulistiani et al. 2012; Yong et al. 2014; Neves et al. 2015; Luhan and Mateo 2017), using inorganic nutrient media, could generate great numbers of potential plantlets for out-planting successfully. More recently, the use of a commercial seaweed extract, manufactured from the open atmosphere, alkali hydrolysis of the temperate brown seaweed Ascophyllum nodosum—so named Ascophyllum Marine Plant Extract Powder (AMPEP and AMPEP K+) when used singly, or in combination with PGR (IAA + Kinetin) (Hurtado et al. 2009; Yunque et al. 2011), and/or colchicine or oryzalin (Tibubos et al. 2017; Ali et al. 2018), led to the mass generation of healthy microplantlets which was highly encouraging and should be researched further.
Personal communications of the senior author with several farmers in Semporna, Sabah, found that Kappaphycus cultivation has stopped since 2018 to date in: Sepangkat Is., Omadal Is., Selakan Is., Manis Is. and Pantau-Pantau Is. Reasons given were mainly due to disease outbreaks which had been brought about by increased surface seawater temperatures, excessive grazing by turtles as well as fluctuating farmgate prices. Further investigations revealed that only Gelam Is., Silungun Is. and Timur Mata Is. continued to cultivate, but all at very reduced rates of 30, 20 and 10%, of their prior volumes, respectively. Disease outbreaks such as ‘ice–ice’ and epi-endophytism regularly lead to the lack of availability of quality cultivars for the next growth cycle. These cyclical events and loss of income consequently dampen the enthusiasm of seaweed entrepreneurs to continue farming. It is in this context that a management tool was developed, to produce laboratory to land, with transfer to sea-based nursery-grown healthy plantlets as potential sources of cultivars for successful commercial cultivation in the present study. However, there is a desperate need to scale-up to a level where sufficient cultivars can be generated to provide for those interested in commercial cultivation. This study was conducted as a proof of concept in order to generate a modest number of plantlets as starters for out-planting purposes in Malaysia.
Materials and methods
Kappaphycus alvarezii (tambalang brown and green), K. striatus (sacol green) and K. melasianus plants were collected from a seaweed farm in Selakan Is., Semporna Sabah (4° 34′ 35.4″ N; 118° 41′ 50.6″ E). Whilst on site, the seaweeds were cleaned of any adhering particles and organisms such as sand, shells and macro-epiphytes, packed and covered with seawater-moistened cheesecloth and transported in an ice chest box to the Seaweed Laboratory, University of Malaysia-Sabah. Following the procedures described by Hurtado and Cheney (2003), the seaweeds were acclimated in a fiberglass tank (5 × 1.5 m) for 5–7 days using UV-treated seawater provided with mild aeration. Daily changes of UV-treated seawater were made.
Laboratory conditions
Preparation of explants followed the methods already described by Ali et al. (2018). Briefly, only clean, healthy and epiphyte-free apical segments (3–5 cm long) were cut and used as explants. The apical segments of each strain/species were cleaned and sterilized using povidone iodine (1%) by brushing the surface with a soft artist’s brush, which were then rinsed three times with autoclaved seawater in order to provide clean, healthy and diatom-free explants. The apical segments were cut into 2–3-mm-thick segments, washed and rinsed three times with UV-treated seawater. Two hundred (200) segments were inoculated in a 3-L wide-mouth plastic jar for each treatment at 0.1, 0.5, 1.0, 3.0 and 5.0 L−1 concentrations of AMPEP K+ (Tibubos et al. 2017), singly and in combination with PGR (i.e. 1 mg L−1 each of IAA + kinetin) in order to induce formation of direct axis shoots. A total of 60–3 L plastic jars each were used in the present study for the Treatments using AMPEP K+ (0.1, 0.5, 1.0, 3.0 and 5.0 L−1) with and without PGR and another three jars for the UV-treated seawater acted as control. Due to limited space in the laboratory, the two sets of experiment (i.e. AMPEP K+ with and without PGR) were made at different times, i.e. after 45 days under the same laboratory conditions. All segments were induced to produce direct axes in a walk-in culture room, at 23–24 °C, 13 L:11D, with an irradiance of 30–40 μmol photons m−2 s−1. Moderate aeration was provided to each treatment. Media changes were made every 7 days.
Three batches of inoculation were made each lasting 45 days. The following measurements were recorded: (1) the overall length (mm), (2) number of direct axis shoots per section and (3) percentage of new direct axes formed after 45 days. The length of the shoot per treatment per species/per strain was measured individually after 15 days using a Dino-Lite AM2011 hand held a digital microscope for exact measurements due to their small size. However, at the end of 45 days the shoots of each explant were measured by placing a white bond paper and a plastic ruler beneath a Petri dish containing seawater and the explants with shoots. The percentage of number of explants with direct axes formed was computed as follows:
The percentage (%) of direct axes formed = (number of segments with direct shoots formed at day 45/total number of segments incubated at day 1) × 100 (after Tibubos et al. (2017).
Sea-based nursery
After 45 days of growth, under defined laboratory conditions, microplantlets (i.e. explants with > 7 mm shoots) of each Kappaphycus strain/species, bearing direct shoots (i.e. 500 microplantlets per cage) were grown in a cylindrical net cage (25 × 40 cm) which was suspended on a 2 × 2 m PVC raft for another 45 days from February–August following a modified protocol of Hurtado and Critchley (2019). The optimum combination from the treatments tested was 3 mg L−1 AMPEP K+ + PGR and was used in the sea-based nursery so as to test its efficacy on growth rate. Exactly the same segments, without any AMPEP K+ dip, served as controls for the four strains evaluated.
The cylindrical net cage was cleaned every 3–4 days by brushing the surface of the netting so as to remove any attached debris and allow for free water exchange within. Once the microplantlets grew to young thalli, these were tied to a cultivation rope (polyethylene rope, PER#6) with soft plastic ‘tie-tie’ until the individuals obtained a weight of 90–100 g. The cultivation ropes were attached to a 3 m × 3 m PVC raft and this was enclosed with a net cage (2 mm mesh) cage to prevent grazing by siganids, parrot fish, turtles and escape of the explants. This study was done in a commercial farm at Selakan Is., Semporna Sabah.
The increase in fresh biomass (g) of each strain was determined every 45 days over three separate growth cycles and daily growth rate (DGR) was calculated as the percentage increase in fresh weight day−1.
Statistical analyses
The experimental design was factorial in a completely randomized design, using two factors, i.e. species/strain and AMPEP K+ concentrations. The combined factors resulted in 52 treatments (i.e. 3 species (K. akvarezii, K. striatus and K. malesianus to include 2 strains of K alvarezii (brown and green) × 13 AMPEP K+ concentrations) for the micropropagation of the plantlets and 32 treatments (i.e. 4 strains × 4 growth cycles × 2 AMPEP K+ (and control) for the sea-based nursery. In order to assess the effects of factor combinations on variables such as: length of shoots, number of shoots formed per segment and the total percentage of shoots formed, DGR and with AMPEP K+, plus controls, a one-way ANOVA was employed. A test for homogeneity of variance was verified using Lavene’s test (p = 0.01 and 0.05).
A correlation coefficient analysis was made for the DGR data for AMPEP K+ treatments (with controls) at p = 0.01 and p = 0.05 significance levels.
Results
Length of direct axes formed
The three species used as test plants in the present study (K. alvarezii, K. malesianus and K. striatus) to include two strains of K. alvarezii (brown and green) each demonstrated individual length performances in response to AMPEP K+ concentrations (Fig. 1). The test plants showed that the longest direct shoots (within a range of 2.6–7.2 mm) were formed in the 3 mg L−1 AMPEP K+ + PGR treatment and that these were significantly different from each other (p < 0.05). The shortest direct shoots formed were measured at varying concentrations in the absence of PGRs, viz. the following AMPEP K+ treatments of: K. alvarezii brown, 0.8 mm ± 0.05 at 0.5 mg L−1; K. alvarezii green, 0.9 mm ± 0.08 at 0.01 mg L−1; K. malesianus, 0.9 mm ± 0.0 at 0.5 mg L−1; and K. striatus, 0.7 mm ± 0.0 at control). These were not significantly different from each other (p > 0.05).
Number of direct axes shoots per segment
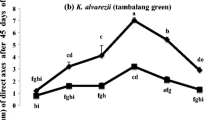
Figure 2 shows that the average, highest number of direct shoots formed per segment was observed in K. striatus (3.4 ± 0.19 shoots), K. alvarezii brown and green (2.7 ± 0.28 shoots) and K. malesianus (2.1 ± 0.06 shoots), all at 3 mg L−1 AMPEP K+ + PGR. However, the number of direct shoots formed per segment were not significantly different from each other (p > 0.05). K. striatus at 5 mg L−1 AMPEP K+ which, at the highest concentration, whilst K. alvarezii brown and green and K. malesianus under control condition produced the lowest number of direct shoots per segment, and these were significantly different from each other (p < 0.05).
Percentage of explants with direct shoots formed after 45 days
All Kappaphycus test plants used in the present study attained the highest average percentage of segments with shoots formed after 45 days following the 3 mg L−1 AMPEP K+ + PGR treatment, over differing time periods (i.e. 85.7–99.3%). These were not significantly different from each other at the level of p > 0.05 (Fig. 3). The lowest percentage of segments with direct shoots formed (i.e. 45.3–52.7%) was from those without AMPEP K+ treatment, i.e. the control and these were not significantly different at p > 0.05 level.
Daily growth rate of young plants whilst in the sea-based nursery
Figure 4 shows the daily growth rates of all Kappaphycus test plants using the optimum combination obtained under the laboratory conditions (i.e. 3 mg L−1 AMPEP K+ + PGR) with their control, over four consecutive growth cycles. All four test plants with 3 mg L−1 AMPEP K+ + PGR treatments produced the highest DGR (i.e. 5.2 ± 0.59–5.5 ± 0.56% day−1) during the fourth growth cycle (July–August) and these were significant at p < 0.05. The lowest DGR was observed in K. alvarezii brown (0.38 ± 0.58%), K. alvarezii green (0.38 ± 0.61%) and K. malesianus (0.96 ± 0.83%) in their respective controls, at the second growth cycle (April–May) whilst K. striatus had its lowest DGR (0.76 ± 0.41%) during the first growth cycle (February–April) and these were not significantly different from each other (p > 0.05).
Water quality
The ranges of seawater characteristics evaluated (pH, 7.4–8.1; salinity, (32–34.1 ppt); surface sea temperature (SST, 27.2–33.8 °C); and turbidity (24.6–25.4 NTU) were very small over the entire period February–August when all Kappaphycus test plants were grown in the sea-based nursery. Salinity and turbidity were positively correlated with the mean daily growth rates and these were significant at p < 0.001. pH was negatively correlated and significantly different at p < 0.05. Furthermore, when these water qualities were correlated with one another, pH was negatively correlated with salinity and turbidity and these were significant at p < 0.001; while SST was positively correlated with salinity and turbidity and were significant at p < 0.001 (Table 1).
Discussion
The results of the present study demonstrated the efficacy of a commercial soluble extract powder from the brown seaweed A. nodosum, which is normally applied as a land plant biostimulant, for the generation of microplantlets from three species of Kappaphycus plus two strains of K. alvarezii (brown and green) under both laboratory and grow-out conditions. It was clearly demonstrated that each species/strain responded differently to AMPEP K+, supplemented with, and without, PGRs (IAA and kinetin) as compared with their respective controls in terms of the direct shoots generated, i.e. length, number and total number of segments bearing direct shoots after 45 days. AMPEP K+ has an enhanced content of potassium, (as compared with basic AMPEP see Tibubos et al. 2017 for details), which is essential for cell/tissue growth and development.
The addition of IAA and kinetin as PGRs with AMPE K+, in the present study, was demonstrated to be more effective than AMPEP K+ alone, or AMPEP (i.e. no additional K+) alone in the micropropagation of the Kappaphycus test plants. Tibubos et al. (2017) obtained the longest direct axis shoots in K. alvarezii (9.6 mm ± 0.33) at 5 mg L−1 AMPEP K+ with PGR, which is a little lower (7.6 mm ± 0.46) than what was obtained in the present study at 3 mg L−1 AMPEP K+ with PGR. However, our present results concurred exactly with the results of Ali et al. (2018) on the longest direct shoots formed, highest number of direct shoots formed per segment and the highest percentage of direct shoots formed at 3 mg L−1 AMPEP K+ with PGR.
It is surmised that the higher plant-hormone-like biostimulant levels naturally within AMPEP were insufficient to cause significant effects in the model plant Arabidopsis (Wally et al. 2013), hence the addition of IAA and kinetin as PGRs to AMPEP K+, in the present study, triggered longer direct shoots and also higher percentage of explants with direct shoots after 45 days. Further, the same authors claimed that there are known elicitors within seaweed extracts that modulate the pathways for the phytohormone biosynthesis in land plants, this may be similar in treated red seaweeds, but the mode(s) of actions remain unknown.
Other in vitro studies of red seaweeds, notably Gracilaria caudata and Laurencia catarinensis also demonstrated the biostimulatory effects of AMPEP related to their growth rate and phycobiliprotein contents of the treated samples (Souza et al. 2019). The results of the present study on types of Kappaphycus were further supported by the recent publication of Umanzor et al. (2019) where juvenile kelp sporophytes of Saccharina latissima and S. angustissima (whilst in the nursery stage) also treated with AMPEP were found to have enhanced thermal tolerances, thus allowing the treated blades to grow larger than their control counterparts, over the same period of lower water temperatures.
It is rather difficult to compare the results obtained from tissue cultured propagated K. alvarezii as reported by Budiyanto et al. (2019) and Febriyanti et al. (2019) with the results of the present study, simply because, the nutrients used in the former studies were entirely different from the present study. These two recent studies used inorganic culture media while the present study used organic media from a seaweed extract of A. nodosum with and without PGRs supplements. During the sea-based nursery stage of the Kappaphycu test plants, salinity and turbidity were positively correlated with DGR and these were significant at the p < 0.001 level, thereby indicating a very strong relationship with the two variables.
Out-planting Kappaphycus microplantlets using cylindrical net cages in sea-based nursery conditions provided an enhanced management protocol—rather than prolonging the biomass in the land-based conditions. As such, the out-planted microplantlets grew in an ambient, or normal environment (Hurtado and Critchley 2019). In so doing, this is also a cost-efficient method, as under the out-planted conditions there was no longer a need for power to generate CO2, or simulate wave action in on-land tanks. Based on the prior experiences of the authors, the microplantlets would grow much faster so as to mature to young plant size. Housing the microplantlets or young plants in the cylindrical net cages also prevented biomass losses due to herbivores.
Results of the present study clearly showed that at 3 mg L−1 AMPEP K+ supplemented with PGR, produced the longest new shoots, obtained the highest number of direct shoots per segment, and the highest percentage of direct shoots formed under laboratory conditions.
A modified protocol for up-scaling the production of microplantlets of Kappaphycus (Hurtado and Critchley 2019) was adopted in the present study (Fig. 5). Once transferred to the farmers it would take approximately 4–5 months to generate the much needed new and improved cultivars for commercial field cultivation. The protocol developed in this study is a far superior option to improving stocks rather than importing foreign materials and complying with the necessary, existing regulations and the need for expensive quarantine procedures.
Schematic flow diagram for the production of new and improved cultivars from micropropagation technique for field cultivation (adapted from Hurtado and Critchley 2019)
References
Ali MM, Sani MZB, Hi KK, Yasir SM, Critchley AT, Hurtado AQ (2018) The comparative efficiency of a brown algal-derived biostimulant extract (AMPEP), with and without supplemented PGRs: the induction of direct, axis shoots as applied to the propagation of vegetative seedlings for the successful mass cultivation of three commercial strains of Kappaphycus in Sabah, Malaysia. J Appl Phycol 30:1913–1919
Azanza RV, Aliaza T (1999) In vitro carpospore release and germination in Kappaphycus alvarezii (Doty) Doty from Tawi-Tawi, Philippines. Bot Mar 42:281–284
Azanza RV, Ask E (2003) Kappaphycus alvarezii (Doty) Doty carposporeling growth and development in the laboratory. Int Seaweed Symp 17:95–99
Budiyanto, Kasim M, Abadi SY (2019) Growth and carrageenan content of local and tissue culture seed of Kappaphycus alvarezii cultivated in floating cage. AACL Bioflux 12:167–178
Campbell R, Hotchkiss S (2017) Carrageenan industry market overview. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on Spinosum and Cottonii of commerce. Springer, Dordrecht, pp 193–206
Dawes C, Koch E (1991) Branch, micropropagule and tissue culture of the red algae Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J Appl Phycol 3:247–257
Dawes CJ, Trono GC Jr, Lluisma AO (1993) Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippine seaweed farms. Hydrobiologia 260:379–383
Doty MS (1973) Farming the red seaweed, Eucheuma, for carrageenans. Micronesica 9:59–73
Doty MS, Alvarez VB (1981) Eucheuma farm productivity. Proc Int Seaweed Symp 8:688–691
Febriyanti F, Aslan LOM, Iba W, Patadjai AB, Nurdin AR (2019) Effect of various planting distances on growth and carrageenan yield of Kappaphycus alvarezii (Doty) using seedlings produced from mass selection combined with tissue- cultured method. IOP Conf Series: Earth Environ Sci 278:1–8
Hayashi L, Yokoya NS, Kikuchi DM, Oliveira EC (2008) Callus induction and micropropagation improved by colchicines and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol 20:653–659
Hayashi L, Reis RP, Alves dos Santos AA, Castelar B, Robledo D, de Vega GB, Msuya FE, Eswaran K, Yasir S, Ali MJ, Hurtado AQ (2017) The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on Spinosum and Cottonii of commerce. Springer, Dordrecht, pp 55–90
Hurtado AQ, Biter A (2007) Plantlet regeneration of Kappaphycus alvarezii var. adik by tissue culture. J Appl Phycol 19:783–786
Hurtado AQ, Cheney DP (2003) Propagule production of Eucheuma denticulatum (Burman) Collins et Hervey by tissue culture. Bot Mar 46:338–341
Hurtado AQ, Critchley AT (2019) Recent advances in the use of on-land nurseries for commercial production and out-planting of Kappaphycus seedlings, a carrageen-bearing seaweed. Institute of Ocean and Earth Sciences Monograph Series 17: Taxonomy of southeast Asian seaweeds III (In press)
Hurtado AQ, Yunque DA, Tibubos K, Critchley AT (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Luhan MRJ, Mateo JP (2017) Clonal production of Kappaphycus alvarezii (Doty) Doty in vitro. J Appl Phycol 29:2339–2344
Luhan MRJ, Sollesta H (2010) Growing the reproductive cells (carpospores) of the seaweed, Kappaphycus striatum, in the laboratory until out-planting in the field and maturation to tetrasporophyte. J Appl Phycol 22:579–585
Neves FAS, Simioni C, Bouzon ZL, Hayashi L (2015) Effects of spindle inhibitors and phytoregulators on the micropropagation of Kappaphycus alvarezii (Rhodophyta, Gigartinales). J Appl Phycol 27:437–445
Reddy CRK, Kumar GRK, Siddhanta AK, Tewari A (2003) In vitro somatic embryogenesis and regeneration of somatic embryos from pigmented callus of Kappaphycus alvarezii (Doty) Doty (Rhodophyta, Gigartinales). J Phycol 39:610–616
Souza JMC, Castro JZ, Critchley AT, Yokoya NS (2019) Physiological responses of the red algae Gracilaria caudata (Gracilariales) and Laurencia catarinensis (Ceramiales) following treatment with a commercial extract of the brown alga Ascophyllum nodosum (AMPEP). J Appl Phycol 31:1883–1888
Sulistiani E, Soelistyowati DT, Alimuddin YSA (2012) Callus induction and filaments regeneration from callus of cottonii seaweed Kappaphycus alvarezii (Doty) collected from Natuna Islands, Riau Islands Province. Biotropia 19:103–114
Tibubos K, Hurtado AQ, Chritchley AT (2017) Direct formation of axes in new plantlets of Kappaphycus alvarezii (Doty) Doty, as influenced by the use of AMPEP K+, spindle inhibitors and plant growth hormones. J Appl Phycol 29:2345–2349
Umanzor S, Shin S, Marty-Rivera M, Augyte S, Yarish C, Kim JK (2019) Preliminary assessment on the effects of the commercial seaweed extract, AMPEP, on growth and thermal tolerance of the kelp Saccharina spp. from the Northwest Atlantic. J Appl Phycol. https://doi.org/10.1007/s10811-019-01852-3
Wally OSD, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, Abrams SR, Prithiviraj B (2013) Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J Plant Growth Regul 32:324–339
Yong WTL, Ting SH, Yong YS, Thien VY, Wong SH, Chin WL, Rodrigues KF, Anton A (2014) Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol 26:1597–1606
Yunque DAT, Tibubos KR, Hurtado AQ, Critchley AT (2011) Optimization of culture conditions for tissue culture production of young plantlets of carrageenophyte Kappaphycus. J Appl Phycol 23:433–438
Acknowledgments
The first author thanks Acadian Seaplants, Canada, for the sample of AMPEP K+ and various seaweed farmers in Malaysia for their assistance in the field. The first author also thanks the Seaweed Research Unit, Universiti Malaysia Sabah, for the use of laboratory space provided for the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, M.K.M., Critchley, A.T. & Hurtado, A.Q. Micropropagation and sea-based nursery growth of selected commercial Kappaphycus species in Penang, Malaysia. J Appl Phycol 32, 1301–1309 (2020). https://doi.org/10.1007/s10811-019-02003-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-02003-4