Abstract
This work aimed to establish the nutrient fertilization regime for indoor maintenance of Kappaphycus alvarezii seedlings during periods of low sea temperatures using biofloc effluent as a fertilizer. Moreover, we evaluated the development of seaweed seedlings after transplantation into the sea cultivation in Santa Catarina, Brazil. Seedlings were fertilized with biofloc effluent diluted by 25% in three different nutrient fertilization regimes for 4 weeks: single fertilization (SF): seedlings were fertilized for 1 week and remained in seawater for the following 3 weeks; alternating fertilization (AF): alternated 1 week with nutrient fertilization and 1 week without, and continuous fertilization (CF): seedlings were cultivated continuously with biofloc effluent. After this period, samples of each treatment were transferred into the sea cultivation and kept for 5 more weeks. The daily growth rates of plants cultivated in SF and AF were significantly higher than CF in indoor maintenance. However, in sea cultivation, the daily growth rates from AF and CF were significantly higher than SF. In both cases, there were no differences in the carrageenan yield between treatments. Light microscopy and quantification of floridean starch and pigments showed that after the sea cultivation period, floridean starch presence changed from cortical to medullar cells depending on the treatment, but no significant difference in the quantity was observed. Also, seedlings of SF treatment showed a significantly higher phycocyanin content compared to CF treatment. Based on the results, we suggest the continuous fertilization for indoor maintenance in tanks of seaweed seedlings with biofloc effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kappaphycus alvarezii and Eucheuma spp. were the second most produced aquatic plants in 2018, producing 11 million tonnes of biomass (FAO 2020). These species are the main sources of carrageenan, a colloid extracted from red seaweeds, used in food, pharmaceutical, and cosmetics industries as a thickening and stabilizing agent (Loureiro et al. 2017). In 2018, Brazil imported 2,005 t of carrageenan at the cost of US$ 17.9 million (MDIC 2019).
In Santa Catarina, K. alvarezii was introduced in 2008 and cultivation in the sea was proved to be technically feasible in the spring, summer, and autumn, planting seedlings in tubular nets, essential in the region due to strong sea currents. In winter, when the water temperature can reach values below 18 °C, growth is impaired (Hayashi et al. 2011a). One alternative to avoid this production loss during this period is the maintenance of the seaweed in indoor conditions, cultivating it in tanks with effluents from other aquaculture activities to minimize costs.
Previous works have shown that the enrichment of seaweeds in tanks with inorganic nutrients and effluents from other aquatic organisms is effective and thereafter, these seedlings can be transplanted and grown into sea cultivation (Nelson et al. 2001; Nagler et al. 2003; Hayashi et al. 2008). Moreover, seaweeds can act as bioremediators when these effluents are treated. In sustainable systems as the biofloc technology (BFT) system, several authors have demonstrated that seaweeds can use these nutrients to grow and treat these effluents at the same time (Brito et al. 2014, 2016; Fourooghifard et al. 2017; Brito et al. 2018a, b). BFT is an interaction of aggregates (bioflocs) of bacteria, microalgae, fungi, protozoa, nematodes, rotifers, and other organisms, maintaining the water quality and recycling residues. For this reason, aquatic organisms can be cultivated in high densities and with minimum or no water exchange (Crab et al. 2012; Emerenciano et al. 2017). Furthermore, the effluent generated can be rich in nitrogen and phosphorus compounds from the feed, essential nutrients for seaweed growth (Silva et al. 2013).
Promising results have recently been obtained, specifically with K. alvarezii cultivated in BFT effluents. Pires et al. (2021) found that BFT effluent from shrimp Litopenaeus vannamei, when diluted by 25% (BFT25), can substitute von Stosch 50% enrichment solution in K. alvarezii cultivated in vitro. These authors also concluded that K. alvarezii cultivated in BFT25 had carrageenan with high gel strength and viscosity compared to those cultivated just in seawater and that BFT25-enriched seaweeds could be efficient in phosphate and nitrogen bioremediation. Under in vitro conditions, Pedra et al. (2017) observed that when the same species are enriched with BFT25, the growth rate is higher (1.56 ± 0.08% day−1) than when cultivated only sterilized water (0.51 ± 0.05% day−1); moreover, the enriched seaweeds increased the production of secondary metabolites. Berchof (2018) found that the maintenance of K. alvarezii in tanks for 4 weeks in continuous fertilization with BFT25 at a density of 21 g L−1 is also feasible, and the seaweed did not show any damage when transplanted into the sea cultivation.
Based on the results mentioned above, the present work aims to establish the nutrient fertilization regime for indoor maintenance of K. alvarezii using BFT25 as a fertilizer, based on the daily growth rate, carrageenan yield, extraction and characterization of carrageenans, light microscopy, floridean starch concentration, and photosynthetic pigments analyses. We also evaluated the development of these seedlings in sea cultivation after indoor nutrient fertilization regimes.
Material and methods
Biological material
For indoor maintenance, 3.15 kg of the green tetrasporophyte of Kappaphycus alvarezii from the Experimental Marine Farm of Sambaqui (27°29′19″ S; 48°32′18″ W) of Federal University of Santa Catarina (UFSC) were used. These seedlings were harvested at the end of March and transported to the Seaweed Laboratory (LCM Macroalgas-UFSC) in coolers. The samples were washed in seawater and cleaned of epiphytes and debris. Then, they were acclimated and kept for 25 weeks in the laboratory in plastic boxes filled with 50 L of sterilized seawater at a density of 7 g L−1, 12 h photoperiod, 165 ± 7 µmol photons m−2 s−1 irradiance, constant aeration, 23 ± 0.5 °C temperature, and 35‰ salinity (Pedra et al. 2017). During this period, the enrichment with biofloc effluent diluted at 25% (Pires et al. 2021) was carried out in alternated weeks with sterilized seawater. The sterilization of seawater was done by filtration in 25, 10, and 5 mm sequence of filters, followed by sterilization with ultraviolet (UV) light.
Effluent from shrimp biofloc system
The effluent was collected weekly from a 50 m3 tank of marine shrimp Litopenaeus vannamei rearing in a biofloc system of the Marine Shrimp Laboratory (LCM-UFSC). Shrimp were fed four times a day with commercial feed (35% crude protein), according to the feeding table proposed by Van Wyk (1999). After collection, the effluent was filtered in a 25 µm bag filter to remove the solids and diluted with sterilized seawater to a concentration of 25% (Pires et al. 2021). From this diluted effluent, the following water quality parameters were evaluated: total ammonia nitrogen, nitrite, dissolved orthophosphate (Grasshoff et al. 1983), total suspended solids, volatile solids, alkalinity (APHA 2005), and nitrate (HACH method, by cadmium reduction).
Indoor maintenance
Before the nutrient enrichment, the seaweeds were kept for 2 weeks just in sterilized seawater at 35‰ salinity and 23 ± 0.5 °C temperature. During the experimental period, seawater or BFT25 were changed once a week.
Three treatments with different nutrient fertilization regimes in BFT25 were tested in tanks:
-
Single fertilization (SF): 1 week of cultivation in BFT25 followed by 3 weeks of continuous cultivation in sterilized seawater;
-
Alternating fertilization (AF): 1 week of cultivation with BFT25 alternated with 1 week of cultivation in sterilized seawater;
-
Continuous fertilization (CF): every week of continuous cultivation with BFT25.
All treatments were in triplicates (n = 3). Each experimental unit was composed of 74 L plastic boxes (35 cm width × 41 cm height × 68 cm length) with a useful volume of 50 L. The seaweeds were cultivated for 4 weeks at a density of 7 g L−1, 12 h photoperiod, 165 ± 7 µmol photons m−2 s−1 irradiance, 23 ± 0.5 °C temperature, and 35‰ salinity (Pedra et al. 2017), according to each treatment.
Temperature, salinity, pH, and dissolved oxygen were monitored twice a week. BFT25 or sterilized seawater was renewed weekly, depending on the treatment. At the time, boxes were cleaned, and seaweeds were weighed to determine the growth rates, according to Yong et al. (2013):
where DGR = daily growth rate; FB = final biomass; IB = initial biomass, and t = cultivation time. After the cultivation period, samples of each treatment were selected for carrageenan yield, extraction and characterization of carrageenans, light microscopy, floridean starch concentration, and photosynthetic pigments analyses, as described below.
Sea cultivation
After the indoor cultivation period, seedlings were transferred into the Experimental Marine Farm and cultivated for 34 days, between October 2018 and November 2018. Samples of 100 g were taken from each experimental unit in the laboratory and placed in tubular nets 22 ± 1 cm long. The tubular nets were randomly distributed in the cultivation raft and a fishing net was placed below the raft to prevent herbivory by fish. Seaweeds were weighed in the first, fourth, and fifth weeks during sea cultivation, where the daily growth rates were calculated according to Yong et al. (2013). The temperature was recorded daily with an Optic StowAway Tidbit Data Logger at 30 cm from the surface. After the sea cultivation period, samples of each treatment were separated for carrageenan yield, extraction and characterization of carrageenans, light microscopy, floridean starch concentration, and photosynthetic pigments analyses.
Carrageenan yield
Samples of fresh seaweed were dried in an oven at 35 °C for 24 h and then at 60 °C for another 24 h. For the carrageenan extraction, 5 g of dry seaweed was treated in a 6% KOH solution and placed in a water bath at 80 °C with stirring for 2 h. After this period, the solution was filtered and washed for 20 h under running water. Then, the alkali-treated seaweed was kept in distilled water with constant agitation for 4 h at 60 °C. The filtration was carried out with a low-pressure pump and the resulting carrageenan was gelified with 0.2% KCl. The resulting carrageenan was frozen and thawed at least twice and dried at 60 °C. The carrageenan yield was calculated by the percentage of extracted carrageenan in relation to the dry weight of the seaweed (Hayashi et al. 2007a).
Characterization of carrageenans
In order to evaluate the characterization of the carrageenans extracted and eventual alterations in their chemical structures resulting from the treatments, samples were submitted to medium infrared spectroscopy. FTIR spectra were recorded on a Bruker IFS-55 spectrometer (Model Opus 5.0, Bruker Biospin, Germany) equipped with a DGTS detector and single reflection system (45° angle of incidence) with total ATR reflectance attenuation accessory. Spectra of 3 replicates per sample were collected in a spectral window of 4000–600 cm−1, with a resolution of 4 cm−1. The spectra were further processed using the Essential FTIR software (v.1.50.282), considering the definition of the spectral window of interest (i.e., 4000–600 cm−1) baseline correction, normalization, and optimization of the signal-to-noise (smoothing) relation. The identification of the bands was performed by analogy with other FTIR reports in similar conditions (Pereira 2006; Gómez-Ordóñez and Rupérez 2011).
Light microscopy
Samples of fresh seaweeds with approximately 5 mm were fixed in a 2.5% glutaraldehyde solution in 0.1 M sodium phosphate buffer, pH 7.2, for 12 h, at the temperature of 4 °C. Then, the material was washed three times with 0.1 M sodium phosphate buffer and dehydrated in growing ethanol solutions at 30, 50, 70, 90, and 100% (Bouzon et al. 2006). The pre-infiltration and the infiltration of the samples were made in glycolmethacrylate historesin and the blocks were mounted in the same historesin adding a hardener. Sections of 4 µm were prepared with a Leica RM 2125 microtome with a tungsten razor and were placed on slides for the application of histochemical techniques.
Toluidine Blue (AT-O) (Gordon and McCandless 1973): this technique was used to identify acidic polysaccharides through the metachromasia reaction, corresponding mainly to carrageenan. The slides with the sections were stained for about 60 s, washed in running water, and dried at room temperature. Subsequently, the blades were assembled in Canadian balsam.
Schiff’s Periodic Acid (PAS) (Gahan 1984): this technique was used to identify the presence of neutral polysaccharides, mainly floridian starch grains. The slides with the sections were immersed in a 1% aqueous periodic acid solution for 15 min. Then, they were washed in running water for 15 min and the Schiff’s reagent was applied for 20 min in the dark. Then, the sections were rewashed under running water for 15 min, air-dried, and mounted with Canadian balsam.
Floridean starch concentration
For extraction and quantification of floridean starch, 50 mg of dried K. alvarezii (n = 3) were ground in liquid nitrogen and macerated in methanol:chloroform: water solution (MCW, 12:5:3 v/v/v). The MCW extracts were subjected to reactions, as described by Umbreit and Burris (1964), with modifications. For this, 2 mL of anthrone reagent (200 mg of anthrone in 100 mL of concentrated H2SO4) was added in 1 mL aliquots and the final solution was vortexed and heated for 3 min at 100 °C. The solid phase resulting from the extraction was subjected to a 30% perchloric acid reaction (1 mL) and anthrone 2% in H2SO4 (2 mL) following the heating and reading procedures in a spectrophotometer at 630 nm (UV-2000A spectrophotometer, Instrutherm, Brazil). The starch content was calculated from an external calibration curve in concentrations of 10 to 500 μg mL−1 (y = 0.0075x; r2 = 0.997).
Photosynthetic pigments
For the extraction photosynthetic pigments (chlorophyll a, total carotenoids, and phycobiliproteins), 1 g samples of fresh seaweed were stored at − 20 °C. Chlorophyll a and carotenoids were extracted in 1.5 mL of dimethyl sulfoxide (DMSO) at 40 °C for 40 min and quantified according to Hiscox and Israelstam (1979). The phycobiliprotein content was determined by grinding the material to a powder with liquid nitrogen and extraction in 0.05 M phosphate buffer, pH 6.4, at 4 °C in the dark. The levels of phycobiliproteins (phycoerythrin (PE), phycocyanin (PC), and allophycocyanin (APC)) were determined by spectrometry and calculated using the equations of Kursar et al. (1983).
Statistical analysis
Homoscedasticity and normality were tested using Levene and Shapiro–Wilk, respectively. The data were subjected to analysis of variance (ANOVA) unifactorial, followed by the Tukey test to compare means between treatments in the same period of cultivation (indoor or sea). Different periods of cultivation (indoor or sea) inside the same treatment were compared by Student’s t-test. All statistical analyses were performed with a significance level of 5% (p < 0.05).
Results
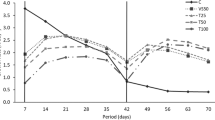
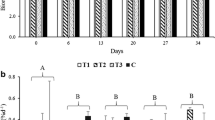
The BFT25 water quality parameters collected weekly used in the indoor maintenance are shown in Table 1. Temperature, salinity, pH, and dissolved oxygen were stable during the indoor maintenance period (Table 2). Seaweeds cultivated in SF and AF treatments did not show significant differences in daily growth rates (0.67 ± 0.15% day−1 and 0.74 ± 0.04% day−1, respectively) and were significantly higher than CF (0.44 ± 0.07% day−1) (p < 0.05) (Fig. 1a). No significant differences were observed in the carrageenan yield of seaweeds from SF, AF, and CF treatments (22.45 ± 1.06%, 22.71 ± 0.45%, and 21.33 ± 0.32%, respectively) (p < 0.05) (Fig. 1b).
Kappaphycus alvarezii cultivated under three different nutrient fertilization regimes: single fertilization (SF); alternating fertilization (AF), and continuous fertililization (CF), for 4 weeks in indoor maintenance and 34 days in sea cultivation. a Daily growth rate (% day−1) and b carrageenan yield (%). Values presented in mean ± confidence interval (n = 3). Different letters indicate significant differences between treatments (p < 0.05)
Parameters of temperature in sea cultivation period were also stable during the period analyzed (Table 3). In this case, seaweeds from AF and CF treatments showed similar daily growth rates (4.16 ± 0.19% day−1 and 4.01 ± 0.06% day−1, respectively) and significantly higher in relation to SF treatment (3.46 ± 0.18% day−1) (p < 0.05) (Fig. 1a). No significant differences were observed in carrageenan yield of seaweeds from SF, AF, and CF treatments (21.63 ± 2.24%, 20.53 ± 1.24%, and 19.91 ± 0.32%, respectively) (p < 0.05) (Fig. 1b).
The carrageenans FTIR spectroscopic profiles (Fig. 2) showed a series of bands typically found in kappa and iota carrageenans (Table 4). The spectra of kappa and iota carrageenans extracted from samples produced indoor revealed peaks with higher intensity patterns for the SF treatment, followed by the AF and CF treatments. Contrarily, seaweed from sea cultivation showed relatively higher amounts of those carrageenan types under CF treatment, with smaller amounts for the AF and SF ones (Fig. 2).
Kappaphycus alvarezii samples observed under light microscopy showed a strong metachromatic reaction to staining with Toluidine Blue (AT-O) (Fig. 3). All treatments presented similar AT-O reactions, showing the presence of carrageenan in the cortical and subcortical cells. An intense reaction to Schiff’s Periodic Acid (PAS) was also observed under light microscopy (Fig. 4) in samples cultivated indoor, evidencing the presence of floridean starch grains in the cortical and subcortical cells in all treatments (Fig. 4a, c, and e) and in the medullary cells in SF and CF treatments (Fig. 4b and f). Samples from SF treatment (11.89 ± 2.35 mg g−1) showed significantly higher amounts of floridean starch grains at the end of indoor cultivation (Ti) compared to AF treatment (6.88 ± 1.57 mg g−1) and similar to CF treatment (8.28 ± 0.81 mg g−1) (p < 0.05) (Table 5). After the sea cultivation period, samples from SF treatment (Fig. 5a) showed a higher presence of floridean starch grains in cortical cells and subcortical cells in relation to the other treatments (Fig. 5c and e). In the medullary cells, few floridean starch grains were observed in SF and AF treatments (Fig. 5b and d) and none in CF treatment (Fig. 5f). However, when the floridean starch grains were quantified, no significant differences were observed between treatments (p < 0.05). Only samples from CF treatment showed a significant reduction in the floridean starch concentration when indoor and sea conditions were compared (8.28 ± 0.81 to 5.05 ± 0.23 mg g−1) (p < 0.05) (Table 5).
Light microscopy of transverse sections of K. alvarezii stained with Toluidine Blue (AT-O). The metachromatic reaction to AT-O in the cell wall of the cortical cell (cc) and subcortical cells (sc) indicates the presence of carrageenan. a to c samples cultivated in indoor maintenance; d to f samples cultivated in sea cultivation. a and d SF treatment; b and e AF treatment; c and f CF treatment. Scale: 50 µm
Light microscopy of transverse sections of K. alvarezii cultivated in indoor maintenance, stained with Schiff’s Periodic Acid (PAS). Arrows point PAS positive floridean starch grains present in the cortical cells (cc), subcortical cells (sc), and some medullary cells (mc). cw, cell wall. a and b SF treatment; c and d AF treatment; e and f CF treatment. Scale: 50 µm
Light microscopy of transverse sections of K. alvarezii cultivated in sea cultivation, stained with Schiff’s Periodic Acid (PAS). Arrows point PAS positive floridean starch grains present in the cortical cells (cc), subcortical cells (sc), and some medullary cells (mc). cw, cell wall. a and b SF treatment; c and d AF treatment; e and f CF treatment. Scale: 50 µm
After the indoor period, a visual variation in the seedlings color was observed according to the treatment. CF treatment showed intense coloration followed by AF and SF treatments, respectively. As the frequency of nutrient fertilization increased, the darker the pigmentation of the thallus became. Nevertheless, the concentration of pigments (chlorophyll a, carotenoids, and phycobiliproteins) was similar between the treatments at the end of the indoor period (Ti). After sea cultivation, despite the seaweeds showing the same color independent of the treatment, a significant difference in phycocyanin content was observed between treatments. Samples of SF treatment had a significantly higher phycocyanin content (7.97 ± 1.72 µg g−1) compared to CF treatment (3.90 ± 1.33 µg g−1) (p < 0.05).
When the treatments were compared between indoor maintenance and sea cultivation, seedlings from AF and CF treatments showed a significant reduction of carotenoids (1.06 ± 0.18 to 0.56 ± 0.13 µg g−1 and 0.95 ± 0.11 to 0.63 ± 0.10 µg g−1, respectively) (p < 0.05). Samples of CF and SF treatments also showed a significant reduction in the phycoerythrin concentration (6.37 ± 1.45 to 2.31 ± 0.25 µg g−1 and 5.31 ± 1.80 to 1.51 ± 0.49 µg g−1, respectively) (p < 0.05). Plants cultivated in SF treatment also presented a significant increase in the phycocyanin concentration (4.73 ± 1.00 to 7.97 ± 1.72 µg g−1) considering the beginning and the end of the sea cultivation (Table 6) (p < 0.05).
Discussion
The results showed that Kappaphycus alvarezii could be maintained indoor by using BFT25 and the nutrient fertilization regimes used did not affect the development of the seaweeds after transplantation into sea cultivation. Light, temperature, salinity, and pH parameters were within the optimal conditions for its development during the indoor period (Trono and Ohno 1989; Granbom et al. 2001; Hayashi et al. 2011b; Araujo et al. 2014; Tee et al. 2015; Borlongan et al. 2016). Seaweeds, in general, use mainly nitrogen and phosphorus as the primary nutrients for their development (Harrison and Hurd 2001) and these were present in the BFT25 in sufficient quantity for their development.
According to Pires et al. (2021), BFT25 can be used to replace the 50% von Stosch solution, a nutrient enrichment solution commonly used to maintain the species in the laboratory. The daily growth rates of SF and AF treatments (0.67 ± 0.15% day−1 and 0.74 ± 0.04% day−1, respectively) were higher than CF treatment (0.44 ± 0.07% day−1) during the indoor period. These results corroborate with those from Paula et al. (2001), with K. alvarezii cultivated in vitro in different regimens of seawater enrichment media. According to these last authors, the high concentrations of nutrients from continuous enrichment can hinder seaweed growth. Furthermore, these rates were lower than those observed by Pedra et al. (2017) and Pires et al. (2021) (1.56 ± 0.14% day−1 in CF and 1.19 ± 0.04% day−1 in SF, respectively) in seedlings grown for 5 weeks with BFT25 and higher than those observed by Berchof (2018) (0.29 ± 0.04% day−1 in CF) in seedlings grown for 4 weeks with BFT25. Nevertheless, the nutrient concentrations of the BFT25 inoculum used by Pedra et al. (2017) and Pires et al. (2021) before dilution were higher, which could explain the difference in the daily growth rates. Despite this, the seedlings probably accumulated the inorganic nutrients available in BFT25, reflected in the growth rates and changes in the color intensity, as observed by Hayashi et al. (2008) and Pires et al. (2021).
During sea cultivation seedlings did not show any damage or signals of “ice-ice” disease. Curiously, the daily growth rates of AF and CF treatments (4.16 ± 0.19% day−1 and 4.01 ± 0.06% day−1, respectively) were higher than SF treatment (3.46 ± 0.18% day−1), a different pattern of that observed in indoor conditions. These values were higher than those observed by Hayashi et al. (2011a) in the same season and location, suggesting that previous BFT25 enrichment in indoor conditions may have an important effect on crops after transplantation into sea condition. The increase in the nutrient fertilization regime indoor can be related to higher daily growth rates in sea conditions. These results corroborate the results of Nelson et al. (2001), Nagler et al. (2003), and Hayashi et al. (2008).
No significant differences were found in carrageenan yield between indoor maintenance and sea cultivation treatments, indicating that the nutrient fertilization regimes did not influence carrageenan yield. In this case, the concept of “Neish Effect,” plants that grow in unenriched seawater have a higher carrageenan content than those grown in N-enriched seawater, was not observed (Chopin et al. 1990). Light microscopy also evidenced this result, where the thickening of the cell walls was similar in both periods of cultivation. The carrageenan yield observed in this present work were similar to those observed by Hayashi et al. (2008) (21.50 ± 0.50%) in K. alvarezii cultivated with fish effluent in the sea and similar to K. alvarezii cultivated in Santa Catarina (22.93 ± 0.20%) and São Paulo (21.84% and 20–32%) (Brazil) in sea cultivation (Hayashi et al. 2007a; b; Hayashi 2011a), but lower than those observed by Pires et al. (2021) (29.90 ± 1.35%) in seedlings fertilized in BFT25 and cultivated in laboratory. The carrageenan analysis in seedlings cultivated indoor was made just as a comparison parameter, with no intention by authors to develop tank cultivation for the carrageenan industry. The fact that there is no change in carrageenan yield between indoor maintenance and sea cultivation was an interesting result and indicates that the BFT25 has no effect on the production of carrageenan at the beginning of the production cycle at sea.
Mid-infrared vibrational spectroscopy (IR) has been frequently used for the study of the chemical composition of phycocolloids, requiring minimum amounts of sample (milligrams) and providing spectra with reliable accuracy (Pereira et al. 2009). In the present work, carrageenan FTIR spectra revealed a predominance of kappa and iota carrageenan peaks, and no bands for the lambda type have been detected. For instance, two bands at 846 cm−1 and 805 cm−1 were observed, assigned to D-galactose-4-sulfate and D-galactose-2-sulfate, respectively, typical of kappa and iota carrageenan (Pereira 2006). Bands around 1150 cm−1 and 1010 cm−1 were found and related to CO and CC’s stretching vibrations of the pyranose ring typical to those polysaccharides (Gómez-Ordóñez and Rupérez 2011). Besides, the characteristic bands at 1068 cm−1 (C–O, 3, 6-anhydrogalactose) have been attributed to kappa and iota carrageenans, respectively (Pereira 2006; Pereira et al. 2009,2013; Gómez-Ordóñez and Rupérez 2011). In fact, the FTIR spectra of kappa and iota carrageenans extracted from samples indicate a clear effect of the indoor and sea conditions concerning the types and proportion of carrageenans produced.
According to the treatment and cultivation condition, the location of floridean starch grains changed, as observed in light microscopy. The floridean starch grains are produced by the Calvin cycle biosynthetic pathway when nutrients are present, mainly in active photosynthetic cortical cells, which have several chloroplasts. This accumulation begins in the cortical cells and extends to the medullary cells through the pit connections (Mayanglambam and Sahoo 2015), as observed in seedlings cultivated in laboratory conditions in the present work. However, the concentration reduction was confirmed only in CF treatment (8.28 ± 0.81 to 5.05 ± 0.23 mg g−1) after transplantation from indoor into sea conditions. Probably, seedlings from this treatment used the reserve of floridean starch grains to grow in the sea conditions. Floridean starch grains are responsible for the energy and carbon reserves of red seaweeds for several cellular processes (e.g., growth) and protection from stress factors (Vitova et al. 2015). This could explain why at the end of the nutrient fertilization period (Ti), the SF treatment showed a higher floridean starch grains concentration. Since this treatment has a lower nutrient fertilization frequency, the seaweed accumulated the floridean starch grains as a reserve, but this was insufficient to improve its growth after transplantation into the sea cultivation. Previous studies have shown that stress factors such as nutrient limitation and salinity variations can increase florid starch grains reserves in cell structures as a protection strategy (Hayashi et al. 2011b; Prabhu et al. 2019). A decrease in starch grains is due to possible degradation pathway activation. According to Pereira et al. (2018), the degradation pathway can be used to activate the biosynthesis of defense compounds, such as the production of cell wall components, the thickening of cell walls, and the consequent production of new cells.
During indoor maintenance, the pigment concentration (chlorophyll a, total carotenoids, and phycobiliproteins) remained stable, with no significant differences. However, after transplantation into sea cultivation, a reduction in photosynthetic pigments was observed in all treatments. The nitrogen available in cultivation environment influences the production of photosynthetic pigments, mycosporins, and seaweed growth, contributing to acclimation and photoprotection (Davison et al. 2007; Barufi et al. 2011). Although this reduction was considered significant for carotenoids (AF treatment) and phycoerythrin (CF treatment), it did not compromise the seaweed’s photosynthetic capacity, as observed in their respective the daily growth rates.
The present work results are important for defining strategies and protocols for the maintenance of K. alvarezii in indoor conditions. AF and CF treatment showed interesting results considering the response to sea cultivation after nutrients enrichment. Both treatments showed no signs of stress when transferring from indoor maintenance into this environment, and both the daily growth rates and carrageenan yields were similar. CF treatment showed significantly lower daily growth rates indoor, which can be interesting if considering the maintenance costs. The increase in biomass means increased maintenance costs in indoor conditions, especially in pumps and air blowers. CF treatment could also be considered in integrated multitrophic cultivation in biofloc systems since the effluent could continuously circulate within the system.
The CF treatment is indicated for maintaining seaweeds in indoor cultivation fertilized with BFT25. This treatment helps the seedlings acclimation and protection after transplantation into the sea cultivation and improves their daily growth rate without affecting the carrageenan yield and quality.
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files. The data also may be available upon request from some of the participants but not for all due to relevant data protection laws.
References
APHA (2005) Standard methods for the examination of the water and wastewater, 21st edn. American Public Health Association Washington, DC, USA
Araújo PG, Ribeiro ALN, Yokoya NS, Fujii MT (2014) Temperature and salinity responses of drifting specimens of Kappaphycus alvarezii (Gigartinales, Rhodophyta) farmed on the Brazilian tropical coast. J Appl Phycol 26:1979–1988
Barufi JB, Korbee N, Oliveira MC, Figueroa FL (2011) Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under UV radiation. J Appl Phycol 23:457–466
Berchof FF (2018) Influência de diferentes densidades de cultivo da macroalga Kappaphycus alvarezii na manutenção de linhagens em laboratório e no cultivo no mar. Federal University of Santa Catarina, Monograph
Bouzon ZL, Ouriques LC, Oliveira EC (2006) Spore adhesion and cell wall formation in Gelidium floridanum (Rhodophyta, Gelidiales). J Appl Phycol 18:287–294
Borlongan IAG, Luhan MRJ, Padilla PIP, Hurtado AQ (2016) Photosynthetic responses of ‘Neosiphonia sp. epiphyte-infected’ and healthy Kappaphycus alvarezii (Rhodophyta) to irradiance, salinity and pH variations. J Appl Phycol 28:2891–2902
Brito LO, Arantes R, Magnotti C, Derner R, Pchara F, Olivera A, Vinatea L (2014) Water quality and growth of Pacific white shrimp Litopenaeus vannamei (Boone) in co-culture with green seaweed Ulva lactuca (Linaeus) in intensive system. Aquac Int 22:497–508
Brito LO, Chagas AM, Da Silva EP, Soares RB, Severi W, Gálvez AO (2016) Water quality, Vibrio density and growth of Pacific white shrimp Litopenaeus vannamei (Boone) in an integrated biofloc system with red seaweed Gracilaria birdiae (Greville). Aquac Res 47:940–950
Brito LO, Junior LC, Abreu JL, Severi W, Moraes LB, Galvez AO (2018a) Effects of two commercial feeds with high and low crude protein content on the performance of white shrimp Litopenaeus vannamei raised in an integrated biofloc system with the seaweed Gracilaria birdiae. Spanish J Agric Res 16:e0603
Brito LO, Junior LDOC, Lavander HD, Abreu JLD, Severi W, Gálvez AO (2018) Bioremediation of shrimp biofloc wastewater using clam, seaweed and fish. Chem Ecol 34:901–913
Chopin T, Hanisak MD, Koehn FE, Mollion J, Moreau S (1990) Studies on carrageenans and effects of seawater phosphorus concentration on carrageenan content and growth of Agardhiella subulta (C.Agardh) Kraft and Wynne (Rhodophyta, Solieriaceae). J Appl Phycol 2:3–16
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture 356:351–356
Davison IR, Jordan TL, Fegley JC, Grobe CW (2007) Response of Laminaria saccharina (Phaeophyta) growth and photosynthesis to simultaneous ultraviolet radiation and nitrogen limitation. J Appl Phycol 43:636–646
Emerenciano MGC, Martínez-Córdova LR, Martínez-Porchas M, Miranda-Baeza A (2017) Biofloc technology (BFT): a tool for water quality management in aquaculture. In: Tutu H (ed) Water Quality. IntechOpen, London, pp 91–109
FAO (2020) The state of world fisheries and aquaculture: sustainability in action. Food And Agriculture Organization Of The United Nations, Rome
Fourooghifard H, Matinfar A, Mortazavi MS, Ghadikolaee RK, Mirbakhsh M (2017) Growth parameters of whiteleg shrimp Litopenaeus vannamei and red seaweed Gracilaria corticata in integrated culturing method under zero water exchange system. Aquac Res 48:5235–5242
Gahan PB (1984) Plant histochemistry and cytochemistry. Academic Press, London
Gómez-Ordóñez E, Rupérez P (2011) FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids 25:1514–1520
Gordon EM, McCandless EL (1973) Ultrastructure and histochemistry of Chondrus crispus Stack. Proc Nova Scot Inst Sci 27:111–133
Granbom M, Pedersén M, Kadel P, Lüning K (2001) Circadian rhythm of photosynthetic oxygen evolution in Kappaphycus alvarezii (Rhodophyta): dependence on light quantity and quality. J Appl Phycol 37:1020–1025
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Verlag Chemie, Weinheim, p 419
Harrison PJ, Hurd CL (2001) Nutrient physiology of seaweeds: application of concepts to aquaculture. Cah Biol Mar 42:71–82
Hayashi L, Paula EJ, Chow F (2007) Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State, Brazil. J Appl Phycol 19:393–399
Hayashi L, Oliveira EC, Bleicher-Lhonneur G, Boulenguer P, Pereira RT, von Seckendorff R, Shimoda VT, Leflamand A, Vallée P, Critchley AT (2007) The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. J Appl Phycol 19:505–511
Hayashi L, Yokoya NS, Ostini S, Pereira RTL, Braga ES, Oliveira EC (2008) Nutrients removed by Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in integrated cultivation with fishes in recirculating water. Aquaculture 277:185–191
Hayashi L, Santos AA, Nunes BG, Souza MS, Fonseca ALD, Barreto PLM, Oliveira EC, Bouzon ZL (2011) Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) cultivated in subtropical water in Southern Brasil. J Appl Phycol 23:337–343
Hayashi L, Faria GS, Nunes BG, Zitta CS, Scariot LA, Rover T, Felix MRL, Bouzon ZL (2011) Effects of salinity on the growth rate, carrageenan yield, and cellular structure of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultured in vitro. J Appl Phycol 23:439–447
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Kursar TA, van Der Meer J, Alberte RS (1983) Light-harvesting system of the red alga Gracilaria tikvahiae: I. Biochemical analyses of pigment mutations. Plant Physiol 73:353–360
Loureiro RR, Cornish ML, Neish IC (2017) Applications of carrageenan: with special reference to iota and kappa forms as derived from the eucheumatoid seaweeds. In: Hurtado A, Critchley A, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities. Springer, Cham, pp 165–171
Mayanglambam A, Sahoo D (2015) Red algae. In: Sahoo D, Seckbach J (eds) The Algae World. Springer, Dordrecht, pp 205–234
MDIC (2019) Exportação e importação geral (Comex Stat). Ministério do Desenvolvimento, Indústria e Comércio. http://comexstat.mdic.gov.br/pt/geral. Accessed 11 January 2019
Nagler PL, Glenn EP, Nelson SG, Napolean S (2003) Effects of fertilization treatment and stocking density on the growth and production of the economic seaweed Gracilaria parvispora (Rhodophyta) in cage culture at Molokai. Hawaii Aquaculture 219:379–391
Nelson SG, Glenn EP, Conn J, Moore D, Walsh T, Akutagawa M (2001) Cultivation of Gracilaria parvispora (Rodophyta) in shrimp-farm effluent ditches and floating cages in Hawaii: a two-phase polyculture system. Aquaculture 193:239–248
Paula EJ, Erbert C, Pereira RTL (2001) Growth rate of the carrageenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales) in vitro. Phycol Res 49:155–161
Pedra AGLM, Ramlov F, Maraschin M, Hayashi L (2017) Cultivation of the red seaweed Kappaphycus alvarezii with effluents from shrimp cultivation and brown seaweed extract: effects on growth and secondary metabolism. Aquaculture 479:297–303
Pereira L (2006) Identification of phycocolloids by vibrational spectroscopy. In: Critchley AT, Ohno M, Largo DB (eds.), World seaweed resources: an authoritative reference system. ETI Information Services, Wokingham, Berkshire
Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids 23:1903–1909
Pereira L, Gheda SF, Ribeiro-Claro PJA (2013) Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int J Carbohydr Chem 2013: 537202
Pereira DT, Simioni C, Ouriques LC, Ramlov F, Maraschin M, Steiner N, Chow F, Bouzon ZL, Schmidt EC (2018) Comparative study of the effects of salinity and UV radiation on metabolism and morphology of the red macroalga Acanthophora spicifera (Rhodophyta, Ceramiales). Photosynthetica 56(3):799–810
Pires CM, Bazzo GC, Barreto PL, do Espírito Santo GM, Ventura TF, Pedra AG, Rover T, McGovern M, Hayashi L (2021) Cultivation of the red seaweed Kappaphycus alvarezii using biofloc effluent. J Appl Phycol 33:1047–1058
Prabhu M, Chemodanov A, Gottlieb R, Kazir M, Nahor O, Gozin M, Israel A, Livney YD, Golberg A (2019) Starch from the sea: the green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res 37:215–227
Silva KR, Wasielesky W, Abreu PC (2013) Nitrogen and phosphorus dynamics in the biofloc production of the Pacific white shrimp Litopenaeus vannamei. J World Aquacult Soc 44:30–41
Tee MZ, Yong YS, Rodrigues KF, Yong WTL (2015) Growth rate analysis and protein identification of Kappaphycus alvarezii (Rhodophyta, Gigartinales) under pH induced stress culture. Aquac Reports 2:112–116
Trono GC, Ohno M (1989) Seasonality in the biomass production of Eucheuma strains in northern Bohol, Philippines. In: Umezaki I (ed.), Scientific survey of marine algae and their resources in the Philippine Islands. A Technical Report of the Ministry of Education, Science and Culture, Japan: 71–80
Umbreit WW, Burris RH (1964) Method for glucose determination and other sugars. In: Umbreit WW, Burris RH, Staufer UP (eds) Manometric Techniques. Burgess Publishing, NY, pp 163–168
Van Wyk P (1999) Nutrition and feeding of Litopenaeus vannamei in intensive culture systems. In: Van Wyk P, Davis-Hodgkins M, Laramoreb R, Main KL, Mountain J, Scarpa J (eds) Farming marine shrimp in recirculating freshwater systems. Florida Department of Agriculture and Consumer Services - Harbor Branch Oceanic Institute, Florida, pp 125–140
Vitova M, Bisova K, Kawano S, Zachleder V (2015) Accumulation of energy reserves in algae: from cell cycles to biotechnological applications. Biotechnol Adv 33:1204–1218
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Acknowledgements
LH thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Productivity Fellowship (Process number 308631/2017-0).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Martino, R., Mariot, L.V., da Silva, F.Z. et al. Effects of biofloc effluent in different regimes as a fertilizer for Kappaphycus alvarezii: indoor maintenance and sea cultivation. J Appl Phycol 33, 3225–3237 (2021). https://doi.org/10.1007/s10811-021-02539-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02539-4