Abstract
Diatoms are considered to have great potential as new biofuel sources because they can effectively accumulate triacylglycerols (TAGs). Detailed structure information of TAG in diatoms is much needed not only for the assessment of biofuel quality such as fatty acid chain length and unsaturation degree but also for the tracing of biosynthetic precursors because the biosynthesis of TAG is typically completed by utilizing the diacylglycerol acyltransferase in the cytoplasm. In this report, a comprehensive characterization of TAGs in marine diatoms was performed using ultra performance liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry. Many types of major TAGs were identified for the first time in these diatoms: 12 TAGs in Chaetoceros debilis, 9 TAGs in Phaeodactylum tricornutum Bohlin, 16 TAGs in Nitzschia closterium f. minutissima, 16 TAGs in Thalassiosira weissflogii, 13 TAGs in Thalassiosira sp., 16 TAGs in Stephanodiscus asteaea and 7 TAGs in Skeletonema costatum. Semi-quantification of TAGs in these diatoms was also carried out, and it was found that the contents of individual TAGs ranged from 0.5 ± 0.1 to 217.9 ± 8.1 nmol mg−1 total lipids. In addition, the total lipid contents in diatoms ranged from 143.6 ± 16.3 to 201.1 ± 16.3 mg g−1 dry microalgae and the total TAG contents ranged from 36.8 ± 9.5 to 793.2 ± 54.4 nmol mg−1 total lipids. By comparative analysis of the compositions and concentrations of major TAGs in the seven algal strains, N. closterium f. minutissima with high abundance of TAGs containing the most monounsaturated fatty acids (mainly palmitoleic acid) was considered as one of the most promising diatom strains for microalgal biofuel production. Additionally, based on the information of sn-2 fatty acid obtained (mainly C16 in the sn-2 position), we propose the hypothesis that TAGs in diatoms are mainly derived from lipids in chloroplasts through the prokaryotic biosynthesis pathway, including monogalactosyldiacylglycerol and digalactosyldiacylglycerol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are potential new sources of biofuels and biomaterials (Hu et al. 2008; Beer et al. 2009). Triacylglycerol (TAG) is the major component of algae-derived oil, which contains three fatty acid esters with glycerol (Durrett et al. 2008). The chain length and degree of unsaturation of the fatty acids play an important role in determining the fuel properties. Generally, monounsaturated fatty acids are the most desirable choice (Durrett et al. 2008). Besides, the acyl group at the sn-2 position is significant for tracing the biosynthetic precursors of TAGs and regulating TAG biosynthesis artificially on a cellular or molecular level. It will be of interest to those who work in the field of biofuel research. It is therefore important to fully characterize TAGs from microalgae (MacDougall et al. 2011; Samburova et al. 2013; Holguin and Schaub 2013).
Previous analysis of TAGs in microalgae primarily focused on the fatty acid composition, involving the initial separation of TAGs by thin-layer chromatography or liquid chromatography, derivatization and detection by GC/MS (Sushchik et al. 2010; Lepage and Roy 1986). These methods are time-consuming and cannot confirm the acyl position in TAGs (Miao and Wu 2004; Guedes et al. 2010). Mass spectrometry using a variety of ionization techniques has been applied for the characterization of TAGs, including electron impact (Murphy 1993; Demirbuker et al. 1992), chemical ionization (Evershed et al. 1996; Manninen et al. 1995a, b; Kallio and Rua 1994), field desorption (Lehmann and Kessler 1983; Evans et al. 1974), desorption chemical ionization (Laakso and Kallio 1996), fast atom bombardment (Lamberto and Saitta 1995; Hori et al. 1994; Evans et al. 1991), thermospray (Sundin et al. 1992), electrospray ionization (ESI; Duffin et al. 1991) and atmospheric pressure chemical ionization (APCI; Neff and Byrdwell 1995). Recently, complete structural characterizations of TAGs in some microalgae by matrix-assisted laser desorption ionization/time-of-flight and ESI linear ion trap (LTQ-Orbitrap) mass spectrometry are reported (Danielewicz et al. 2011). However, because no chromatographic separation was used, matrix ion suppression might cause significant loss of ion signal to those lower content components in the analysed complex samples (Jessom and Volmer 2006). In addition, characterization of the lipid fractions in microalgae by multidimensional nuclear magnetic resonance and ESI-MS techniques has been reported (MacDougall et al. 2011; Samburova et al. 2013; Basconcillo et al. 2009; Hiraga et al. 2008). The application of HPLC/APCI-MS for the analysis of TAGs in a range of vegetable oils has been reported (Mottram et al. 1997).
Quadrupole time-of-flight mass spectrometry, which has high mass accuracy (>10,000 resolving power at m/z 1,000), makes it possible to differentiate the nominally isobaric species (Bristow 2006) and has been demonstrated as a superior tool in the rapid characterization of complex lipids (Xu et al. 2009, 2010; Chen et al. 2008). For assessing the potential of diatoms for biofuel applications, a comprehensive characterization and semi-quantification of major TAGs in seven strains of marine diatoms was performed in this study using ultra performance liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS). The results support the potential advantages of diatoms as biofuel sources.
Materials and methods
The seven marine diatoms—Chaetoceros debilis, Phaeodactylum tricornutum, Nitzschia closterium f. minutissima, Thalassiosira weissflogi, Thalassiosira sp., Stephanodiscus asteaea, Skeletonema costatum—were obtained from the Marine Biotechnology Laboratory of Ningbo University, China. Seawater (pH 8.30, 28 ‰ salinity) for culture was filtered using cellulose acetate membranes (0.45 μm) and sterilized. The algae were grown in f/2 medium (Borowitzka 1988) in 2,500-mL conical flasks at 20 ± 2 °C under natural daylight and shaken three times every day. Cells were sampled daily for cell counting with a haemocytometer. Microalgae were harvested at stationary phase by centrifugation at 5,000 rpm for 15 min and freeze-dried. All experiments were performed in triplicate and are reported as the mean ± one standard deviation.
Total lipid extraction and standard TAGs
A modified Bligh and Dyer method (Bligh and Dyer 1959) was used to obtain the total lipid from microalgae. This method had been used in our other studies (Xu et al. 2009, 2010; Chen et al. 2008). Briefly, 50 mg of freeze-dried microalgae was extracted with chloroform/methanol/water (1:2:0.8, v/v/v). The extract was evaporated on a rotary evaporator and the residue stored at −20 °C. TAG standards (>99 %) including tristearin (18:0–18:0–18:0)-TAG, trilinolenin (18:3–18:3–18:3)-TAG (n-9,12,15), trigammalinolenin (18:3–18:3–18:3)-TAG (n-6,9,12), 1-stearin-2-olein-3-linolein (18:0–18:1–18:2)-TAG, 1,2-palmitolein-3-olein (16:1–16:1–18:1)-TAG, 1,3-palmitolein-2-olein (16:1–18:1–16:1)-TAG, 1,3-palmitin-2-olein (16:0–18:1–16:0)-TAG and triolein (glycerol [3-13C] (18:1–18:1–18:1)-TAG were purchased from Larodan (Sweden) and used without further purification. Standard TAGs were dissolved in chloroform/methanol (1:4, v/v) at a final concentration of 10 pmol mL−1, respectively. Lithium acetate was then added to this solution to achieve a final [Li]+ of 2 mM.
Solid phase extraction
Total lipid sample was dissolved in 500 μL chloroform/methanol (1:1, v/v) and subjected to solid phase extraction separation using an LC-Si column containing 500 mg adsorbent (Waters), which was primed with methanol. The column was eluted sequentially with 5 mL hexane/diethylether (4:1, v/v), 5 mL hexane/diethyl ether (1:1, v/v), 5 mL methanol and 5 mL chloroform/methanol/water (3:5:2, v/v/v; Xu et al. 2009). The fractions of the methanol and chloroform/methanol/water, which contained the polar lipids, were detached; the fractions of the hexane and diethyl ether, which contained the neutral lipids, were combined, dried and dissolved using methanol to give a concentration of 10 μg mL−1 for LC-MS analysis. Before using solid phase extraction for separating the total lipids of the algae samples, TAG standards were used to verify recovery (>99 %, not shown).
UPLC condition
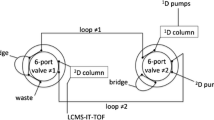
Reversed-phase analysis was performed on a Waters ACQUITY Ultra Performance LC system (UPLC) using an ACQUITY UPLC BEH C8 analytical column (2.1 × 100-mm i.d.; particle size, 1.7 μm). A 1:4 split of the column effluent was used to achieve a flow rate of approximately 50 μL min−1 into the ESI source. Lithium acetate (0.01 %) was added to the mobile phase as the electrolyte in order to produce ions that can be readily fragmented. To obtain efficient separation of the TAGs, water/acetonitrile (1:2, v/v) was used as mobile phase A and acetonitrile/isopropyl alcohol/tetrahydrofuran (1:1:1, v/v/v) as mobile phase B. The initial composition of mobile phase B was 40 %, which was kept for 5 min, then reached 80 % in 60 min and held for 5 min, returned to the initial 40 % in 1 min and equilibrated for 6 min. The temperature of the sample chamber was set at 4 °C; the column temperature was set at 40 °C and injection volume was 5 μL for each analysis. Before injection, samples were filtered using a 0.2-μm ultrafiltration membrane (Millipore, USA).
Mass spectrometric condition
Mass spectrometry was performed on a Waters Q-TOF Premier mass spectrometer operating in the positive ion mode. The mass range was from 150 to 1,200, with a scan duration of 0.3 s. High-purity nitrogen was used as the nebulizer and drying gas. The nitrogen drying gas was at a constant flow rate of 400 L h−1 and the source temperature was 120 °C. TAGs were detected in the positive ion mode. The capillary voltage was set at 3.0 kV and the sampling cone voltage was set at 40 V. MS2 analysis was performed at a collision energy of 40 V. The time-of-flight analyser was used in V mode and tuned for maximum resolution (>10,000 resolving power at m/z 1,000). The instrument was previously calibrated with sodium formate and the lock mass spray for precise mass determination was set by leucine enkephalin at m/z 556.2771 at a concentration 0.2 ng μL−1 in the positive ion mode.
Semi-quantification
Triolein (glycerol 3-13C, 18:1–18:1–18:1-TAG) was spiked (5 nmol mL−1) into the samples as the stable isotope internal standard. When changing the proportion of mobile phase B (40, 50, 60, 70 and 80 %), different ionization efficiencies of the TAG standards were obtained (not shown), respectively. The extracted ion chromatogram of each TAG was obtained from the low-collision-energy scan (6 V) and the peak area was integrated. After being calibrated for variable ionization efficiencies in different proportions of the mobile phases, semi-quantitative analysis was performed according to the ratio of the area of each TAG to that of the internal standard.
Data processing
The raw LC-MS data were analysed using MassLynx (v4.1) software (Waters). Identification of each TAG class and acyl chain was achieved using tandem mass spectrometry in positive mode and described in “Results”.
Results
Optimization of chromatographic conditions
To separate the complex TAGs in the samples, several mobile phases were tested, including different ratios of acetonitrile in water (phase A) and different ratios of isopropyl alcohol/tetrahydrofuran in acetonitrile (phase B). Finally, we found that most TAGs, including some regioisomers of TAGs such as trilinolenin (18:3–18:3–18:3)-TAG (n-9,12,15)] and trigammalinolenin (18:3–18:3–18:3)-TAG (n-6,9,12) that differ only in the position of the double bond, can be well separated when 2:1 acetonitrile/water (v/v) as phase A and 1:1:1 acetonitrile/isopropyl alcohol/tetrahydrofuran (v/v/v) as phase B were used. However, positional isomers of TAGs such as (16:1–18:1–16:1)-TAG and (16:1–16:1–18:1)-TAG cannot be separated (Fig. 1), consistent with other studies (Herrera et al. 2010). The [M+Li]+ ions of TAGs could be obtained in the positive mode by adding lithium acetate into the mobile phase at a final [Li]+ concentration of 2 mM.
Identification of the fatty acid composition and position in TAG standards
The fragmentation mechanism of TAG was studied by analysing the tandem mass spectra of lithiated adducts of TAG standards containing three fatty acid substituents. The positive ion mass spectra of the TAG standards displayed the precursor ion [M+Li]+. The MS/MS spectra of [M+Li]+ showed: [R n CO-18]+, [R n CO]+, [R2CH=CHCOOH+Li]+, [R n COOH+Li]+, [M+Li-R n COOH-R2CH=CHCOOH]+, [M+Li-R n COOLi]+ and [M+Li-R n COOH]+ (Fig. 2a, b).
The difference between [M+Li]+ and [M+Li-R n COOH]+ was utilized to determine the identity of fatty acids in TAGs. Previous studies suggested that it is the position of the fatty acid substituent on the glycerol, not the identity of the fatty acid, that governs the relative abundances of ions [M+Li-R n COOH]+ and [M+Li-R n COOLi]+ (Hsu and Turk 1999). Meanwhile, other studies suggested that the number of double bonds and the nature of the metal ions have a major effect on the relative abundance of TAG product ions (Herrera et al. 2010). In this study, we found that the abundances of [M+Li-R1/3COOH]+ were always stronger than the ion [M+Li-R2COOH]+ under different collision energies (6–80 V, not shown), suggesting that the loss of acyl chain at the sn-1/3 position was easier than the elimination of acyl chain at the sn-2 of TAG. A similar observation on the ion intensities was made for lithium salts [M+Li-R n COOLi]+. Therefore, the positions of the acyl chains of TAGs could be differentiated readily by ESI-MS/MS in the positive mode, which is consistent with the previous report (Hsu and Turk 1999). However, the [R n CO-18]+ and [RnCO]+ product ions of the (18:0–18:1–18:2)-TAG (Fig. 2a) molecule did not show a similar trend in signal intensity to that of the diglyceride product ions.
Identification of positional isomeric TAG standards (ABA, AAB)
AAB denotes a TAG containing two different fatty acids, A and B. Positional isomers are written as AAB and ABA (symmetric regioisomer). The two product ions [M+Li-R1COOH-R2CH=CHCOOH]+ and [M+Li-R3COOH-R2CH=CHCOOH]+ are of approximately equal abundance and are separated by the difference in the masses of both sn-1/3 fatty acid substituents. For the positional isomers (16:1–18:1–16:1)-TAG and (16:1–16:1–18:1)-TAG, the sn-1/3 fatty acids of the ABA isomer (16:1–18:1–16:1)-TAG are the same. Therefore, the tandem spectrum contains a single ion at m/z 301.28 [M+Li-R1/3COOH-R2CH=CHCOOH]+ (Fig. 3a). For (16:1–16:1–18:1)-TAG (AAB), a pair of ions with the near abundance occur at m/z 329.31 [M+Li-R1COOH-R2CH=CHCOOH]+ and m/z 301.28 [M+Li-R3COOH-R2CH=CHCOOH]+(Fig. 3b).
Tandem mass spectra of lithiated adducts of the positionally isomeric TAG standards. a Tandem spectrum of the lithiated adduct of (16:1–18:1–16:1)-TAG. b Tandem spectrum of its positional isomer (16:1–16:1–18:1)-TAG. c Tandem spectrum of the lithiated adduct of equate mixed (16:1–18:1–16:1)-TAG and (16:1–16:1–18:1)-TAG)
Although RP-HPLC typically cannot separate positional isomers of TAGs (i.e. (16:1–16:1–18:1)-TAG and (16:1–18:1–16:1)-TAG), in Fig. 1, potentially co-eluting positional isomeric TAGs could be distinguished based on the fragment abundances of [M+Li-R1COOH-R2CH=CHCOOH]+ and [M+Li-R3COOH-R2CH=CHCOOH]+ at m/z 301.28 and 329.31 as the intensity difference between [M+Li-R1COOH-R2CH=CHCOOH]+ and [M+Li-R3COOH-R2CH=CHCOOH]+ in mixed positional isomers (AAB and ABA; Fig. 3c) was more obvious than in individual TAG (AAB; Fig. 3b).
Identification of TAGs from the lipid extract of diatom samples
Based on the fragmentation patterns discussed above, many types of TAGs were identified for the first time in these diatoms, including 12 TAGs in C. debilis, 9 TAGs in P. tricornutum Bohlin, 16 TAGs in N. closterium f. minutissima, 16 TAGs in T. weissflogii, 13 TAGs in Thalassiosira sp., 16 TAGs in S. asteaea and 7 TAGs in S. costatum. The dominating TAG structure profiles from seven algae strains have been determined extensively, indicating that the sn-2 position of the glycerol backbone in TAGs carried mainly C16 fatty acids. Based on the information of sn-2 fatty acid obtained so forth, we put forward a hypothesis that TAGs in diatoms are mainly derived from lipids in chloroplasts through the prokaryotic biosynthesis pathway, including monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG; exclusively C16 in the sn-2 position; Xu et al. 2010; Chen et al. 2008; Yan et al. 2011). It is generally accepted that TAGs are end products that do not participate in any known pathway of fatty acid or lipid metabolism (Khozin-Goldberg et al. 2000). The biosynthetic pathway of TAGs containing C16 fatty acids at position sn-2 in diatoms needs further verification. One reason may be that phosphatidylcholine (PtdCho), a proposed intermediate in lipid trafficking between the endoplasmic reticulum (ER) and the plastid in cells (Li-Beisson et al. 2010), is absent from the diatom. The type of acyl group (C16 or C18) present in the sn-2 position of the glycerol backbone is the key distinguishing feature between lipids assembled by the ER pathway and that by the plastid pathway. This is thought to be due to the difference in substrate specificity of acyltransferases at the ER compared to at the plastid envelope. However, it should be noted that this class of enzymes has not been studied universally in microalgae at present (Liu and Benning 2012).
Compared with higher plants, diatom TAGs presented many unique characteristics in acyl component. In vegetable oils, palmitic acid (16:0) and stearic acids (18:0) are typically located at the sn-1/3 positions of TAGs, whilst unsaturated fatty acids and oleic (18:1) and linoleic acids (18:2) are mostly found at the sn-2 position; the uncommon fatty acids are often found at the sn-1/3 position (Zhu et al. 2011). In contrast, animal oils show different distribution patterns, with the saturated fatty acids (palmitic and stearic acids) at all positions (Perona and Ruiz-Gutierrez 2004). TAGs of microalgae presented many similarities in acyl position to that of higher plants. Microalgae TAGs showed high levels of unsaturated fatty acids at the sn-2 position, including 16:1, 16:2, 16:3. Oleic (18:1) and linoleic acids (18:2) are mostly found at the sn-2 position in TAGs of higher plants (Herrera et al. 2010). However, C16 acids account for nearly 100 % of the total fatty acids at the sn-2 position of TAGs in diatoms, which include mainly palmitoleic 16:1 and unsaturated fatty acids C16:2 and C16:3 at a minor content. The sn-1/3 fatty acids of TAGs in microalgae are complex. In addition to C16:0 and C18:0 fatty acids and some unusual fatty acids, C14:0 and C22:6, there are many polyunsaturated fatty acids (20:5, 22:6 and 16:4). The position distribution of fatty acids indicates that the biosynthetic pathways for TAGs in diatoms may be different from those of higher plants.
Semi-quantitative analysis of TAGs in the lipid extract
Evidence suggests (Wang et al. 2010) that storage lipid TAGs are mainly accumulated in the later exponential growth phase and plateau phase. Hence, semi-quantitative analyses of TAGs in diatom samples were carried out during this phase. By use of the modified version of Bligh and Dyer’s method (Bligh and Dyer 1959), total lipids were extracted from 50 mg dry weight of the diatom and the content of individual TAGs ranged from 0.5 ± 0.1 to 217.9 ± 8.1 nmol mg−1 total lipids. In addition, the total lipid content in the diatoms ranged from 143.6 ± 16.3 to 201.1 ± 16.3 mg g−1 dry microalgae, and total TAG contents ranged from 36.8 ± 9.5 to 793.2 ± 54.4 nmol mg−1 total lipids.
Shui et al. (2007) reported that the ion response on ESI-Q-TOF-MS was linear over the biologically relevant ranges for a number of different lipids, and they added internal lipid standards into samples for the semi-quantitative analysis. Xu et al. (2010) performed a similar semi-quantitative analysis of complex lipid mixtures. In this work, the dynamic range/linearity of TAG was determined with the concentration of mixed standards up to 5 nmol mL−1 and showed good linearity. As the effect of the number of carbon atoms in individual lipid species on the quantification with one internal standard is generally not considered in bioactive lipid analysis (Xu et al. 2010; Shui et al. 2007), the results of this paper were also not corrected for variations in response to acyl chain length, although the numbers of carbon atoms in individual TAG species could affect the ionization efficiencies of the corresponding compound (Yang and Han 2011). According to the study of Herrera et al. (2010), if co-eluting positional isomers of TAGs be found in samples, the corresponding TAG standards could be purchased and their calibration curve data derived from [M+Li]+ adducts could be applied to the quantification of the regioisomers.
Thus, the TAGs were semi-quantified in comparison with the internal standards in this study; the total TAG contents in seven diatoms are presented in Table 1. Algal biodiesel production could be improved significantly by increasing the yields of TAGs. The content of (16:1–16:1–16:0)-TAG in N. closterium f. minutissima was 217.9 ± 8.1 nmol mg−1 total lipid, which is the highest TAG content among all diatom samples. In contrast, the content of (16:1–16:2–16:1)-TAG in Thalassiosira sp. was the lowest, with merely 0.5 ± 0.1 nmol mg−1 total lipids (Table 1). Additionally, (20:5–16:3–22:6)-TAG was only detected in S. asteaea, indicating that this kind of particular TAG might be utilized as a biomarker of microalgae chemotaxonomy.
Previous studies suggest that the fatty acid profile of TAGs is an important factor to assess the suitability of microalgae as a biofuel feedstock. Typical oils from microalgae mainly consisted of monounsaturated fatty acids such as palmitoleic (16:1) and oleic (18:1) acids, which exhibit desirable oxidative stability and cold flow properties (Durrett et al. 2008; Meng et al. 2009). In this study, the isolated TAGs contained large amounts of C16 fatty acids, up to 56.9–88.5 % of total fatty acids. These C16 fatty acids consisted of mainly (over 90 %) unsaturated C16 (C16:1 and C16:3), indicating that diatoms are excellent sources of biofuel. Of the diatoms investigated, N. closterium f. minutissima was the most promising microalage for biofuel production based on its high TAG yield and desirable fatty acid profile compared with the other species (Table 2).
In conclusion, the acyl chain at the sn-2 position of the glycerol backbone of TAGs in all the studied diatom species carried mainly C16 fatty acids, suggesting that diatom TAGs were mainly derived from photosynthetic lipids in chloroplasts because only MGDG and DGDG were biosynthesized through the “prokaryotic” pathway within the chloroplast with fatty acid species in the sn-2 position, mainly C16 (Xu et al. 2010; Chen et al. 2008; Yan et al. 2011). Furthermore, the results highlighted the potential advantages of diatoms in biofuel production, in which N. closterium f. minutissima were chosen as the most promising biofuel production organisms based on criteria such as high abundance of TAGs yields containing palmitoleic acid compared with individual species.
References
Basconcillo LS, Zaheer R, Finan TM, McCarry BE (2009) A shotgun lipidomics approach in Sinorhizobium meliloti as a tool in functional genomics. J Lipid Res 50:1120–1132
Beer LL, Boyd ES, Peters JW, Posewitz MC (2009) Engineering algae for biohydrogen and biofuel production. Curr Opin Biotech 20:264–271
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1988) Algal growth media and sources of algal cultures. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 456–465
Bristow AW (2006) Accurate mass measurement for the determination of elemental formula: a tutorial. Mass Spectrom Rev 25:99–111
Chen DY, Xu JL, Yan XJ, Li HY (2008) Analysis of glycolipid compoundby UPLC-Q-TOF-MS method. Chin J Anal Chem 11:54–58
Danielewicz MA, Anderson LA, Franz AK (2011) Triacylglycerol profiling of marine microalgae by mass spectrometry. J Lipid Res 52:2101–2108
Demirbuker M, Blomberg LG, Olsson NU, Bergqvist M, Herslof BG, Jacobs FA (1992) Characterization of triacylglycerols in the seeds of Aquilegia vulgaris by chromatographic and mass spectrometric methods. Lipids 27:436–441
Duffin KL, Henion JD, Shieh JJ (1991) Electrospray and tandem mass spectrometric characterization of acylglycerol mixtures that are dissolved in nonpolar solvents. Anal Chem 63:1781–1788
Durrett TP, Benning C, Ohlrogge J (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54:593–607
Evans N, Games DE, Harwood JL, Jackson AH (1974) Field desorption mass spectrometry of triglycerides and phosphoglycerides. J Biochem Soc Trans 2:1091–1093
Evans C, Traldi P, Bambigiotti-Alberti M, Gianelli V, Coran SA, Vincieri FF (1991) Positive and negative fast atom bombardment mass spectrometry and collision spectroscopy in the structural characterization of mono-, di-, and triglycerides. Biol Mass Spectrom 20:351–356
Evershed RP (1996) High-resolution triacylglycerol mixture analysis using high-temperature gas chromatography/mass spectrometry with a polarizable stationary phase, negative ion chemical ionization, and mass-resolved chromatography. J Am Soc Mass Spectrom 7:350–361
Guedes AC, Meireles LA, Amaro HM, Malcata FX (2010) Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. J Am Oil Chem Soc 87:791–801
Herrera LC, Potvin MA, Melanson JE (2010) Quantitative analysis of positional isomers of triacylglycerols via electrospray ionization tandem mass spectrometry of sodiated adducts. Rapid Commun Mass Spectrom 24:2745–2752
Hiraga Y, Shikano T, Widianti T, Ohkata K (2008) Three new glycolipids with cytolytic activity from cultured marine dinoflagellate Heterocapsa circularisquama. Nat Prod Res 22:649–657
Holguin FO, Schaub T (2013) Characterization of microalgal lipid feedstock by direct-infusion FT-ICR mass spectrometry. Algal Res 2:43–50
Hori M, Sahashi Y, Koike S, Yamaoka R, Sago M (1994) Molecular species analysis of polyunsaturated fish triacylglycerols by high performance liquid chromatography/fast atom bombardment mass spectrometry. Anal Sci 10:719–724
Hsu FF, Turk J (1999) Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 10:587–599
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Jessom LL, Volmer DA (2006) Ion suppression: a major concern in mass spectrometry. LC GC N Am 24:498–510
Kallio H, Rua P (1994) Distribution of the major fatty acids of human milk between sn-2 and sn-1,3 positions of triacylglycerols. J Am Oil Chem Soc 71:985–992
Khozin-Goldberg I, Yu HZ, Adlerstein D, Didi-Cohen S, Heimer YM, Cohen Z (2000) Triacylglycerols of the red microalga porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids 35:881–889
Laakso P, Kallio H (1996) Optimization of the mass spectrometric analysis of triacylglycerols using negative ion chemical ionization with ammonia. Lipids 31:33–42
Lamberto M, Saitta M (1995) Principal component analysis in fast atom bombardment–mass spectrometry of triacylglycerols in edible oils. J Am Oil Chem Soc 72:867–871
Lehmann WD, Kessler M (1983) Characterization and quantification of human plasma lipids from crude lipid extracts by field desorption mass spectrometry. Biomed Mass Spectrom 10:220–226
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Li-Beisson YH, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD et al (2010) The Arabidopsis book. American Society of Plant Biologists, Rockville
Liu B, Benning C (2012) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotech 24:300–309
Macdougall KM, Mcnichol J, Mcginn PJ, O’Leary SJ, Melanson JE (2011) Triacylglycerol profiling of microalgae strains for biofuel feed stock by liquid chromatography high-resolution mass spectrometry. Anal Bioanal Chem 401:2609–2616
Manninen P, Laakso P, Kallio H (1995a) Separation of γ- and α-linolenic acid containing triacylglycerols by capillary supercritical fluid chromatography. Lipids 30:665–671
Manninen P, Laakso P, Kallio H (1995b) Method for characterization of triacylglycerols and fat-soluble vitamins in edible oils and fats by supercritical fluid chromatography. J Am Oil Chem Soc 72:1001–1008
Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M (2009) Biodiesel production from oleaginous microorganisms. Renew Energy 34:1–5
Miao X, Wu Q (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110:85–93
Mottram HR, Woodbury SE, Evershed RP (1997) Identification of triacylglycerol positional isomers present in vegetable oils by high performance liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 11:1240–1252
Murphy RC (1993) Mass spectrometry of lipids. In: Snyder F (ed) Handbook of lipid research. Plenum, New York, pp 213–243
Neff WE, Byrdwell WC (1995) Soybean oil triacylglycerol analysis by reversed phase high performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. J Am Oil Chem Soc 72:1185–1191
Perona JS, Ruiz-Gutierrez V (2004) Analysis of neutral lipids: triacylglycerols. In: Nollet LML (ed) Handbook of food analysis. Marcel Dekker, New York, pp 275–312
Samburova V, Lemos MS, Hiibel S, Hoekman SK, Cushman JC, Zielinska B (2013) Analysis of triacylglycerols and free fatty acids in algae using ultra performance liquid chromatography mass spectrometry. J Am Oil Chem Soc 90:53–64
Shui G, Bendt AK, Pethe K, Dick T, Wenk MR (2007) Sensitive profiling of chemically diverse bioactive lipids. J Lipid Res 48:1976–1984
Sundin P, Larsson P, Wesen C, Odham G (1992) Chlorinated triacylglycerols in fish lipids: chromatographic and mass spectrometric studies of model compounds. Biol Mass Spectrom 21:633–641
Sushchik N, Gladyshev M, Ivanova E, Kravchuk E (2010) Seasonal distribution and fatty acid composition of littoral microalgae in the Yenisei River. J Appl Phycol 22:11–24
Wang JN, Yan XJ, Zhou CX, Xu JL (2010) Screening of oil-producing microalgae and detecting dynamics of neutral lipid accumulation. Acta Biophysica Sinica 26:472–480
Xu JL, Chen DY, Yan XJ, Li HY (2009) Ultra performance liquid chromatography–quadrupole-time of flight mass spectrometric analysis of photosynthetic glycolipids mixture. Chin J Anal Chem 4:511–516
Xu JL, Chen DY, Yan XJ, Chen JJ, Zhou CX (2010) Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization–quadrupole-time of flight mass spectrometry. Anal Chim Acta 663:60–68
Yan XJ, Chen DY, Xu JL, Zhou CX (2011) Profiles of photosynthetic glycerolipids in three strains of Skeletonema determined by UPLC-Q-TOF-MS. J Appl Phycol 23:271–282
Yang K, Han XL (2011) Accurate quantification of lipid species by electrospray ionization mass spectrometry—meets a key challenge in lipidomics. Metabolites 1:21–40
Zhu TH, Fan L, Qian XM, Wang Y, Jing YC (2011) A review of analyzing triacylglycerols profiling in plant oils based on HPLC analysis. China Oils and Fats 36:59–63
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31172448), National Key Technology R&D Program, China (2011BAD13B08), Zhejiang Natural Science Foundation, China (Y3100534 and Z3100565), Ningbo Science and Technology Research Projects, China (2011C11003), Zhejiang Marine Biotechnology Innovation Team, China (2012R10029), and Ningbo Marine Algae Biotechnology Team, China (2011B81007) and partly sponsored by the K. C. Wong Magna Fund in Ningbo University, Renewable Energy Project of National Ocean Bureau China, GHME2001SW02, and Zhejiang Science and Technology Team (2009R50012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Xu, J., Chen, J. et al. Characterization of the triacylglycerol profile in marine diatoms by ultra performance liquid chromatography coupled with electrospray ionization–quadrupole time-of-flight mass spectrometry. J Appl Phycol 26, 1389–1398 (2014). https://doi.org/10.1007/s10811-013-0159-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0159-4