Abstract
The paper presents the ultra-performance liquid chromatography (UPLC) and high-resolution mass spectrometric analysis and comparison of total lipid profiles of two green algal species, Chlamydomonas reinhardtii and Scenedesmus (Acutodesmus) obliquus. The targeted UPLC-mass spectroscopy (MS) analysis revealed that both the green algae showed the presence of almost similar types of lipids. However, there were differences in the presence of three triacylglycerol (TAG) species (TAG 54:4, TAG 54:5 and TAG 54:8) and two diacylglycerol (DAG) species (DAG 36:3 and DAG 36:4) in C. reinhardtii that were found to be completely absent in Scenedesmus obliquus. The triacylglycerol content in S. obliquus was five times more than that in C. reinhardtii. In addition, amount of diacylglycerol-O-(N,N,N-trimethyl) homoserine, a characteristic algal lipid, in S. obliquus was only half of that in C. reinhardtii. The paper also discusses the metabolic roles of the lipids produced by these algal species with reference to the lipids identified by UPLC-MS analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fossil fuel reserves of the world are depleting fast whereas the demand for energy is increasing. Biomass, a renewable energy source, can replace the role played by fossil fuels not only in supplying fuels but also even in supplying the feedstock for chemical and petroleum industries. The world has moved to biomass for the supply of biofuels (Hu et al. 2008; Deeba et al. 2012). However, recent food-versus-fuel controversy has turned the attention of the scientific community towards algae for the supply of third-generation biofuels (Chisti 2007; Kumar et al. 2013; Gautam et al. 2013). Microalgae are responsible for the production of 40 % of the total organic matter of 2 × 1011 t year−1 by utilizing 40 % of the total of 8 × 1010 t of photosynthetically fixed carbon dioxide (Ravishankar and Sarada 2007). Algae are also responsible for the production of at least 50 % oxygen in the atmosphere (Anderson 2005). As algae are considered a promising target for obtaining lipids, there is a need to understand their lipid metabolism for the possibility of extending research in enhancing their lipid production. Another important aspect is the photosynthetic efficiency of algae, which surpasses that of flowering plants and results in sequestration of much more atmospheric carbon dioxide (Sayre 2010). Pioneering work on algae was initiated by the US Department of Energy (USDOE) through the Aquatic Species Program (ASP). Under this programme, 2000 algae strains were screened to select 300 potential strains that produced higher amount of lipids (Sheehan et al. 1998). These mainly included algae from Chlorophyceae and Bacillariophyceae (green algae and diatoms, respectively).
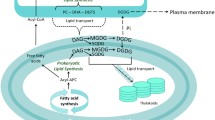
Algae produce a wide range of lipids, which are either polar (membrane) or neutral (storage) lipids. Neutral lipids in algae are mainly composed of monoacylglycerol (MAG), diacylglycerol (DAG) and triacylglycerol (TAG). Algal chloroplasts have monogalactosyldiacylglycerols (MGDG) as their main lipid, with smaller amounts of digalactosyldiacylglycerols (DGDG) and the two negatively charged lipids, sulfoquinovosyldiacylglycerols (SQDG) and phosphatidylglycerols (PG) (Canto de Loura et al. 1987). Algal thylakoid membranes have a lipid composition similar to those in higher plants and higher bacteria. Thus, amount of MGDG, DGDG, SQDG and PG will probably be 40–55, 15–35, 10–20 and 10–20 %, respectively (Harwood 1998; Harwood and Guschina 2009). Diacylglycerol-O-(N,N,N-trimethyl) homoserine (DGTS) is a characteristic lipid of algae, bacteria and some spore-forming plants (Schlapfer and Eichenberger 1983). DGTS, although not a phospholipid, appears to completely replace phosphatidylcholine in Chlamydomonas reinhardtii (Schlapfer and Eichenberger 1983; Giroud et al. 1989). In the Phaeophyceae (brown algae), DGTS can be present in the form of its β-alanine analogue (Vogel and Eichenberger 1992; Guschina and Harwood 2006).
Lipids, apart from playing a structural role in the membranes, have also been known to function as signals, activators of enzymes and interaction molecules with signaling molecules (Mashburn-Warren et al. 2008). Thus, they have an important role in controlling and directing different metabolic networks in algae. There exists a wide scope of manipulating the algal metabolic systems by exploiting the use of the signaling molecules. Therefore, it would be important to study the presence of these lipid molecules in algae through lipidomics research.
Lipid analysis in photosynthetic organisms poses a challenge due to the presence of many types of pigments present in them. In addition, many glycerolipids, phospholipids and neutral lipids may also be present in saturated or unsaturated forms (Seiwert et al. 2010). This makes algal and plant lipids much more diverse as compared to the other animal lipids. Thus, no single technique has been able to detect as well as quantify all different kinds of lipids that may be present in the photosynthetic organisms. Hence, there is a need to use different lipid analysis techniques and integrate them for analysis of such diverse lipids.
Chromatographic techniques such as thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) have been reported to be the most important and useful techniques for lipid analysis (Seiwert et al. 2010). However, TLC is considered to be a less reproducible, time consuming and labour intensive process. On the other hand, HPLC offers the possibility of full automation and higher speed, resolution, sensitivity and specificity (Lima and Abdalla 2002). Another benefit of HPLC is that fractions containing single lipid classes can be easily collected for detailed mass spectroscopy (MS)-based analysis or identification of molecular species. Seiwert et al. (2010) have analyzed the MGDG fraction from lipids of C. reinhardtii using HPLC-electrospray ionization/mass spectroscopy (ESI/MS). The strain used for the study contained 14 different types MGDG species, all of which contained either 34 or 36 carbon atoms. The unsaturations in these species ranged from two to eight. In another study, Vieler et al. (2007) analyzed and compared lipids from C. reinhardtii and Cyclotella meneghiniana by analyzing the same using matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) and TLC. The results showed the detection and identification of at least 49 lipid classes. It was seen that TLC could be successfully used for detecting the presence of different lipid classes in the two algal species such as DGTS and phosphatidylcholine (PC). Hence, TLC may be used for a basic qualitative study of the lipids; however, for high throughput analysis, use of other advanced techniques like MALDI and HPLC equipped with MS should be preferred.
Most of the advanced lipid analysis techniques detect and identify different lipid species by forming adducts with various positive and negative ions such as H+, Na+, NH4 +, etc. In a project report by Nevada Desert Research Institute (2011), 18 different strains of Dunaliella were selected for lipid extraction and analysis. Lipids were extracted using hexane-dichloromethane solvent system and the TAGs were analyzed using ultra-high-performance liquid chromatography-mass spectroscopy (UPLC-MS). TAGs were eluted and detected as ammonium adducts. The analysis results for TAG were obtained in the form of the three acyl chains involved in the formation of TAG molecule. The TAGs ranged from 50 to 56 carbon atoms. The fatty acids involved in the formation of TAG included 16:0, 16:1, 16:3, 16:4, 18:0, 18:1, 18:2, 18:3 and 22:0. In another study, glycolipids from Chaetoceros, a marine diatom, were analyzed using electrospray ionization-quadrupole ion trap mass spectrometry (ESI-QITMS). The analysis could identify 11 different types of DGDG and 10 types of MGDG (Guella et al. 2003). Photosynthetic polar glycerolipids in the microalga Tetraselmis chuii were analyzed using ultra-performance liquid chromatography-electrospray ionization-quadrupole-time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS) by direct analysis of the total lipids extract. As a result, more than 40 lipid molecular species, including 11 MGDG species, 7 DGDG species, 16 SQDG species and 9 PG species, were detected in T. chuii (Li et al. 2008). Reverse-phase liquid chromatography electrospray ionization mass spectrometry method has also been used. It was found to be a better technique for the detection of phospholipids such as phosphatidic acid and phosphatidylserine (Ogiso et al. 2008). He et al. (2011) used liquid chromatography-mass spectrometry (LC-MS) followed by electrospray ionization and mass analysis with a linear ion trap (LTQ) coupled with 14.5T Fourier transform ion cyclotron resonance mass spectrometry (FT-ICRMS) for the first time for analysis of polar lipids in Nannochloropsis oculata. About 20 polar lipids including fatty acids were identified in this analysis. Membrane lipids such as MGDG, DGDG, DGMG, SQDG, PG, LysoPG, DGTS, PE, PC, monogalactosyl trimethyl homoserine (MGTS), sulfoquinovosylmonoacylglycerol (SQMG) and inositol phosphorylceramide (IPC) were detected, out of which SQDG was detected with the highest intensity followed by IPC.
Recently, techniques like nuclear magnetic resonance (NMR) spectroscopy and Fourier transform infrared (FTIR) spectroscopy have also been used for analysis and quantification of lipids in stressed and non-stressed cells (Beal et al. 2010; Laurens and Wolfrum 2011). A more complete analysis was reported by Jones et al. (2012) wherein Chlorella lipids were analyzed using HPLC-MS. The technique could analyze the presence of polar as well as nonpolar lipids. However, the study lacked a detailed analysis in terms of the carbon number, number of unsaturations, types of fatty acids involved in the formation of different lipid species and their corresponding intensities. There has been a dearth of complete information in terms of a single technique being able to analyze all possible classes of lipids in an alga. The use of different techniques only for detection of a particular class of lipids may not result in correct quantification of that fraction in the total lipid sample.
In the present study, the authors have reported the UPLC-MS analysis of the total lipid sample of two algae species C. reinhardtii and Scenedesmus obliquus. The genomic information of C. reinhardtii is known, and S. obliquus seems to be a potential alga for biodiesel production. Therefore, a comparative analysis of their lipids could be of potential interest. The identification of lipid species is based on accurate measurement of mass values. Such an analysis of the total lipids of S. obliquus has not been reported earlier and this work is the first report of its comparison with that of C. reinhardtii. The metabolic/signaling roles of lipids so identified by UPLC-MS analysis have also been discussed.
Materials and methods
S. obliquus 276-1 (the currently accepted name is Acutodesmus obliquus; Hegewald and Hanagata 2000) was procured from EPSAG culture collection maintained by the University of Göttingen, Germany. C. reinhardtii strain was kindly donated by Dr. Laurent Cournac, FEA, France. S. obliquus and C. reinhardtii were grown in BG–11 medium (pH 8) (Stanier et al. 1971) and TAP medium (pH 7.2) (Gorman and Levine 1965), respectively. The cultures were grown at 25–28 °C with continuous shaking at 80–100 rpm. The light intensity was maintained at 34–36 μmol photons m−2 s−1 with a 16:8 h light and dark photoperiod. Inoculum of 10 % (v/v) of fresh exponentially growing cells was used to inoculate 200 mL culture medium. The optical density of the primary culture used for inocula at 650 nm was equal to 1. The cells were harvested in the late exponential phase after 7–8 days of growth.
Lipid extraction and ultra-performance liquid chromatography and high-resolution mass spectrometric analysis
The biomass concentration in the algal culture used for lipid analysis during the late exponential phase was 0.27 and 0.33 g L−1 in case of C. reinhardtii and S. obliquus, respectively. Lipids of a packed algal cell pellet of approximately 1 mL of culture were extracted from three independent biological replicates using 1 mL methanol:methyl tertiary butyl ether (1:3) mixture (Hummel et al. 2011). For the phase separation of polar and lipophilic compounds, 0.5 mL of water:methanol were added, which resulted in an upper organic phase, containing the lipids, and a lower phase containing the polar and semipolar metabolites as described in Hummel et al. (2011). The mass spectrometric analysis of lipids was carried out using a UPLC system (Waters Acquity) equipped with C8 reversed phase column (100 mm × 2.1 mm × 1.7 μm particles, Waters) coupled to a high-resolution mass spectrometer (Exactive, Thermo-Fisher, Germany) as previously described in Hummel et al. (2011). The data analysis of the lipids was performed in a targeted way by using the manually annotated retention times of the lipids. Peak intensities were then extracted from the three replicates using the ToxID software package (Thermo-Fisher). By using this method, we accepted that several hundred peaks remained unannotated and unanalyzed thus providing a far unused resource of information. The individual peak intensities were added to give the total intensity. The relative intensity was calculated for each peak as a percent of total intensity and is shown in the form of bar graphs. The amount of each lipid fraction is given as “per total ion intensity of all annotated lipid peaks” (/TIAL).
Lipid extraction for GC-MS analysis
Standard protocol was used to extract oil from the lyophilized algal biomass using chloroform:methanol (2:1) solvents (Bligh and Dyer 1959). About 10–20 mg of lipid sample was transesterified using 25 mL of 2 % sulphuric acid prepared in methanol at 65–70 °C for 5–6 h. The fatty acid methyl esters (FAME) were extracted using ethyl acetate as described by Gautam et al. (2013). The extract was passed over a bed of anhydrous sodium sulphate to remove moisture. FAME were analyzed by GC (Shimadzu GC-2010) equipped with FID using SP–2560 capillary column (100 m × 0.25 mm 1D 0.20 μm film). The temperature programming was adjusted to 140 to 240 °C at a rate of 4 °C min−1. The FAME(s) were identified by comparing their fragmentation pattern with a 37-component standard (Sigma). It has been known from earlier studies that the two lipid extraction procedures, i.e. methyl tert-butyl ether and chloroform-methanol, give similar results (Matyash et al. 2008).
Results
Analysis of lipids of C. reinhardtii and S. obliquus and their comparison
The UPLC-MS method described in the present study has been used for identification and quantification of both polar and nonpolar lipids for the first time. Analysis of the two algal species revealed varied lipid patterns. The representative chromatograms (positive mode) for the UPLC-MS analysis for C. reinhardtii and S. obliquus are shown in Fig. 1. It was observed that polar glycerol lipids eluted from the column between 5 and 12 min, followed by triacylglycerol which eluted between 12 and 17 min. All the lipid classes eluted from the column within 16 min of sample injection. The peaks in the chromatograms show the intensity of each lipid fraction. Fig. 2 shows the distribution of lipid classes obtained by the UPLC-MS analysis. In this analysis, at least 8 different lipid classes and 83 lipid subclasses were observed. The lipid classes include chlorophyll, DGDG, MGDG, DGTS, TAG, DAG, PG and phaeophytin.
Representative chromatograms (positive mode) obtained from the UPLC-MS analysis of lipids of C. reinhardtii (upper) and S. obliquus (lower) shows the resolution of various lipid classes between 0 and 20 min. The chromatogram shows the elution of polar glycerol lipids between 5 and 12 min followed by triacylglycerols between 12 and 17 min
A comparison of the lipid profiles of the two algae shows that five lipid species, i.e. DAG 36:3, DAG 36:4, TAG 54:4, TAG 54:5 and TAG 54:8, were completely absent in S. obliquus as compared to that of C. reinhardtii (Table 1). The amount of total DAG in both the algae was more or less similar. However, the amount of TAG varied drastically from 6.39/TIAL in C. reinhardtii to 33.04/TIAL in S. obliquus, the latter over five times more than the former (Fig. 1 and 3). It was also observed that 36-carbon-atom DAGs were found to be completely absent in S. obliquus. This was indicative of the absence of another cycle of fatty acid elongation pathway governed by Ketoacyl ACP Synthases I and II, which is responsible for production of fatty acids with 6–16 and >16 carbon atoms, respectively. Generally, carbon number of TAGs ranged from 48 to 54, with 1–9 unsaturations. DAG, however, was present in 34 and 36 carbon atom range, wherein a 36-carbon-atom species was present with higher unsaturations (Fig. 4). The DAG species eluted from 6.87 to 10 min. However, TAGs were among the last ones to elute from 13.6 to 15.31 min. TAGs and DAGs were mainly eluted as [M + NH4]+ and [M + Na]+ ions. DAG 34:6 was eluted in the form of sodium adduct. DAG 36:2 in C. reinhardtii and DAG 36:3 and DAG 36:4 in S. obliquus remained undetected in the form of adducts. Both the algae species produce all the TAG with 48 to 54 carbon atoms. However, 4, 5 and 8 unsaturations of TAG 54 were absent in S. obliquus. The enzyme thioesterase introduces the unsaturations in the fatty acyl chains and the above result demonstrates its lower activity or turnover number in S. obliquus.
Distribution of various species of TAG in Chlamydomonas reinhardtii and Scenedesmus obliquus. The analysis was carried out in triplicates using three independent culture samples. The percentage on the basis of per total ion intensity of all annotated lipid peaks (/TIAL) is shown as an average of triplicates
Distribution of various species of DAGs in Chlamydomonas reinhardtii and Scenedesmus obliquus. The analysis was carried out in triplicates using three independent culture samples. The percentage on the basis of per total ion intensity of all annotated lipid peaks (/TIAL) is shown as an average of triplicates
The amount of the cellular pigments such as phaeophytin, chlorophyll a and chlorophyll b was comparable in the two algal species. However, the membrane lipids existed in variable amounts. Chlorophylls and phaeophytin were both detected as [M + H]+ and [M + Na]+ ions. DGTS was also detected in the form of [M + H]+ and [M + Na]+ ions. 32:3, 34:3 and 34:4 DGTS species were also detected as [M + NH4]+ ion in addition to [M + H]+ and [M + Na]+.
The amounts of DGTS and PG in C. reinhardtii were approximately double of that in S. obliquus. DGTS was present in the form of 34–36 carbon atoms with one to six unsaturations (Fig. 5). In both the algae species, C34 fraction was present in maximum amount, wherein C34:3 and C34:4 fractions were the most dominant. DGTS elution started with C32:5 at 6.21 min till 9.47 min, when C36:2 were eluted from the column. DGTS, however, replaces PC completely in C. reinhardtii, and thus, PC was not detected in its lipid sample. S. obliquus was also found to have DGTS, while PC was absent in its lipid sample. This signifies the presence of DGTS synthetase (BTA1Cr) enzyme responsible for the synthesis of DGTS in the two algae. The difference in the amounts of DGTS could be attributed to genetic phenomena specific to the algal strain or species. Membrane lipid MGDG eluted between 7.3 and 9.4 min and was represented by 34:1, 34:2, 34:3 and 34:4 carbon atom fractions. Amount of MGDG was comparatively more in case of S. obliquus. MGDG and DGDG were detected as [M + NH4]+ and [M + Na]+ ion adducts (Fig. 6). In case of the highly abundant DGDG, 34:1, 34:3, 34:4 and 36:5 adducts were also detected as [M + H]+ ion. A similar analysis on the MGDG class of C. reinhardtii was carried out by Seiwert et al. (2010). The analysis showed the chromatographic separation of 14 MGDG species. However, the present analysis carried out on the total analysis of C. reinhardtii lipids could identify only 4 MGDG species. The elution of the first MGDG (32:7) from the study by Seiwert and colleagues (2010) started at around 6 min and continued till about 19.7 min. The 14 fractions included 32:5, 32:6, 32:7, 34:2, 34:3, 34:4, 34:5, 34:6, 34:7, 34:8, 36:4, 36:5, 36:6 and 36:7. 34:5 and 34:6 were detected with highest intensity, while many fractions had negligible intensities. The MGDG species in this study was detected with sodium ions and eluted between 7.3 and 9.5 min. The percent relative intensity (/TIAL) of 34:4 MGDG fraction was detected to be maximum in both C. reinhardtii and S. obliquus. The concentration of DGDG was around 16/TIAL in the two algae. DGDG species were represented by 32, 34 and 36 carbon atoms. The 32 carbon species was present with lower unsaturations, the 36 carbon species with higher unsaturations and the 34-carbon species with one to six unsaturations. DGDG first eluted at 5.62 min with 34:6 species and ended with 34:0 at 9.49 min.
Distribution of various species of DGTS in Chlamydomonas reinhardtii and Scenedesmus obliquus. The analysis was carried out in triplicates using three independent culture samples. The percentage on the basis of per total ion intensity of all annotated lipid peaks (/TIAL) is shown as an average of triplicates
Distribution of various species of PG (a), MGDG (b) and DGDG (c) in Chlamydomonas reinhardtii and Scenedesmus obliquus. The analysis was carried out in triplicates using three independent culture samples. The percentage on the basis of per total ion intensity of all annotated lipid peaks (/TIAL) is shown as an average of triplicates
All the PG eluted from the column between 6 and 7 min, containing 32:0, 32:1, 34:1, 34:2, 34:3 and 34:4 lipid species. PG was detected as all three [M + H]+, [M + NH4]+ and [M + Na]+ ion adducts. PG was present in very low concentrations in both the algal classes, reflecting a value of 2.07/TIAL in C. reinhardtii and 1.2/TIAL in S. obliquus. In both the algal species, the amount of chla was about 6/TIAL and chlb was about 1.8/TIAL. Phaeophytin is a breakdown product of chlorophyll and lacks the central magnesium ion. It is present in C. reinhardtii and S. obliquus in 6/TIAL and 8.2/TIAL, respectively (refer to Fig. 7). The presence of other glycerolipids like SQDG and phospholipids such as PE, PI and PS were not detected in the lipid analysis results, which could be due to the strictly targeted peak extraction procedure used.
Distribution of chlorophylls (a) and phaeophytin (b) in Chlamydomonas reinhardtii and Scenedesmus obliquus. The analysis was carried out in triplicates using three independent culture samples. The percentage on the basis of per total ion intensity of all annotated lipid peaks (/TIAL) is shown as an average of triplicates
Algal fatty acids showed maximum percentage of palmitic, oleic and linoleic fatty acids. Docosahexaenoic was not detected at all in the fatty acid sample of C. reinhardtii. In the algal strains, myristic acid, hexadecadienoic acid, hexadecatrienoic acid and behenic acid were found in traces. PUFAs are present in small amounts as compared to mono- and di-unsaturated fatty acids as shown in Table 2.
Discussion
In this study, the total lipid extracts of C. reinhardtii and S. obliquus have been analyzed using a UPLC-MS-based method. It was thus found that UPLC is an effective method for determination lipid profile in algae. The method can be used for determination of single (targeted analysis) lipid or all (untargeted analysis) the lipid species present in algae. Lipids identified in the present analysis play many important metabolic roles which are as follows.
Lipids apart from having a structural role are also involved in different pathways and their activation by acting as secondary messengers and signaling molecules. All lipid components especially DAG and phospholipids are key signaling molecules that are involved in switching of the various metabolic pathways.
DGDG serves as markers for cellular recognition because of their association with cell membranes (Holdt and Kraan 2011). DGDG is also required for better photosynthetic growth of Synechocystis under phosphate limitation (Awai et al. 2007) as it is involved in the structural organization of photosynthetic apparatus (Sato et al. 2000).
UPLC-MS analysis of S. obliquus total lipid sample detected about 1.2/TIAL of PG. Phospholipids act as important signal precursors or signaling molecules (Munnik et al. 1998; Munnik and Testerink 2009). Phospholipids in plants affect the hormone levels of auxin and abscisic acid (ABA) which regulates the root/shoot elongation and stress responses for stomatal opening and closure, respectively (Xue et al. 2007). They also promote vesicle trafficking, membrane recycling and secretion. PG helps in regeneration of vitamin E in Spirulina maxima (Miranda et al. 1998).
PG is a very important molecule responsible for driving many pathways. Being a signaling molecule, it is required in small quantities in the cell. Thus, it was present in small amounts in the algal cell. PG plays a very essential role in photosynthesis. Biological membranes have a specific protein and lipid composition. The membrane lipids form a barrier between the inside and the outside of the membranes, supply the membrane proteins with sites for their action, and regulate the functions of the membrane proteins. The X-ray crystallographic studies of protein complexes that are involved in photosynthesis have revealed that PG is present in both photosystem I (PSI) (Jordan et al. 2001) and photosystem II (PSII) protein complexes (Loll et al. 2005) and can be found, also, in the light-harvesting complex (LHC) attached to the PSII core complex (Liu et al. 2004). PG is also found to interact with PSI which results in reduction in intensity of chlorophyll a peak bands which further improve the absorption of light by these photosynthetic pigments. Chlorophylls and phaeophytin are pigments which are also lipids in nature. Thus, present solvent extraction method results in their extraction also.
In the present analysis of C. reinhardtii and S. obliquus, lipid samples of both the species contained DGTS. As already known in case of C. reinhardtii, the entire PC is replaced by DGTS, and thus, it contained over 60 % DGTS. Incorporation of positively charged DGTS lipid instead of phospholipids, which carry negatively charged phosphate groups, will influence the net charge of the cell envelope and could influence cell surface interactions (Danhorn et al. 2004).
DAG is a neutral lipid which contains two chains of fatty acid bound to a glycerol molecule. DAG was found to be present in very small quantities in both C. reinhardtii (0.16/TIAL) and S. obliquus (0.13/TIAL) in the present study. DAG is considered to be a secondary messenger and helps in activation of protein kinase C and significantly reduces farnesol-induced apoptosis (Nickerson et al. 2006). Effect of farnesol especially in germ tube formation or inhibiting growth is reversed by DAG or 1-oleoyl-2 acetyl-sn-glycerol (Langford 2010). It has also been observed that under conditions which inhibit phospholipid biosynthesis, DAG which is predestined to form phospholipid is then utilized for formation of TAG (Jackowski et al. 2000). DAG is also involved in the activation of some biotic/abiotic stress responses thus helping in signaling as a response to stress (Arisz et al. 2009).
TAG is one of the most important molecules for biodiesel production. Amount of TAG was detected to be 6.39/TIAL in C. reinhardtii and 32.04/TIAL in S. obliquus. TAG synthesis is involved in the regulation of DAG and fatty acids which are involved in signal transduction and TAG mobilization is mediated by cAMP cascade-dependent mobilization and acts as a source of fatty acid for phospholipid biosynthesis (Ducharme and Bickel 2008). TAGs are involved in the regulation of many steps in membrane trafficking, vesicular formation and transport along actin and tubulin networks and membrane fusion (Yu et al. 2011).
Based on the above study, the lipidomics data obtained for both the algae showed variations in terms of absence of five lipid species in S. obliquus. It contains a much higher TAG content, i.e. almost five times greater than that of C. reinhardtii, however, lacked three TAG species. This is indicative of the fact that either some of the fatty acid elongation genes responsible for synthesis of long chain fatty acids and their unsaturation are less active. The other reason could be that the activation of a feedback inhibition reaction changes the regulatory mechanisms in S. obliquus leading to this result. Longer chain TAGs indeed elute later from the column and longer fatty acid chain lipids could also be detected. However, a more targeted than complete analysis of this data was performed in this study. The TAGs with summed chain length up to 62 carbons in their fatty acids were also targeted, however, since these are not very abundant they were not included in the target list. Thus, TAGs with a summed fatty acid chain length up to 54 carbons were included in the target list as these are the most abundant.
On the basis of the literature available on the possible combinations of fatty acid for the five TAG and DAG species, the following combinations of fatty acids may be present in these species. In case of TAG 54:4, TAG 54:5 and TAG 54:8, the possible combinations of fatty acids are [18:0, 18:0, 18:4], [(18:1, 18:0, 18:4)/(18:0, 18:0, 18:5)] and [(18:4, 18:0, 18:4)/(18:2, 18:3, 18:3)], respectively. These fatty acids may not be favoured for good biodiesel quality due to the presence of high unsaturation. Similarly, in case of DAG, both fractions, i.e. DAG 35:3 and DAG 35:4, involve the presence of undesirable fatty acid, i.e. [15:0/20:3] and [15:0/20:4], respectively, due to the presence of C15:0 which is an odd number carbon. Thus, the absence of these TAG and DAG species and high TAG content renders S. obliquus more suitable for biodiesel production. The present study clearly demonstrates how powerful lipidomics-based research in algae can be to address major biological questions.
References
Anderson RA (2005) Algal culturing techniques. Elsevier Academic Press, London
Arisz SA, Testerink C, Munnik T (2009) Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta 1791:869–875
Awai K, Watanabe H, Benning C, Nishida I (2007) Digalactosyldiacylglycerol is required for better photosynthetic growth of Synechocystis sp. PCC6803 under phosphate limitation. Plant Cell Physiol 48:1517–1523
Beal CM, Webber ME, Ruoff RS, Hebner RE (2010) Lipid analysis of Neochloris oleoabundans by liquid state NMR. Biotechnol Bioeng 106:573–583
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Phys 37:911–917
Canto de Loura I, Duabacq JP, Thomas JC (1987) The effects of nitrogen deficiency on pigments and lipids of cyanobacteria. Plant Physiol 83:838–843
Chisti Y (2007) Biodiesel from algae. Biotechnol Adv 25:294–306
Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C (2004) Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J Bacteriol 186:4492–4501
Deeba F, Kumar V, Gautam K, Saxena RK, Sharma DK (2012) Bioprocessing of Jatropha curcas seed oil and deoiled seed hulls for the production of biodiesel and biogas. Biomass Bioenerg 40:13–18
Ducharme NA, Bickel PE (2008) Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinol 149:942–949
Gautam K, Pareek A, Sharma DK (2013) Biochemical composition of green alga Chlorella minutissima in mixotrophic cultures under the effect of different carbon sources. J Biosci Bioeng 116:624–627
Giroud C, Gerber A, Eichenberger W (1989) Lipids of Chlamydomonas reinhardtii: analysis of molecular species and intracellular site(s) of biosynthesis. Plant Cell Physiol 29:587–595
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 54:1665–1669
Guella G, Frassanito R, Mancini I (2003) A new solution for an old problem: the regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass Spectrometry. Rapid Commun Mass Spectrom 17:1982–1994
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Harwood JL (1998) Membrane lipids in algae. In: Siegenthaler P-A, Murata N (eds) Lipids in photosynthesis: structure, function and genetics. Springer, Netherlands, pp 53–64
Harwood JL, Guschina IA (2009) The versatility of algae and their lipid metabolism. Biochimie 91:679–684
He H, Rodgers RP, Marshall AG, Hsu CS (2011) Algae polar lipids characterized by online liquid chromatography coupled with hybrid linear quadrupole ion trap/Fourier transform ion cyclotron resonance mass spectrometry. Energ Fuels 25:4770–4775
Hegewald E, Hanagata N (2000) Phylogenetic studies on Scenedesmaceae (Chlorophyta). Algol Stud/Arch Hydrobiol Suppl 100:29–49
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Hummel J, Segu S, Li Y, Irgang S, Jueppner J, Giavalisco P (2011) Ultra performance liquid chromatography and high resolution mass spectrometry for the analysis of plant lipids. Front Plant Sc 2:1–17
Jackowski S, Wang J, Baburina I (2000) Activity of the phosphatidylcholine biosynthetic pathway modulates the distribution of fatty acids into glycerolipids in proliferating cells. Biochim Biophys Acta 1483:301–315
Jones J, Manning S, Montoya M, Keller K, Poenie M (2012) Extraction of algal lipids and their analysis by HPLC and mass spectrometry. J Am Oil Chem Soc 89:1371–1381
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 41:909–917
Kumar G, Srivastava R, Singh R (2013) Exploring biodiesel: chemistry, biochemistry, and microalgal source. Intl J Green Energ 10:775–796
Langford ML (2010) Farnesol signaling in Candida albicans. PhD Thesis, University of Nebraska – Lincoln, USA
Laurens LML, Wolfrum EJ (2011) Feasibility of spectroscopic characterization of algal lipids: chemometric correlation of NIR and FTIR spectra with exogenous lipids in algal biomass. Bioenerg Res 4:22–35
Li H, Yan X, Xu J, Zhou C (2008) Precise identification of photosynthetic glycerolipids in microalga Tetraselmis chuii by UPLC-ESI-Q-TOF-MS. Sci China C Life Sci 51:1101–1107
Lima ES, Abdalla DSP (2002) High-performance liquid chromatography of fatty acids in biological samples. Anal Chim Acta 465:81–91
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438:1040–1044
Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, Brandenburg K, Whiteley M (2008) Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol Microbiol 69:491–502
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D (2008) Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 49:1137–1146
Miranda MS, Cintra RG, Barros SBM, Mancini-Filho J (1998) Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res 31:1075–1079
Munnik T, Testerink C (2009) Plant phospholipid signaling-in a nutshell. J Lipid Res 50:260–265
Munnik T, Irvine RF, Musgrave A (1998) Phospholipid signalling in plants. Biochim Biophys Acta 1389:222–272
Nevada Renewable Energy Consortium (2011) Algal-based biofuels, final report, subtask 1.3. Desert Research Institute, Reno, p 38
Nickerson KW, Atkin AL, Hornby JM (2006) Quorum sensing in dimorphic fungi: farnesol and beyond. Appl Environ Microbiol 72:3805–3813
Ogiso H, Suzuki T, Taguchi R (2008) Development of a reverse-phase liquid chromatography electrospray ionization mass spectrometry method for lipidomics, improving detection of phosphatidic acid and phosphatidylserine. Anal Biochem 375:124–131
Ravishankar GA, Sarada R (2007) Proc. Discussion meeting on energy biosciences, Department of Biotechnology. Minist Sci Technol
Sato N, Hagio M, Wada H, Tsuzuki M (2000) Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci 97:10655–10660
Sayre R (2010) Microalgae: the potential for carbon capture. Bioscience 60:724–727
Schlapfer P, Eichenberger W (1983) Evidence for the involvement of diacylglyceryl(N, N, N-trimethyl)-homoserine in the desaturation of oleic and linoleic acids in Chlamydomonas reinhardtii (Chlorophyceae). Plant Sci 32:243–252
Seiwert B, Giavalisco P, Willmitzer L (2010) Advanced mass spectrometry methods for analysis of lipids from photosynthetic organisms. In: Wada M, Murata N (eds) Lipids in photosynthesis: essential and regulatory functions. Springer, Dordrecht, pp 445–461
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae by the National Renewable Energy Laboratory. Report NREL/TP-580-24190, National Renewable Energy Laboratory, Golden
Stanier RY, Kunisawa R, Mandel M, CohenBazire G (1971) Purification and properties of unicellular bluegreen algae (order Chroococcales). Bacteriol Rev 35:171–205
Vieler A, Wilhelm C, Goss R, Suß R, Schiller J (2007) The lipid composition of the unicellular green alga Chlamydomonas reinhardtii and the diatom Cyclotella meneghiniana investigated by MALDI-TOF MS and TLC. Chem Phys Lipids 150:143–155
Vogel G, Eichenberger W (1992) Betaine lipids in lower plants. Biosynthesis of DGTS and DGTA in Ochromonas danica (Chrysophyceae) and the possible role of DGTS in lipid metabolism. Plant Cell Physiol 33:427–436
Xue H, Chen X, Li G (2007) Involvement of phospholipid signaling in plant growth and hormone effects. Curr Opin Plant Biol 10:483–489
Yu WL, William A, Schoepp NG, Hannon MJ, Mayfield SP, Burkart MD (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Fact 10:91–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, D.K., Gautam, K., Jueppner, J. et al. UPLC-MS analysis of Chlamydomonas reinhardtii and Scenedesmus obliquus lipid extracts and their possible metabolic roles. J Appl Phycol 27, 1149–1159 (2015). https://doi.org/10.1007/s10811-014-0407-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0407-2