Abstract
An exhaustive qualitative and quantitative profiling of the photosynthetic glycerolipids in three strains of the marine diatom Skeletonema sp. was carried out by ultra performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. In the diatom thylakoid membrane, monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) account for about 45–70% and 5–15% of the total membrane lipids, respectively. The anionic sulfoquinovosyldiacylglycerol (SQDG) as well as the likewise anionic phosphatidylglycerol (PG) contribute between 10–40% and 4–10% each. The predominant species of MGDG were those with C16:3/C16:3, C20:5/16:1, and C20:5/C16:3. Three main molecular species of DGDG contained C20:5/C16:1, C20:5/C16:2, and C16:1/C16:1. The major molecular species of SQDG were those containing combinations of C14:0/C14:0, C14:0/C16:0, C14:0/C16:1, and C14:0/C16:3. All the PG classes contained the C18:1/C18:1 as the main molecular species. Based on the fatty acid species in sn-2 position, it is indicated that MGDG and DGDG are biosynthesized through prokaryotic pathway exclusively within the chloroplast, whereas PG and SQDG have a typical mixed biosynthetic pathway (both prokaryotic pathway and eukaryotic pathways). The chemical characteristics of photosynthetic glycerolipids related with ecological physiology are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thylakoid membranes of organisms from cyanobacteria to seed plants, where oxygenic photosynthesis takes place, possess a characteristic membrane lipid composition. They contain two neutral galactolipids: monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) as major components. The anionic phospholipid phosphatidylglycerol (PG) and the anionic nonphosphorous glycolipid sulfoquinovosyldiacylglycerol (SQDG) are components that provide the lipid matrix with a negatively charged lipid–water interface (Brügger et al. 1997). Photosynthetic glycerolipids interact with photosynthetic protein complexes, affecting the function and activity of proteins in the thylakoid membranes in plants and algae. Furthermore, photosynthetic glycerolipids are crucial for membrane bilayer stabilization, and they also play an essential role during phosphate limitation in plants and bacteria by replacing phospholipids and thus facilitating the survival in phosphate-limited environments (Hartel and Benning 2000; Andersson et al. 2003; Páli et al. 2003; Frentzen 2004; Sato 2004; Fyfe et al. 2005; Jones 2007). The lipid composition of the photosynthetic glycerolipids also relates to the ability of the organisms to adapt themselves to extreme environment conditions (Thompson 1996; Singh et al. 2002).
On the other hand, their perceived importance has increased due to new descriptions of their biological activities, mainly related to their anti-inflammatory, antitumor-promoting, antialgal, hemolytic, and antiviral properties (Reshef et al. 1997). For example, glycol analogs of ceramides and of PG with antithrombotic and anti-inflammatory activities have been reported in cyanobacteria (Antonopoulou et al. 2005).
Diatoms are unicellular, photosynthetic, eukaryotic algae found throughout the world’s oceans and freshwater systems. They form the base of short, energetically efficient food webs that support large-scale coastal fisheries. Photosynthesis by marine diatoms generates as much as 40% of the 45–50 billion tonnes of organic carbon produced each year in the sea (Nelson et al. 1995). Over geological time, diatoms may have influenced global climate by changing the flux of atmospheric carbon dioxide into the oceans (Brzezinski et al. 2002).
Skeletonema is a universially distributed diatom genus. The cells are cylindrical in shape and are linked together by silica tubes to form long chains that look like short beads joined by many fine threads. Skeletonema is one of the most important phytoplankton genera in China coastal waters and is also often part of the spring bloom. Therefore, it was selected for photosynthetic glycerolipid characterization in our study.
Typically, methods for intact lipid analysis involve lipid extraction followed by thin-layer chromatography (TLC) separation of lipid classes and their detection by mass spectrometry. Obtaining fatty acid distributions within each lipid class requires removal of TLC spots, extraction into an organic solvent, and analysis of the extract by gas chromatography coupled to mass spectrometry (Xu et al. 2002). The conventional method is time consuming, and its major drawback is the large amounts of lipids required; moreover, chromatography methods often suffer from poor lipid recoveries and changes to the distribution of molecular species (DeLong et al. 2001). Recently, approaches based on electrospray ionization tandem mass spectrometry (ESI-MS/MS) have been developed to comprehensively analyze lipid composition in animals, yeast, bacteria, and plants (Brügger et al. 1997; Welti et al. 2002, 2003; Welti and Wang 2004; Han and Gross 2005; Wenk 2005; Han et al. 2006; Rainville et al. 2007; Basconcillo et al. 2009; De Souza et al. 2009). It requires only simple sample preparation and small samples to identify and quantify lipid molecular species.
In this paper, we reported the composition of photosynthetic glycerolipids obtained from three strains of Skeletonema sp., using the ultra performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (UPLC-ESI-Q-TOF-MS) measurements. The ESI-MS/MS analysis of the photosynthetic glycerolipids can provide information regarding not only the nature of the fatty acyl chains but also their relative positions (sn-1, sn-2) in individual lipid classes. Such biochemical information can be used to compare the ecophysiology of different strains.
Materials and methods
The diatom strains SKSPXS0711 (Xiangshan Bay, Zhejiang Province, China), SCXMBO2 (Xiamen, Fujian Province, China), and ATOX (Azores, Atlantic Ocean) were obtained from the Marine Biotechnology Laboratory of Ningbo University. The seawater for culture was sterilized (pH 8.30, 27‰ salinity) and enriched with nutrients: 100 mg L−1 KNO3, 10 mg L−1 KH2PO4, 20 mg L−1 Na2SiO3, 0.25 mg L−1 MnSO4·H2O, 2.50 mg L−1 FeSO4·7H2O, 10 mg L−1 EDTA-Na2, 6 μg L−1 Vitamin B1, and 0.05 μg L−1 Vitamin B12. Cultures were grown in a 10-L air-lift photobioreactor with aeration of 3 L min−1. Each culture was grown at 25°C at an irradiance of 120 μmol photons m−2 s−1 in continuous light. Cells were harvested at stationary phase by centrifugation at 5,000 rpm for 15 min and freeze-dried. All experiments were performed in triplicate and reported as average ± one standard deviation.
Total lipid extraction and standard glycolipids
Microalgae samples (freeze-dried) were extracted in CHCl3/CH3OH/H2O (1:2:0.8, v/v/v) by a modified version of Bligh and Dyer (1959) method to obtain the total lipid and then dried in a rotary evaporator, and the residue was stored at −20°C. PG standards (di14:0, di17:0) were obtained from the Avanti Polar Lipids, Inc. (Alabaster, AL). Glycolipid standards including MGDG, DGDG, and SQDG were purchased from Lipid Products, UK. In order to confirm the relative position and content of the glycolipid standards, the sn-2 acyl lysoglycolipids were synthesized, employing the known enzyme lipase XI (from the fungus Rhizopus arrhizus) and analyzed by LC/MS (detailed information discussed in Xu et al. 2009).
Cleanup of polar and neutral lipids
The residue of lipid fraction (10 mg) was spiked with a cocktail of standards, dissolved in methanol, which were utilized as internal standards for different lipid classes. The content of each standard MGDG (18:3–18:3), MGDG (18:3–18:2), DGDG (18:3–16:0), DGDG (18:3–16:3), SQDG (16:0–18:2), SQDG (18:3–18:3), and PG standards (di14:0, di17:0) in the total lipids of Skeletonema sp. was 5 nmol mg−1, respectively, and these standards were not observed in the sample in preliminary experiments. According to Pacetti et al. (2007), after being dried by nitrogen flow, they were dissolved in 200 μL of chloroform/methanol (2:1, v/v) and subjected to silica gel column chromatography using LC-Si tubes, 6-mL volume; 1 g of adsorbent (Supelco) was treated with a sequential elution of 5 mL hexane/diethyl ether (4:1, v/v), 5 mL hexane/diethyl ether (1:1, v/v), 5 mL methanol, and 5 mL chloroform/methanol/water (3:5:2, v/v/v). The fractions of the hexane and diethyl ether containing neutral lipids were discarded; the fractions of the methanol and chloroform/methanol/water, containing polar lipids such as glycolipids and phospholipids, were combined, dried, dissolved in methanol to give a concentration of 100 μg mL−1, and used for LC/MS analysis.
UPLC analytical conditions
Reversed-phase analysis was performed on a Waters ACQUITY Ultra Performance LC system using an ACQUITY UPLC BEH C18 analytical column (i.d. 2.1 mm × 50 mm, particle size 1.7 m, pore size 130 Å), and a 3:7 split of the column effluent was used to achieve a flow rate of approximately 100 L min−1 into the ESI source. Added to the mobile phase as electrolyte were 0.001 mM sodium formate and 15 mM formic acid for ESI positive mode and 15 mM ammonium hydroxide for negative ion mode. To obtain efficient separation of the lipids, H2O/isopropyl alcohol (85:15, v/v) was used as mobile phase A and acetonitrile/isopropyl alcohol (85:15, v/v) as mobile phase B. The composition of mobile phase B was changed from 50% to 92% in 10 min and kept for 10 min, then reached 100% in 1 min, and held for 5 min. The temperature of sample manager was set at 4°C, and injection volume was 5 μL for each analysis.
Mass spectrometric conditions
Mass spectrometry was performed on a Waters Q-TOF Premier mass spectrometer operating in negative and positive ion electrospray mode. The instrumental parameters were set as follows: mass range scanned from 100 to 1,200 with a scan duration of 0.3 sec; high-purity nitrogen was used as nebulizer and drying gas. The nitrogen drying gas was at a constant flow rate of 400 L h−1, and the source temperature was 120°C. MGDG and DGDG were determined in the positive ion mode, and simultaneously SQDG and PG were analyzed in negative ion mode. For positive mode, the capillary voltage was set at 3.0 kV; the sampling cone voltage was set at 60 V. For negative mode, the capillary voltage was set at 2.5 kV, and the sampling cone voltage was set at 50 V. MSE analysis was performed on the mass spectrometer set at 5 V for low collision energy and ramp of 30∼65 V for high collision energy. MS2 analysis was performed on the mass spectrometer set at different collision energy of 30∼70 V according to different kinds of lipids. The time of flight analyzer was used in V mode and was tuned for maximum resolution (>10,000 resolving power at m/z 1,000). The instrument was previously calibrated with sodium formate; the lock mass spray for precise mass determination was set by leucine enkephalin at m/z 556.2771 with concentration 0.5 ng μL−1 in the positive ion mode, while the leucine enkephalin at m/z 554.2615 was set in the negative ion mode.
Data processing
The raw data files obtained from LC/MS runs were analysed using MassLynx v4.1 and MarkerLynx 4.1 software (Waters). The application detects, integrates, and normalizes the intensities of the peaks to the sum of peaks within the sample. The resulting multivariate dataset consisting of the peak number (based on the retention time and m/z), sample name, and the normalized peak intensity was exported and analyzed by principal components analysis (PCA) using SIMCA-P + 11.5 (Umetrics AB, Sweden).
Qualitative and semiquantitative analysis of the photosynthetic glycerolipids
The use of the Q-TOF analyzer operating in MSE mode allows the selection and isolation of precursor ions with high efficiency, sensitivity, and mass accuracy. Characteristic fragmentations can be used to establish the component of photosynthetic glycerolipids and their acyl composition. The signal intensity of the fragment ions leads to a clear-cut determination of the positional distribution of the sn-glycerol-bound fatty acyl chains. Quantitative determination of a photosynthetic glycerolipid class requires the addition of two internal standards because the ionization efficiency between different photosynthetic glycerolipid classes of ESI-MS may differ significantly with respect to experimental conditions. The lipids in each class were quantified in comparison to two internal standards of that class.
Results
ESI-MS/MS profiling and semiquantitation of photosynthetic glycerolipids
The molecular species of photosynthetic glycerolipids were identified by characteristic fragment ions. The novel MSE technique was used in the analysis of photosynthetic glycerolipids, whereby both precursor and fragment mass spectra are simultaneously acquired by alternating between high and low collision energy (5 V) during a single chromatographic run. Chromatograms obtained from the UPLC-Q-TOF-MS in negative and positive ion modes can be separated effectively on the BEH C18 column. In the positive mode, the ion m/z 243.09 was for MGDG detection, while the ion m/z 405.14 was for DGDG detection; the ion at m/z 225.01 and 171.01 was for SQDG and PG detection in the negative mode, respectively. Considering that both precursor and product ion exist in the MSE chromatogram, extracted ion chromatograms (EIC) for characteristic product ion of each individual lipid class was first extracted respectively from the high collision energy (20–65 V) scans using MassLynx 4.1; then relevant parent ions at the same retention time of characteristic product ion could be detected in the low collision energy scans (5 V). Labels of each compound of the three strains of Skeletonema sp. have been added on the EIC of the characteristic product ion of each lipid class in Figs. 1, 2, and 3.
a1 TIC of the lipid mixture extracted from the diatom ATOX at the low collision energy of 5 V in the positive mode with MSE technique; 2 EIC of m/z 243.09 for characteristic product ion of MGDG in the diatom ATOX from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified MGDGs shown in Table 2; 3 EIC of m/z 405.14 for characteristic product ion of DGDG in the diatom ATOX from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified DGDGs shown in Table 3; b1 TIC of the lipid mixture extracted from the diatom ATOX at the low collision energy of 5 V in the negative mode with MSE technique; 2 EIC of m/z 225.01 for characteristic product ion of SQDG in the diatom ATOX from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified SQDGs shown in Table 4; 3 EIC of m/z 171.01 for characteristic product ion of PG in the diatom ATOX from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified PGs shown in Table 5
a1 TIC of the lipid mixture extracted from the diatom SKSPXS0711 at the low collision energy of 5 V in the positive mode with MSE technique; 2 EIC of m/z 243.09 for characteristic product ion of MGDG in the diatom SKSPXS0711 from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified MGDGs shown in Table 2; 3 EIC of m/z 405.14 for characteristic product ion of DGDG in the diatom SKSPXS0711 from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified DGDGs shown in Table 3; b1 TIC of the lipid mixture extracted from the diatom SKSPXS0711 at the low collision energy of 5 V in the negative mode with MSE technique; 2 EIC of m/z 225.01 for characteristic product ion of SQDG in the diatom SKSPXS0711 from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified SQDGs shown in Table 4; 3 EIC of m/z 171.01 for characteristic product ion of PG in the diatom SKSPXS0711 from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified PGs shown in Table 5
a1 TIC of the lipid mixture extracted from the diatom SCXMBO2 at the low collision energy of 5 V in the positive mode with MSE technique; 2 EIC of m/z 243.09 for characteristic product ion of MGDG in the diatom SCXMBO2 from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified MGDGs shown in Table 2; 3 EIC of m/z 405.14 for characteristic product ion of DGDG in the diatom SCXMBO2 from the high collision energy scans (ramp of 20–65 V) in the positive mode with MSE technique. The marked peaks are consistent with the identified DGDGs shown in Table 3; b1 TIC of the lipid mixture extracted from the diatom SCXMBO2 at the low collision energy of 5V in the negative mode with MSE technique; 2 EIC of m/z 225.01 for characteristic product ion of SQDG in the diatom SCXMBO2 from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified SQDGs shown in Table 4; 3 EIC of m/z 171.01 for characteristic product ion of PG in the diatom SCXMBO2 from the high collision energy scans (ramp of 20–65 V) in the negative mode with MSE technique. The marked peaks are consistent with the identified PGs shown in Table 5
Characteristic fragment ions can be used to establish the component of photosynthetic glycerolipids and their acyl composition. The signal intensity of the fragment ions leads to a clear-cut determination of the positional distribution of the sn-glycerol-bound fatty acyl chains. In the positive ion ESI-MS/MS spectrum of the MGDG and DGDG, the loss of the carboxylic acid linked to the sn-1 glycerol position always produced a more intense peak than that derived from the loss of the sn-2 linked acyl chain (Guella et al. 2003; Xu et al. 2009). Fragment analysis in the negative mode allowed identification of fatty acyl groups of PG and SQDG and tentative assignment of these groups to the sn-1 and sn-2 positions. MS2 fragmentation of SQDG yields the fatty carboxylate anions [RxCOO]−; the ratio of the [R2COO]−/[R1COO]− increases gradually as the collision energy rises (30–65 V); in the ESI-MS2 spectrum of PG, sn-2 carboxylate fragment ions [R2COO]− are more abundant than sn-1 carboxylate ions [R1COO]− under different collision energies (20–60 V). Using this basic rule, tandem mass spectrometric analyses of lipids can provide information regarding not only the nature of the fatty acyl chains but also their relative positions (sn-1 vs. sn-2) in individual lipids.
The accurate masses obtained were processed using the elemental composition calculator incorporated in the elemental composition analyst software (Waters). The parameter settings were as follows: C 0–60, H 0–100, and N 0–6; O 0–20. The double bond equivalent (DBE) was set from 0 to 50. Where phosphorus was a possible constituent of the ion, P was set from 0 to 3, and if sulfur was supposed to be a constituent, S was set from 0 to 1. A margin of error up to 10 ppm for unknown compounds was allowed.
By using the Q-TOF analyzer, it was possible to obtain highly accurate m/z values or to isolate different precursor ions and to study their MS/MS fragmentation pattern. For all these compounds, their possible molecular formulae were calculated as indicated above. To determine the most probable molecular formula, different criteria were considered such as the general rule of the number of nitrogen atoms, the DBE index, as well as the detection of several product ions and complementary ions that could confirm the proposed composition. The DBE parameter, corresponding to the probable molecular formula, was definitive in rejecting those formulae which did not account for the number of unsaturations found (Herrero et al. 2007). The compounds with the lowest molecular masses were successfully identified as the fatty acids, gamma-linolenic acid, linoleic acid, palmitic acid, etc., from their molecular formulae with errors smaller than 8 ppm (Table 1).
It was reported by Shui et al. (2007) that the ion response was linear over biologically relevant ranges for a number of different lipids, and internal lipid standards were added into the sample for the semiquantitative analysis. A simple and robust LC/MS-based methodology used for quantitation via external standards which allows for a semiquantitative characterization of the total composition was also described by Sommer et al. (2006). In our work, the dynamic range/linearity of each lipid class was determined with the concentration of mixed standard lipids up to 5 nmol mL−1 and the four lipid classes showed good linearity. According with this, the lipids in each class are quantified in comparison with two internal standards in this work. Nineteen MGDGs (Table 2), 9 DGDGs (Table 3), 22 SQDGs (Table 4), and 10 PGs (Table 5) molecular species have been unequivocally identified in the three strains of Skeletonema sp.
The proportion of each class in total photosynthetic glycerolipids (nmol mg−1 dry weight algae) is reported in Table 6. In the three strains of Skeletonema sp., MGDG and DGDG account for about 45–70% and 5–15% of the total membrane lipids, respectively. The anionic SQDG, as well as the likewise anionic PG, contribute between 10–40% and 4–10% each (Table 6).
The lipids in each class were quantified in comparison with two internal standards of that class. The amount and fatty acid composition of each class as determined by ESI-MS/MS, and the molecular species composition of photosynthetic glycerolipids is shown in Tables 2, 3, 4, and 5. Total amount of molecular species of photosynthetic glycerolipids in each class had extensive ranges, with MGDG (1.7–57.7 nmol mg−1 dry algae, Table 2), DGDG (0.5–15.1 nmol mg−1 dry algae, Table 3), SQDG (0.3–43.4 nmol mg−1 dry algae, Table 4), and PG (0.7–8.0 nmol mg−1 dry algae, Table 5), respectively.
Molecular species composition of photosynthetic glycerolipids in the three diatoms
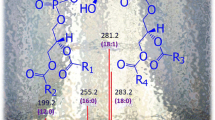
In Table 2, the predominant species of MGDG were those with C16:3/C16:3, C20:5/16:1, and C20:5/C16:3 (Table 2); the three main molecular species of DGDG contained C20:5/C16:1, C20:5/C16:2, and C16:1/C16:1 (Table 3); the major molecular species of SQDG were those containing combinations of C14:0/C14:0, C14:0/C16:0, C14:0/C16:1, and C14:0/C16:3 (Table 4); All the PG classes contained C18:1/C18:1 as the main molecular species (Table 5).
Morphological characteristics of the three strains of Skeletonema sp.
Based on morphological characteristics, the SCXMBO2 (Fig. 4, 1) and SKXPXS0711 (Fig. 4, 2) strains were identified as Skeletonema costatum. Both valves are at least slightly convex while the cells of ATOX were silicified more heavily than those of the other strains and had irregular spacing of cells which are usually lenticular in shape. Moreover, the valves of individual cells are flattened (Fig. 4- 3). Thus, SCXMBO2 and SKSPXS0711 belong to S. costatum, whereas ATOX was proposed as another species of Skeletonema sp.
Cluster analysis in Skeletonema sp. classification
The PCA was used here to find the clustering tendency of the three strains of Skeletonema sp. The raw data files obtained from LC/MS runs in the positive (Fig. 5a) and negative ion (Fig. 5b) modes were analysed using MassLynx v4.1. According to peak area data, about 1,789 peaks of positive ions (ESI+) and 1,163 peaks of negative ions (ESI−) were detected by MarkerLynx 4.1 using the same acquisition method from 0.3 to 20 min of retention time, and then the normalized data were analyzed by PCA and Fig. 3 shows the score plot. In both ion scan modes, three kinds of Skeletonema sp. can be separated clearly in the score plot by the first two components, which explained 53.04% (ESI+, Fig. 5a) and 67.59% (ESI−, Fig. 5b) of all variances. The results indicated that ATOX is obviously different to the two Skeletonema sp strains.
Discussion
Differences in the molecular composition of the two species of Skeletonema sp
Based on morphological characteristics and the application of cluster analysis SCXMBO2 and SKSPXS0711 are S. costatum, whereas ATOX is another species of Skeletonema sp. In the three diatoms, the difference in the composition between MGDG and DGDG was remarkable; unlike the SKSPXS0711 and SCXMBO2, the main MGDG and DGDG species containing C20:4/C16:1, C20:4/C16:3 (Table 2), or C20:4/C16:0, C20:4/C16:2 (Table 3) were only present in the ATOX. Considering the fatty acid composition of the three strains, docosapentaenoic acid (DPA, C22:5; Table 3) and arachidonic acid (AA, C20:4; Tables 2 and 3) were also only present in ATOX as a component of MDGD and DGDG. In addition, SQDG contained high amount of the species with C14:0/C16:0 in ATOX, but was low in the two other strains.
Differences in the molecular composition of the two S. costatum strains
The two strains of SKSPXS0711 and SCXMBO2 are distributed in different geographic locations in China. Comparing the molecular species composition of photosynthetic glycerolipids of SCXMBO2 and SKSPXS0711, the main MGDG species were those with C16:3/C16:3, C16:1/C16:3, and C20:5/C16:3 which was high in SKSPXS0711, but lower in SCXMBO2 (Table 2). DGDG also contained the species with C20:5/C16:1, C20:5/C16:2, and C20:5/16:3 in SKSPXS0711 but low in SCXMBO2 (Table 3). So it is obvious that an unsaturated fatty acid whose carbon chain has four, six, seven, and eight double per molecule is found chiefly in SKSPXS0711. The high degree of fatty acid unsaturation of MGDG and DGDG increased significantly in SKSPXS0711; this is speculated to enhance membrane fluidity and is thought to be an important strategy in increasing low-temperature tolerance. MGDG is roughly cone-shaped and will not form complete lamellar bilayer phases, even in combination with other thylakoid lipids. It is proposed that this MGDG plays a role in: (a) stabilizing regions of concave curvature in thylakoids and (b) packaging hydrophobic proteins in planar bilayer regions by means of inverted micelles. This model predicts substantial asymmetries in the distribution of lipids both across and along the thylakoid bilayer plane (Murphy 1982). Although MGDG and DGDG are, respectively, non-bilayer- and bilayer-forming lipids, their effects on membrane stability during low temperature were in some respects surprisingly similar. One physical characteristic that both lipids have in common is that they are very poorly hydrated (Hincha et al. 1998). However, the composition of the PG and SQDG in SCXMBO2 was similar to that of the SKSPXS0711; SQDG (C14:0/14:0, C14:0/16:0, and C14:0/16:1) and PG (C18:1/18:1) were the main component in both lipid extracts.
The lipid composition of plant cells is dominated by three characteristic glyceroglycolipids and one phospholipid, namely PG, constituting the main polar lipid component of the photosynthetic membranes. These highly specialized membrane systems are enriched in MGDG and DGDG which account in higher plant thylakoids for about 50% and 30% of the total membrane lipids, respectively. The anionic SQDG as well as the likewise anionic PG contribute between 5% and 12% each (Murata and Siegenthaler 1998). In the three strains of Skeletonema sp., MGDG and DGDG account for about 45–70% and 5–15% of the total membrane lipids, respectively. The anionic SQDG as well as the likewise anionic PG contribute between 10–40% and 4–10% each (Table 1).
In our work, it is evident that the content of acidic lipids (SQDG and PG) is significantly higher in the three strains of Skeletonema sp. than in the higher plants, with an average of about 30% of the total lipid. This situation strongly resembles the lipid composition of cyanobacteria. Wada and Murata (1989) have reported that Synechocystis cells contain 59% MGDG, 17% DGDG, 16% SQDG, and 8% PG. This may be of particular interest, as the cyanobacterial photosynthetic membranes, like diatom thylakoids, are unable to form grana stacks typical of higher plants. This furthermore emphasizes the different phylogenetic origin of the diatom chloroplast, which originates from a secondary endosymbiosis event (Armbrust et al. 2004). Particularly, in thylakoid membrane of chloroplast, MGDG accounts for > 45% of all membrane lipids in the three diatoms. MGDG is also the most abundant lipid in all membrane lipids of whole plant and algae cells. MGDG is considered to be the most abundant polar lipid on earth since the chloroplast membrane is the most developed membrane system in plant cells (Gounaris and Barber 1983). In higher plants, the molar ratio of MGDG/DGDG is generally conserved in thylakoid and inner envelope membranes in chloroplast (approximately MGDG/DGDG = 2:1; Ohta et al. 2000), but in our work, the content of MDDG is far higher than DGDG.
Biosynthesis pathway of galactolipid biosynthesis in the Skeletonema strains
Glycerolipid synthesis in higher plant leaves involves two discrete pathways (Roughan and Slack 1982; Browse and Somerville 1991; Somerville and Browse 1991). Fatty acids synthesized in the chloroplasts may either enter the “prokaryotic” chloroplastidial pathway or be exported to the endoplasmic reticulum (ER), where they are incorporated into lipids through the “eukaryotic pathway”; the former pathway is situated in the chloroplast envelope and involves the sequential action of acyl-ACP glycerol 3-phosphate acyltransferase and acyl-ACP: lysophosphatidic acid acyltransferase. These enzymes produce phosphatidic acid, with almost exclusively C16 fatty acids at the sn-2 position of glycerol (Frentzen et al. 1983). This lipid is either used for the synthesis of phosphatidylglycerols (PG) or is hydrolyzed by the phosphatidate phosphatase to form diacylglycerols. Diacylglycerol moieties containing C16 fatty acids at the sn-1 position are the precursors for the synthesis of prokaryotic galactolipids (Joyard et al. 1991).
On the other hand, the microsomal acyltransferases, which are responsible for phosphatidic acid synthesis by the “eukaryotic pathway” in the ER, give rise to lipids that contain C18 fatty acids exclusively at the sn-2 position and either C16 or C18 fatty acids at the sn-1 position (Frentzen et al. 1983). Based on the positional distribution of fatty acids on the individual lipid class of the three strains of Skeletonema sp., it is indicated that MGDG and DGDG are biosynthesized through “prokaryotic” pathway exclusively within the chloroplast because all the fatty acids linked at sn-2 position is several C16 fatty acids in MGDG and DGDG. On the other hand, PG and SQDG have a typical mixed biosynthetic pathway (both “prokaryotic” pathway and “eukaryotic” pathway) because the fatty acids at sn-2 position include both C16 and C18 fatty acids.
Two types of photosynthetic glycerolipids biosynthetic pathways between higher plant and algae reflect the significant differences in the evolutionary process. Exogenous fatty acids gradually can be used to synthesize their photosynthetic membrane lipids in the chloroplasts. Therefore, the loss of the plastidial glycerolipid synthesis pathway in the higher plants can be regarded as a loss of plastidial autonomy concerning lipid synthesis. The only difference between plants having either both or only one metabolic pathway(s) is assumed to be the presence or the absence of plastidial phosphatidate phosphatase activity. Mongrand et al. (1998) suggested that the plastidial pathway was lost during evolution. The question arises as to whether the loss of phosphatidate phosphatase activity is beneficial for the plant? Could this change depend on the environmental condition? That needs further investigation.
In conclusion, quantitative and comprehensive lipid profiling is an emerging technology. As described above, the lipidomics approach has demonstrated great potential to facilitate understanding of the function of membrane lipids in algae adaptation and tolerance to temperature stresses. This work greatly speeds up and simplifies the analysis of microalgae photosynthetic glycerolipids and should pave the way for better understanding of their role in algae performance; it also can be applied to study the ecological physiology of marine microalgae by monitoring the changes of lipid profiles.
References
Andersson MX, Stridh MH, Larsson KE, Liljenberg C, Sandelius AS (2003) Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett 537:128–132
Antonopoulou S, Nomikos T, Oikonomou A, Kyriacou A, Andriotis M, Fragopoulou E et al (2005) Characterization of bioactive glycolipids from Scytonema julianum (Cyanobacteria). Comp Biochem Physiol B Biochem Mol Biol 140:219–231
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Basconcillo LS, Zaheer R, Finan TM, McCarry BE (2009) A shotgun lipidomics approach in Sinorhizobium meliloti as a tool in functional genomics. J Lipid Res 50:1120–1132
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Browse J, Somerville C (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42:467–506
Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD (1997) Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci 94:2339–2344
Brzezinski MA, Pride CJ, Franck VM, Sigman DM, Sarmiento JL, Matsumoto K et al (2002) A switch from Si(OH)4 to NO −3 depletion in the glacial Southern Ocean. Geophys Res Lett 29:1564
De Souza LM, Müller-Santos M, Iacomini M, Gorin PA, Sassaki GL (2009) Positive and negative tandem mass spectrometric fingerprints of lipids from the halophilic Archaea Haloarcula marismortui. J Lipid Res 50:1363–1373
DeLong CJ, Baker PR, Samuel M, Cui Z, Thomas MJ (2001) Molecular species composition of rat liver phospholipids by ESI-MS/MS: the effect of chromatography. J Lipid Res 42:1959–1968
Frentzen M (2004) Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: anionic membrane lipids and phosphate regulation. Curr Opin Plant Biol 7:270–276
Frentzen M, Heinz E, McKeon TA, Stumpf PK (1983) Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phos-phate acyltransferase from pea and spinach chloroplasts. Eur J Biochem 129:629–636
Fyfe PK, Hughes AV, Heathcote P, Jones MR (2005) Proteins, chlorophylls and lipids: X-ray analysis of a three-way relationship. Trends Plant Sci 10:275–282
Gounaris K, Barber J (1983) Monogalactosyldiacylglycerol—the most abundant polar lipid in nature. Trends Biochem Sci 8:378–381
Guella G, Frassanito R, Mancini I (2003) A new solution for an old problem: the regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 17:1982–1994
Han X, Gross RW (2005) Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev 24:367–412
Han X, Yang K, Yang J, Fikes KN, Cheng H, Gross RW (2006) Factors influencing the electrospray intrasource separation and selective ionization of glycerol phospholipids. J Am Soc Mass Spectrom 7:264–274
Hartel H, Benning C (2000) Can digalactosyldiacylglycerol substitute for phosphatidylcholine upon phosphate deprivation in leaves and roots of Arabidopsis? Biochem Soc Trans 28:729–732
Herrero M, Vicente MJ, Cifuentes A, Ibanez E (2007) Characterization by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry of the lipid fraction of Spirulina platensis pressurized ethanol extract. Rapid Commun Mass Spectrom 21:1729–1738
Hincha DK, Oliver AE, Crowe JH (1998) The effects of chloroplast lipids on the stability of liposomes during freezing and drying. Biochim Biophys Acta 1368:150–60
Jones MR (2007) Lipids in photosynthetic reaction centres: structural roles and functional holes. Prog Lipid Res 46:56–87
Joyard J, Block MA, Douce R (1991) Molecular aspects of plastid envelope biochemistry. Eur J Biochem 199:489–509
Mongrand S, Bessoule JJ, Cabantous F, Cassagne C (1998) The C16:3/C18:3 fatty acid balance in photosynthetic tissues from 357 plant species. Phytochemistry 49:1049–1064
Murata N, Siegenthaler PA (1998) Lipids in photosynthesis: an overview, in lipids in photosynthesis: structure, function and genetics. Kluwer Academic, Dordrecht, pp 1–20
Murphy DJ (1982) The importance of non-planar bilayer regions in photosynthetic membranes and their stabilisation by galactolipids. FEBS Lett 150:19–26
Nelson DM, Treguer P, Brzezinski MA, Leynaert A, Queguiner A (1995) Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob Biogeochem Cycle 9:359–372
Ohta H, Awai K, Takamiya K (2000) Glyceroglycolipids of photosynthetic organisms—their biosynthesis and evolutionary origin. Trends Glycosci Glycotechnol 12:241–253
Pacetti D, Boselli E, Lucci P, Frega NG (2007) Simultaneous analysis of glycolipids and phospholipids molecular species in avocado (Persea americana Mill) fruit. J Chromatogr A 1150:241–251
Páli T, Garab G, Horváth LI, Kóta Z (2003) Functional significance of the lipid–protein interface in photosynthetic membranes. Cell Mol Life Sci 60:1591–1606
Rainville PD, Stumpf CL, Shockcor JP, Plumb RS, Nicholson JK (2007) Novel application of reversed-phase UPLC-Q-TOF-MS for lipid analysis in complex biological mixtures: a new tool for lipidomics. J Proteome Res 6:552–558
Reshef V, Mizrachi E, Maretzki T, Silberstein C, Loya S, Hizi A, Carmeli S (1997) New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J Nat Prod 60:1251–1260
Roughan PG, Slack CR (1982) Cellular organization of glycerolipid metabolism. Annu Rev Plant Physiol 33:97–32
Sato N (2004) Roles of the acidic lipids sulfoquinovosyl diacylglycerol and phosphatidylglycerol in photosynthesis: their specificity and evolution. J Plant Res 117:495–05
Shui G, Bendt AK, Pethe K, Dick T, Wenk MR (2007) Sensitive profiling of chemically diverse bioactive lipids. J Lipid Res 48:1976–1984
Singh SC, Sinha RP, Hader DP (2002) Role of lipids and fatty acids in stress tolerance in cyanobacteria. Act Protoz 41:297–308
Somerville C, Browse J (1991) Plant lipids: metabolism, mutants, and membranes. Science 252:80–87
Sommer U, Herscovitz H, Welty FK, Costello CE (2006) LC-MS based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res 47:804–814
Thompson GA Jr (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Wada H, Murata N (1989) Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol 30:971–978
Welti R, Wang X (2004) Lipid species profiling: a high-throughput approach to identify lipid compositional changes and determine the function of genes involved in lipid metabolism and signaling. Curr Opin Plant Biol 7:337–344
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE et al (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277:31994–32002
Welti R, Wang X, Williams TD (2003) Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Anal Biochem 314:149–152
Wenk MR (2005) The emerging field of lipidomics. Nat Rev Drug Discov 4:594–610
Xu YN, Wang ZN, Yan XJ, Lin W, Li LB, Kuang TY (2002) Positional distribution of fatty acids on the glycerol backbone during the biosynthesis of glycerolipids in Ectocarpus fasciculatus. Chin Sci Bull 47:1802–1806
Xu JL, Chen DY, Yan XJ, Li HY (2009) Ultra performance liquid chromatography-quadrupole-time of flight mass spectrometric analysis of photosynthetic glycolipids mixture. Chinese J Anal Chem 37:511–516
Acknowledgments
This research was supported by Program for Changjiang Scholars and Innovative Research Team in University, China (PCSIRT) IRT0734; Ningbo Natural Science Foundation, China (Y506131); National Key Technology R&D Program, China (2007BAD43B09), project of Minsitry of Eductaion China, (NO. 200816460002), and also partly sponsored by K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 918 KB)
Rights and permissions
About this article
Cite this article
Yan, X., Chen, D., Xu, J. et al. Profiles of photosynthetic glycerolipids in three strains of Skeletonema determined by UPLC-Q-TOF-MS. J Appl Phycol 23, 271–282 (2011). https://doi.org/10.1007/s10811-010-9553-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9553-3