Abstract
Autistic disorders (ADs) are heterogeneous neurodevelopmental disorders arised by the interaction of genes and environmental factors. Dysfunctions in social interaction and communication skills, repetitive and stereotypic verbal and non-verbal behaviours are common features of ADs. There are no defined mechanisms of pathogenesis, rendering curative therapy very difficult. Indeed, the treatments for autism presently available can be divided into behavioural, nutritional and medical approaches, although no defined standard approach exists. Autistic children display immune system dysregulation and show an altered immune response of peripheral blood mononuclear cells (PBMCs). In this study, we investigated the involvement of cannabinoid system in PBMCs from autistic children compared to age-matched normal healthy developing controls (age ranging 3–9 years; mean age: 6.06 ± 1.52 vs. 6.14 ± 1.39 in autistic children and healthy subjects, respectively). The mRNA level for cannabinoid receptor type 2 (CB2) was significantly increased in AD-PBMCs as compared to healthy subjects (mean ± SE of arbitrary units: 0.34 ± 0.03 vs. 0.23 ± 0.02 in autistic children and healthy subjects, respectively), whereas CB1 and fatty acid amide hydrolase mRNA levels were unchanged. mRNA levels of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D gene were slightly decreased. Protein levels of CB-2 were also significantly increased in autistic children (mean ± SE of arbitrary units: 33.5 ± 1.32 vs. 6.70 ± 1.25 in autistic children and healthy subjects, respectively). Our data indicate CB2 receptor as potential therapeutic target for the pharmacological management of the autism care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autistic disorders (ADs) are variable heterogeneous neurodevelopmental disorders defined by deficits in social interaction, adaptive functioning, and communication skills, combined with repetitive and stereotypical behaviours (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR), American Psychiatric Association 2000; Levy et al. 2009). While autism pathogenesis remains unclear, efforts to define valid treatments for ADs are being pursued. Numerous biochemical and cellular events are associated with ADs (i.e. oxidative stress, mitochondrial dysfunction, intestinal dysbiosis and immune dysregulation) (Ashwood et al. 2006; de Magistris et al. 2010). Among the immunological dysfunctions described in ADs, peripheral blood mononuclear cells (PBMCs) are reported (Enstrom et al. 2010; Siniscalco et al. 2012). AD-PBMCs show increased levels of pro-inflammatory cytokines and interleukins with resultant long-term immune alterations (Molloy et al. 2006; Onore et al. 2009). Recently, it has been demonstrated that AD-PBMCs show altered expression and activation of several caspases (Siniscalco et al. 2012). These caspases are a phylogenetically conserved structurally-related family of aspartate-specific, cysteine-dependent proteases (Lamkanfi et al. 2002). They regulate apoptosis and inflammatory signalling pathways. However, beyond apoptosis, these enzymes show other functions. Caspases are pleiotropic enzymes, functioning in cell differentiation and proliferation, as well as in activation and nuclear reprogramming pathways (Algeciras-Schimnich et al. 2002).
The endocannabinoid system consists of arachidonic acid derived compounds (endocannabinoids), their receptors and the associated enzymes (Li et al. 2011). This represents an intricate network of lipid signalling pathways (Barna and Zelena 2012). Accumulating evidence highlights that the endocannabinoid system is involved in several psychiatric disorders (i.e. autism, anxiety, major depression, bipolar disorder and schizophrenia), as well as developmental disorders (Schneider and Koch 2005; Ishiguro et al. 2010; Robinson et al. 2010; Garcia-Gutierrez and Manzanares 2011; Minocci et al. 2011).
Endocannabinoids, such as N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG), are synthesized and released upon demand in a receptor-dependent way (Mouslech and Valla 2009). They exert their effects through the G-protein-coupled cannabinoid receptors CB1 and CB2, which, in turn, are negatively coupled to adenylate cyclase enzyme (Pertwee et al. 2010). After receptor binding, endocannabinoids are transported into cells by a specific uptake system and degraded by the fatty acid amide hydrolase (FAAH).
Recent studies suggested that endocannabinoids exhibit potent anti-inflammatory and immunosuppressive properties. Therefore, this pathway presents therapeutic potential for autoimmune and inflammatory diseases (Klein and Cabral 2006; Nagarkatti et al. 2009).
Schultz hypothesized acetaminophen contributes to the risk of autism via activation of the endocannabinoid system (Schultz 2010). To our knowledge, however, no studies have specifically investigated the endocannabinoid system in the development of autism. Herein we address the issue of whether these disorders are associated with changes in the expression of CB1/2 receptors and endocannabinoid metabolism enzymes, the AEA biosynthetic enzyme N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) and the AEA degradative catabolic enzyme FAAH in PBMCs from AD patients.
Materials and Methods
Subjects
Informed consent was obtained from all subjects enrolled in this study in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
We investigated 17 children with autism, and compared them to 22 age and sex matched healthy children used as control group (age ranging 3–9 years; mean age: 6.06 ± 1.52 vs. 6.14 ± 1.39 in autistic and healthy individuals, respectively). The 17 subjects with autism were recruited into the study from the outpatient Centre for Autism of La Forza del Silenzio, Naples-Caserta, Italy. The cohort included 14 boys and 3 girls. Before entering the study, all of the children were administered the Autism Diagnostic Interview-Revised version (Lord et al. 1994), the Childhood Autism Rating Scales (Schopler et al. 1993), and the Autism Diagnostic Observation Schedule-Generic (Lord et al. 2000) to document the diagnosis of autism. All included patients met the Diagnostic and Statistical Manual of Mental Disorders-IV criteria for autism (DSM-IV-TR) (American Psychiatric Association 2000). In addition to meeting the criteria for autistic disorder (AD), subject children were required to score at least 30 points on the CARS scale. Twenty-two healthy children (females 4, males 18) were recruited among staff family members. Potential subjects were excluded if they had any of the following: a neurological or comorbid psychiatric disorder, epilepsy, history of liver, renal or endocrine disorders, current infection of any origin. Mental retardation or behavioural disorders, including Pervasive Developmental Disorder—not otherwise specified (PDD-NOS), inclusion criteria for attention deficit-hyperactivity disorder, were all considered exclusion criteria for control children. Children diagnosed with Asperger’s syndrome, fragile X syndrome and tuberous sclerosis were also excluded from the study. IQ test was not performed. Neither AD subjects nor controls had special diets or other pharmacological interventions. Other exclusion criteria were coeliac disease and/or other major diseases of the intestinal tract, such as inflammatory bowel disease or hepatic disorders.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Fresh peripheral blood samples from AD subjects and control donors were drawn and collected in sterile EDTA tubes (Becton–Dickinson, Franklin Lakes, NJ, USA). Peripheral blood mononuclear cells (PMBCs) were isolated by centrifugation over Histopaque 1,077 density gradient (Sigma Chemical, St Louis, MO, USA). Briefly, blood was diluted 1:1 in phosphate buffer saline (PBS) (Sigma, St. Louis, MO, USA), overlaid onto lymphocyte separation media (Lymphocyte Separation Medium—Lonza, Walkersville, MD, USA), centrifuged at 2,200 rpm for 30 min at room temperature and plasma was removed. Mononuclear cell fraction was harvested and washed twice in PBS. The final pellet was re-suspended in Tri-Reagent solution (Molecular Research Center Inc., Cincinnati, OH, USA) or protein lysis buffer for further molecular analysis.
RNA Extraction and RT-PCR
The RNA was extracted from PBMCs using a RNA Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH, USA) according to the manufacturer’s protocol. The total RNA concentration and integrity were determined by Nanodrop ND-1000 UV spectrophotometer (Nano-Drop® Technologies, Thermo Scientific, Wilmington, DE, USA). The mRNA levels of the endocannabinoid genes under analysis were measured by RT-PCR amplification, as previously reported (Siniscalco et al. 2012). Reverse Transcriptase from Avian Myeloblastosis Virus (AMV-RT; Promega, Madison, WI, USA) was used. For first-strand cDNA synthesis 200 ng total RNA, random examers, dNTPs (Promega, Madison, WI, USA), AMV buffer, AMV-RT and recombinant RNasin ribonuclease inhibitor (Promega, Madison, WI, USA) were assembled in diethyl-pyrocarbonate-treated water to a 20 μl final volume and incubated for 10 min at 65 °C and 1 h at 42 °C. RT minus controls were carried out to check potential genomic DNA contamination. These RT minus controls were performed without using the reverse transcriptase enzyme in the reaction mix. Aliquots of 2 μl cDNA were transferred into a 25 μl PCR reaction mixture containing dNTPs, MgCl2, reaction buffer, specific primers and GoTaq Flexi DNA polymerase (Promega, Madison, WI, USA), and amplification reactions using specific primers and conditions for human genes under analysis cDNA were carried out. Sequences for the human mRNAs from GeneBank (DNASTAR INC., Madison, WI, USA) were used to design specific primer pairs for RT-PCRs (OLIGO 4.05 software, National Biosciences Inc., Plymouth, MN, USA) (Table 1). Each RT-PCR was repeated at least three times to achieve the best reproducibility data. The mean of the inter-assay variability of each RT-PCR assay was 0.07. The levels of mRNA measured were normalized with respect to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was chosen as the housekeeping gene. Indeed GAPDH is one of the most stably expressed genes in human peripheral blood (Stamova et al. 2009). To our knowledge, there is no molecular evidence of variation in GAPDH mRNA-levels in autism disorders (Siniscalco et al. 2012). The gene expression values were expressed as arbitrary units ± SEM. Amplification of the genes of interest and GAPDH was performed simultaneously. PCR products were resolved into 2.0 % agarose gel. A semi-quantitative analysis of mRNA levels was carried out by the “Gel Doc 2000 UV System” (Bio-Rad, Hercules, CA, USA).
Protein Extraction and Western Blot Analysis
For protein extraction, PBMCs were suspended in protein lysis buffer [HEPES 25 mM; EDTA 5 mM; SDS 1 %; Triton X-100 1 %; PMSF 1 mM; MgCl2 5 mM; Protease Inhibitor Cocktail (Roche, Mannheim, Germany); Phosphatase Inhibitor Cocktail (Roche, Mannheim, Germany)]. Protein concentration was determined using the method described by Bradford (1976). Each sample was loaded, electrophoresed in a 15 % SDS-polyacrylamide gel and electroblotted onto a nitrocellulose membrane. The membrane was blocked in 5 % milk, 1X Tris-buffered saline and 0.05 % Tween-20. Primary antibodies to detect CB2 (Calbiochem-Merck, Darmstadt, Germany) were used according to the manufacturer’s instructions at 1:250 dilutions. The rabbit anti-CB2 antibody detects endogenous levels of the human 45 kDa fragment of CB2 receptor protein. The antibody does not cross-react with the CB1 receptor protein and, according to the manufacturer, was validated with a recombinant protein consisting of the first 33 amino acids of human CB2 receptor used as a positive control. Immunoreactive signals were detected with a horseradish peroxidase-conjugated secondary antibody and reacted with an ECL system (Amersham Pharmacia, Uppsala, Sweden). To assess equal loading, protein levels were normalized with respect to the signal obtained with Ponceau S staining, as previously reported (Alessio et al. 2010; Romero-Calvo et al. 2010; Zanichelli et al. 2012). We used Ponceau S staining over actin as equal loading control as this method has a better dynamic range and overcomes the possibility that housekeeping proteins could vary in this pathology or be saturated at the levels of loading (Romero-Calvo et al. 2010). The semi-quantitative analysis of protein levels was carried out by the ChemiDoc-It 5000, using VisionWorks Life Science Image Acquisition and Analysis software (UVP, Upland, CA, USA).
Immunocytochemistry
For immunocytochemical analysis, PBMCs were extracted and plated as previously reported (Siniscalco et al. 2012). In brief, mononuclear cells were re-suspended at 1 × 106 cell/mL in RPMI 1640 complete medium (Lonza, Verviers, Belgium) containing 10 % fetal bovine serum (FBS) (EuroClone-Celbio, Milan, Italy), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (all Lonza), were plated on slides with a 12-well plate and incubated for 4 days at 37 °C with 5 % CO2. Cells were then fixed with 4 % paraformaldheyde fixative. After washing in PBS, non-specific antibody binding was inhibited by incubation for 30 min in blocking solution (1 % BSA in PBS). Primary antibodies were diluted in PBS blocking buffer and slides were incubated overnight at 4 °C in primary antibodies to human CB1 receptor or to human CB2 receptor (either diluted at 1:200; Calbiochem-Merck, Darmstadt, Germany). Fluorescent-labeled secondary antibodies (1:1,000; Alexa Fluor 488 (for CB1) and 568 (for CB2), Molecular Probe; Invitrogen, Carlsbad, CA, USA) specific to the IgG species used as a primary antibody were used to locate the specific antigens in each slide. Cells were counterstained with bisbenzimide (Hoechst 33258; Hoechst, Frankfurt, Germany) and mounted with mounting medium (90 % glycerol in PBS). Fluorescently labelled slides were viewed with a fluorescence microscope (Leica, Wetzlar, Germany). Immunofluorescence images were analyzed with Leica FW4000 software (Leica, Wetzlar, Germany). Only bisbenzimide counterstained cells were considered as positive profiles so as to avoid overcounting cells.
Statistical Analysis
Biomolecular data are expressed as mean ± SEM ANOVA, followed by Student–Neuman–Keuls post hoc test, was used to determine the statistical significance among groups, without correction for multiple test comparison. p < 0.05 was considered statistically significant.
Results
AD- Related Changes in Endocannabinoid System Gene Expressions
We examined endocannabinoid system gene expression mainly by RT-PCR, since this technique is a far more sensitive method for the detection of gene expression than immunocytochemistry (Giordano et al. 2011; Siniscalco et al. 2012). In addition, the genes analysed showed a transcriptional regulative mechanism (Galiègue et al. 1995; Maccarrone et al. 2001; Nong et al. 2002). When compared to controls, the semiquantitative analysis of PBMC-extracted mRNA levels, measured by RT-PCR amplification, showed an increase in the CB2 receptor gene in PBMCs of AD patients (mean ± SE of arbitrary units: 0.34 ± 0.03 vs. 0.23 ± 0.02, p < 0.05, in autistic children and healthy subjects, respectively), whereas mRNA levels of NAPE-PLD gene were slightly decreased (mean ± SE of arbitrary units: 0.25 ± 0.04 vs. 0.39 ± 0.03, p < 0.05, in autistic children and healthy subjects, respectively); mRNA levels of CB1 receptor (mean ± SE of arbitrary units: 0.51 ± 0.05 vs. 0.69 ± 0.07, p > 0.05, in autistic children and healthy subjects, respectively) and FAAH enzyme gene (mean ± SE of arbitrary units: 0.38 ± 0.10 vs. 0.48 ± 0.08, p > 0.05, in autistic children and healthy subjects, respectively) were not different (Fig. 1; Table 2).
Over-expression of CB2 receptor gene, but not of CB1 receptor and FAAH enzyme, and down-expression of NAPE-PLD gene in AD-PBMCs. The measured mRNA levels were normalized with respect to GAPDH (housekeeping gene) and gene expression values were expressed as a percentage of arbitrary units ± SEM open circle indicates significant difference versus healthy controls. p values <0.05 were considered statistically significant. CTL healthy control subjects, AD autistic patients
CB2 Protein Levels are Increased in AD-PBMCs
As G protein-coupled receptors, the cannabinoid receptors (CBs) also show post-translational regulation (Ardura and Friedman 2011; Peralta et al. 2011). To confirm gene expression change, we therefore determined the protein levels of CB2 receptor by western blot analysis, as well as by fluorescence immunocytochemistry.
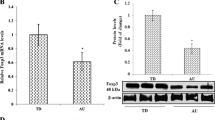
To confirm a lack of change in protein levels for CB1 receptor as implied by mRNA levels, we performed immunocytochemical analysis also for this receptor. Western blot analysis showed a remarkable increase in CB2 protein levels in AD children as compared to healthy controls (Fig. 2) (mean ± SE of arbitrary units: 33.5 ± 1.32 vs. 6.70 ± 1.25, p < 0.05, in autistic children and healthy subjects, respectively). Protein level analysis in AD patients and control groups was performed simultaneously. CB2 protein level was enhanced in all the AD children evaluated. The control group demonstrated no intragroup variances of significance.
Representative western blot analysis of CB2 protein levels in the PBMCs obtained from the autistic children and the healthy controls, respectively. CTL healthy control subjects, AD autistic disorder subjects. The histograms indicate percentage variations in CB2 protein levels in the PBMCs of AD children compared to the healthy controls (CTL). open circle indicates significant differences versus healthy subjects. p < 0.05 was considered as the level of significance
The difference between the increase in CB2 mRNA and in the increase in CB2 protein in AD group is not surprising. Post-translational control of protein function has been described to affect protein levels. Indeed, the CB2 protein, as G protein coupled receptor, show a multilevel system of regulation, that affects the levels of receptor in the cell (Ardura and Friedman 2011; Peralta et al. 2011; Tománková and Myslivecek 2012).
Moreover, the levels of cellular mRNAs can be regulated by controlling the rate at which the mRNA decays (Wilusz et al. 2001). It is noteworthy to consider that there is not a direct correlation between mRNA transcripts and protein levels. Gene expression is also regulated by the control of mRNA degradation, since the steady-state concentration of mRNA is determined both by its rates of synthesis and decay (Rajagopalan and Malter 1997; Meyer et al. 2004). Changes in mRNA half-life do not reflect changes in transcription (Ross 1996). More importantly, the correlation between mRNA and protein abundances in the cell is insufficient to predict protein expression levels from quantitative mRNA data (Gygi et al. 1999; Maier et al. 2009). Determining a direct relationship between mRNA and protein levels can be problematic (Pascal et al. 2008). However, mRNA expression is informative in the prediction of protein expression (Guo et al. 2008). Increasing in both mRNA and correspondent protein is indicative of a positive correlation between mRNA and protein expression levels (Guo et al. 2008; Yang et al. 2013). Using several and different techniques (i.e. RT-PCR, Western blot, immunocytochemistry), as used here, to study changes in gene expression is a valid tool to assess the correlation between these macromolecules inside the cell (Dong et al. 2012).
Immunofluorescence analysis was carried out using an antibody able to detect endogenous levels of the human CB2 receptor protein, without cross-reacting with the CB1 receptor protein. Immunofluorescence staining confirmed that CB2 was over-expressed in the PBMCs in AD children as compared to healthy controls, while no difference in CB1 receptor related signals were observed in AD children respect to healthy controls (Fig. 3).
Representative fluorescent photomicrograph of PBMCs showing immunocytochemistry for CB1 receptor (bottom) and CB2 receptor (top). Top Arrows indicate CB2 positive staining (red fluorescent). Bottom Arrows indicate CB1 positive staining (green fluorescent). To correctly identify cells, their nuclei were counterstained with bisbenzimide (blue fluorescence), as shown in panel a. a healthy control subjects; b autistic disorder patients. Scale bars 15 μm
Discussion
In this study, we demonstrated for the first time the up-regulation of CB2 receptors in PBMCs from ADs subjects. No differences were observed for CB1 receptor regulation. Alterations in endocannabinoid levels are transient adaptive reactions which attempt to re-establish normal homeostasis disrupted by the disease. However, in some conditions, endocannabinoid systems appear to contribute to a chronic maladaptive disease state (Di Marzo and Petrosino 2007). Emerging studies highlight that endocannabinoid signalling through CB2 receptors could activate a protective system. CB2 receptor activation is known to trigger immune suppression (Hegde et al. 2010). After inflammation or tissue injury, there is a rapid increase in local endocannabinoid levels, which appears to mediate immune responses through down-regulation of cytokine expressions (Jean-Gilles et al. 2010; Pacher and Mechoulam 2011). The immunomodulatory effects of endocannabinoids are mainly mediated by the CB2 receptor expressed on immune cells (Klein et al. 2003; Cencioni et al. 2010). The CB2 gene, which is not expressed in the brain, is principally expressed in immune tissues (Kenny 2011); whereas CB1 is abundant in the central nervous system (Galiègue et al. 1995). It’s noteworthy that CB2 receptors regulate cannabinoid- induced immune modulation (Tanikawa et al. 2011). Cannabinoids are involved in B cell activation and maturation through the CB2 receptor. Importantly, B lymphocytes express the highest level of CB2 mRNA relative to other immune cells (Agudelo et al. 2008). In addition, CB2 receptor is able to modulate development, migration, proliferation, and effector functions of immune cells (Basu and Dittel 2011). Alterations in immune system in autism pathogenesis have been reported (Gupta et al. 2010; Suzuki et al. 2011). Moreover, AD-PBMCs show increased activation of both Th1- and Th2- mediated immune response, altered cytokine profiles, decreased lymphocyte numbers, imbalance of serum immunoglobulin levels and caspase-mediated immune response changes (Ashwood et al. 2006; Li et al. 2009; Siniscalco et al. 2012). These observations, when combined with the present study data, are suggestive that CB2 receptor up-regulation in PBMCs could be related to AD-immune dysregulation. It is well established that these cells are key regulators of the immune pathways, and a dysregulation in the PBMC response could result in long-term immune alterations seen in AD (Enstrom et al. 2010). The CB2 receptor alterations we observed in AD-PBMCs indicate the endocannabinoid system may be functionally involved in AD pathogenesis or maintenance. The fact that in PBMCs from autistic children we observed only CB2 receptor changes, but not CB1 and/or the anandamide catabolic enzyme FAAH, could indicate that the main action played by endocannabinoids in these cells is to regulate inflammation and immune responses. CB1 receptors do not seem involved in mediating these events. However, our data cannot exclude CB1 receptor up-regulation in other cell types or within the central nervous system. It is noteworthy that pro-inflammatory stimuli suppress NAPE-PLD expression (Zhu et al. 2011). In fact, the slight down-regulation we observed in mRNA levels for this biosynthetic enzyme could be related to the inflammatory state associated with autism immunopathology.
Another hypothesis could be related to a CB2 protective response to AD-mediated inflammatory stimuli derived from the capacity of CB2 to inhibit pro-inflammatory cytokine synthesis and release (Di Filippo et al. 2004). However, it has been demonstrated that pro-inflammatory cytokines are abundantly increased in the plasma of autistic patients (Ashwood et al. 2011). These data, when combined with our study’s observations, enhance the hypothesis of a correlation between CB2-mediated immune dysfunction and autism pathophysiology. This further indicates that the endocannabinoid system, through CB2 receptors, could mediate a cross-talk between immune and nervous systems.
As previously mentioned, Schultz reviewed the possible autism activation by endocannabinoid system (Schultz 2010). He reviewed data revealing sulfation deficits in acetaminophen (paracetamol) metabolism with the autism population. Acetaminophen administration in the presence of a sulfation deficiency, creates a metabolic by-product, N-arachidonoylphenolamine (AM404), causing an indirect increase of endocannabinoids levels (Högestätt et al. 2005; Soukupová et al. 2010), which in turn activate CB1/2 receptors triggering autism. This hypothesis invites further consideration of the endocannabinoid system regarding autism pathogenesis However, acetaminophen was not routinely taken by any of the subjects of this investigation, so its involvement with endocannabinoids remains speculative. Nevertheless, the question of the endocannabinoid system involvement in autism pathogenesis remains a potentially important concept deserving further investigation. Apart from the endocannabinoid system, other environmental autism risk factors (i.e. environmental toxics exposure, parental age, low birth weight, and maternal infections during pregnancy) are under consideration. Any of these may further interact with the endocannabinoid system as well. Further experiments are needed in order to better characterize the endocannabinoid system’s involvement in AD.
In conclusion, to our knowledge, this is the first study demonstrating an endocannabinoid-CB2 signalling dysregulation in autism, implying the endocannabinoid system may represent a new treatment opportunity for autism pharmacotherapy (Fig. 4). While the therapeutic use of the endocannabinoid systems is inviting, extensive research will be required to further evaluate this complex regulatory pathway and the safety of pharmacological manipulation.
Endocannabinoids, such as N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG), are synthesized and released upon demand in a receptor-dependent way, through the AEA biosynthetic enzyme N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) and the diacylglycerol (DAG) lipase enzyme, respectively. They exert their effects through the G-protein-coupled cannabinoid receptors CB1 and CB2, which, in turn, are negatively coupled to adenylyl cyclase enzyme. After the specific binding with their receptors, endocannabinoids are transported into cells by a specific uptake system and degraded by the enzymes fatty acid amide hydrolase (FAAH). In peripheral blood mononuclear cells, autistic disorders trigger over-production of CB2 receptor gene expression, as well as protein levels, together with a down-expression of NAPE-PLD
References
Agudelo, M., Newton, C., Widen, R., Sherwood, T., Nong, L., Friedman, H., et al. (2008). Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. Journal of Neuroimmune Pharmacology, 3(1), 35–42.
Alessio, N., Squillaro, T., Cipollaro, M., Bagella, L., Giordano, A., & Galderisi, U. (2010). The BRG1 ATPase of chromatin remodeling complexes is involved in modulation of mesenchymal stem cell senescence through RB-P53 pathways. Oncogene, 29(40), 5452–5463.
Algeciras-Schimnich, A., Barnhart, B. C., & Peter, M. E. (2002). Apoptosis independent functions of killer caspases. Current Opinion in Cell Biology, 14, 721–726.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Text Revision. Washington, DC: American Psychiatric Press.
Ardura, J. A., & Friedman, P. A. (2011). Regulation of g protein-coupled receptor function by na +/h + exchange regulatory factors. Pharmacological Reviews, 63(4), 882–900.
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I., & Van de Water, J. (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity, 25(1), 40–45.
Ashwood, P., Wills, S., & Van de Water, J. (2006). The immune response in autism: A new frontier for autism research. Journal of Leukocyte Biology, 80(1), 1–15.
Barna, I., & Zelena, D. (2012). The biochemical complexity of the endocannabinoid system with some remarks on stress and related disorders: A minireview. Endocrine Regulations, 46(2), 107–124.
Basu, S., & Dittel, B. N. (2011). Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunologic Research, 51(1), 26–38.
Bradford, M. M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Cencioni, M. T., Chiurchiù, V., Catanzaro, G., Borsellino, G., Bernardi, G., Battistini, L., et al. (2010). Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE, 5(1), e8688.
de Magistris, L., Familiari, V., Pascotto, A., Sapone, A., Frolli, A., Iardino, P., et al. (2010). Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of Pediatric Gastroenterology and Nutrition, 51(4), 418–424.
Di Filippo, C., Rossi, F., Rossi, S., & D’Amico, M. (2004). Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. Journal of Leukocyte Biology, 75(3), 453–459.
Di Marzo, V., & Petrosino, S. (2007). Endocannabinoids and the regulation of their levels in health and disease. Current Opinion in Lipidology, 18(2), 129–140.
Dong, R., Dong, K., Wang, X., Chen, G., Shen, C., & Zheng, S. (2012). Interleukin-33 overexpression is associated with gamma-glutamyl transferase in biliary atresia. Cytokine, S1043–4666(12), 00771–00775.
Enstrom, A. M., Onore, C. E., Van de Water, J. A., & Ashwood, P. (2010). Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain, Behavior, and Immunity, 24(1), 64–71.
Galiègue, S., Mary, S., Marchand, J., Dussossoy, D., Carrière, D., Carayon, P., et al. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry, 232(1), 54–61.
Garcia-Gutierrez, M. S., & Manzanares, J. (2011). Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. Journal of Psychopharmacology, 25(1), 111–120.
Giordano, C., Siniscalco, D., Melisi, D., Luongo, L., Curcio, A., Soukupova, M., et al. (2011). The galactosylation of N(ω)-nitro-l-arginine enhances its anti-nocifensive or anti-allodynic effects by targeting glia in healthy and neuropathic mice. European Journal of Pharmacology, 656(1–3), 52–62.
Guo, Y., Xiao, P., Lei, S., Deng, F., Xiao, G. G., Liu, Y., et al. (2008). How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochimica et Biophysica Sinica (Shanghai), 40(5), 426–436.
Gupta, S., Samra, D., & Agrawal, S. (2010). Adaptive and innate immune responses in autism: rationale for therapeutic use of intravenous immunoglobulin. Journal of Clinical Immunology, 30, S90–S96.
Gygi, S. P., Rochon, Y., Franza, B. R., & Aebersold, R. (1999). Correlation between protein and mRNA abundance in yeast. Molecular and Cellular Biology, 19(3), 1720–1730.
Hegde, V. L., Nagarkatti, M., & Nagarkatti, P. S. (2010). Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. European Journal of Immunology, 40(12), 3358–33571.
Högestätt, E. D., Jönsson, B. A., Ermund, A., Andersson, D. A., Björk, H., Alexander, J. P., et al. (2005). Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. Journal of Biological Chemistry, 280(36), 31405–31412.
Ishiguro, H., Horiuchi, Y., Ishikawa, M., Koga, M., Imai, K., Suzuki, Y., et al. (2010). Brain cannabinoid CB2 receptor in schizophrenia. Biological Psychiatry, 67(10), 974–982.
Jean-Gilles, L., Gran, B., & Constantinescu, C. S. (2010). Interaction between cytokines, cannabinoids and the nervous system. Immunobiology, 215(8), 606–610.
Kenny, P. J. (2011). Macrophage cannabinoid receptor goes up in smoke. Nature Neuroscience, 14, 1100–1102.
Klein, T. W., & Cabral, G. A. (2006). Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. Journal of Neuroimmune Pharmacology, 1(1), 50–64.
Klein, T. W., Newton, C., Larsen, K., Lu, L., Perkins, I., Nong, L., et al. (2003). The cannabinoid system and immune modulation. Journal of Leukocyte Biology, 74(4), 486–496.
Lamkanfi, M., Declercq, W., Kalai, M., Saelens, X., & Vandenabeele, P. (2002). Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death and Differentiation, 9, 358–361.
Levy, S. E., Mandell, D. S., & Schultz, R. T. (2009). Autism. Lancet, 374(9701), 1627–1638.
Li, X., Chauhan, A., Sheikh, A. M., Patil, S., Chauhan, V., Li, X. M., et al. (2009). Elevated immune response in the brain of autistic patients. Journal of Neuroimmunology, 207(1–2), 111–116.
Li, C., Jones, P. M., & Persaud, S. J. (2011). Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacology & Therapeutics, 129(3), 307–320.
Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Jr, Leventhal, B. L., DiLavore, P. C., et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible Pervasive Developmental Disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685.
Maccarrone, M., De Petrocellis, L., Bari, M., Fezza, F., Salvati, S., Di Marzo, V., et al. (2001). Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Archives of Biochemistry and Biophysics, 393(2), 321–328.
Maier, T., Güell, M., & Serrano, L. (2009). Correlation of mRNA and protein in complex biological samples. FEBS Letters, 583(24), 3966–3973.
Meyer, S., Temme, C., & Wahle, E. (2004). Messenger RNA turnover in eukaryotes: Pathways and enzymes. Critical Reviews in Biochemistry and Molecular Biology, 39(4), 197–216.
Minocci, D., Massei, J., Martino, A., Milianti, M., Piz, L., Di Bello, D., et al. (2011). Genetic association between bipolar disorder and 524A > C (Leu133Ile) polymorphism of CNR2 gene, encoding for CB2 cannabinoid receptor. Journal of Affective Disorders, 134(1–3), 427–430.
Molloy, C. A., Morrow, A. L., Meinzen-Derr, J., Schleifer, K., Dienger, K., Manning-Courtney, P., et al. (2006). Elevated cytokine levels in children with autism spectrum disorder. Journal of Neuroimmunology, 172(1–2), 198–205.
Mouslech, Z., & Valla, V. (2009). Endocannabinoid system: An overview of its potential in current medical practice. Neuro Endocrinology Letters, 30(2), 153–179.
Nagarkatti, P., Pandey, R., Rieder, S. A., Hegde, V. L., & Nagarkatti, M. (2009). Cannabinoids as novel anti-inflammatory drugs. Future Medicinal Chemistry, 1(7), 1333–1349.
Nong, L., Newton, C., Cheng, Q., Friedman, H., Roth, M. D., & Klein, T. W. (2002). Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. Journal of Neuroimmunology, 127(1–2), 169–176.
Onore, C., Enstrom, A., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Van de Water, J., et al. (2009). Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. Journal of Neuroimmunology, 216(1–2), 126–129.
Pacher, P., & Mechoulam, R. (2011). Is lipid signaling through cannabinoid 2 receptors part of a protective system? Progress in Lipid Research, 50(2), 193–211.
Pascal, L. E., True, L. D., Campbell, D. S., Deutsch, E. W., Risk, M., Coleman, I. M., et al. (2008). Correlation of mRNA and protein levels: Cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genomics, 9, 246.
Peralta, L., Agirregoitia, E., Mendoza, R., Expósito, A., Casis, L., Matorras, R., et al. (2011). Expression and localization of cannabinoid receptors in human immature oocytes and unfertilized metaphase-II oocytes. Reprod Biomed Online, 23(3), 372–379.
Pertwee, R. G., Howlett, A. C., Abood, M. E., Alexander, S. P., Di Marzo, V., Elphick, M. R., et al. (2010). Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacological Reviews, 62(4), 588–631.
Rajagopalan, L. E., & Malter, J. S. (1997). Regulation of eukaryotic messenger RNA turnover. Progress in Nucleic Acid Research and Molecular Biology, 56, 257–286.
Robinson, S. A., Loiacono, R. E., Christopoulos, A., Sexton, P. M., & Malone, D. T. (2010). The effect of social isolation on rat brain expression of genes associated with endocannabinoid signaling. Brain Research, 1343, 153–167.
Romero-Calvo, I., Ocón, B., Martínez-Moya, P., Suárez, M. D., Zarzuelo, A., Martínez-Augustin, O., et al. (2010). Reversible Ponceau staining as a loading control alternative to actin in Western blots. Analytical Biochemistry, 401(2), 318–320.
Ross, J. (1996). Control of messenger RNA stability in higher eukaryotes. Trends in Genetics, 12(5), 171–175.
Schneider, M., & Koch, M. (2005). Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: Effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology, 30(5), 944–957.
Schopler, E., Reichler, R. J., & Renner, B. R. (1993). The childhood autism rating scale (CARS). Los Angeles, CA: Western Psychological Services.
Schultz, S. T. (2010). Can autism be triggered by acetaminophen activation of the endocannabinoid system? Acta Neurobiologiae Experimentalis, 70(2), 227–231.
Siniscalco, D., Sapone, A., Giordano, C., Cirillo, A., de Novellis, V., de Magistris, L., et al. (2012). The expression of caspases is enhanced in peripheral blood mononuclear cells of autism spectrum disorder patients. Journal of Autism and Developmental Disorders, 42(7), 1403–1410.
Soukupová, M., Palazzo, E., De Chiaro, M., Gatta, L., Migliozzi, A. L., Guida, F., et al. (2010). Effects of URB597, an inhibitor of fatty acid amide hydrolase (FAAH), on analgesic activity of paracetamol. Neuro Endocrinology Letters, 31(4), 507–511.
Stamova, B. S., Apperson, M., Walker, W. L., Tian, Y., Xu, H., Adamczy, P., et al. (2009). Identification and validation of suitable endogenous reference genes for gene expression studies in human peripheral blood. BMC Medical Genomics, 2, 49.
Suzuki, K., Matsuzaki, H., Iwata, K., Kameno, Y., Shimmura, C., Kawai, S., et al. (2011). Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE, 6(5), e20470.
Tanikawa, T., Kurohane, K., & Imai, Y. (2011). Regulatory effect of cannabinoid receptor agonist on chemokine-induced lymphocyte chemotaxis. Biological and Pharmaceutical Bulletin, 34(7), 1090–1093.
Tománková, H., & Myslivecek, J. (2012). Regulation of receptors coupled to G proteins (GPCRs). Ceskoslovenska Fysiologie, 61(1), 15–23.
Wilusz, C. J., Wormington, M., & Peltz, S. W. (2001). The cap-to-tail guide to mRNA turnover. Nature Reviews Molecular Cell Biology, 2(4), 237–246.
Yang, M., Liu, Y., Lu, S., Wang, Z., Wang, R., Zi, Y., et al. (2013). Analysis of the expression levels of survivin and VEGF in patients with acute lymphoblastic leukemia. Experimental and Therapeutic Medicine, 5(1), 305–307.
Zanichelli, F., Capasso, S., Cipollaro, M., Pagnotta, E., Cartenì, M., Casale, F., et al. (2012). Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr), 34(2), 281–293.
Zhu, C., Solorzano, C., Sahar, S., Realini, N., Fung, E., Sassone-Corsi, P., et al. (2011). Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Molecular Pharmacology, 79(4), 786–792.
Acknowledgments
First and foremost, we thank the many autism families who volunteered as participants in this research study. The authors gratefully thank Mr. Enzo Abate, Ms. Giovanna Gallone and the no-profit organizations “La Forza del Silenzio” and “Cancellautismo”—Italy for their useful assistance. We thank the Autism Research Institute, USA (ARI grant “Research that makes a difference” 2010) for financial support of this study. The authors would like to thank Dr. Sarah Costantino, Second University of Naples, for her useful assistance in image analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siniscalco, D., Sapone, A., Giordano, C. et al. Cannabinoid Receptor Type 2, but not Type 1, is Up-Regulated in Peripheral Blood Mononuclear Cells of Children Affected by Autistic Disorders. J Autism Dev Disord 43, 2686–2695 (2013). https://doi.org/10.1007/s10803-013-1824-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-013-1824-9