Abstract
Autism and autism spectrum disorders (ASDs) are heterogeneous complex neuro-developmental disorders characterized by dysfunctions in social interaction and communication skills. Their pathogenesis has been linked to interactions between genes and environmental factors. Consistent with the evidence of certain similarities between immune cells and neurons, autistic children also show an altered immune response of peripheral blood mononuclear cells (PBMCs). In this study, we investigated the activation of caspases, cysteinyl aspartate-specific proteases involved in apoptosis and several other cell functions in PBMCs from 15 ASD children compared to age-matched normal healthy developing controls. The mRNA levels for caspase-1, -2, -4, -5 were significantly increased in ASD children as compared to healthy subjects. Protein levels of Caspase-3, -7, -12 were also increased in ASD patients. Our data are suggestive of a possible role of the capsase pathway in ASD clinical outcome and of the use of caspase as potential diagnostic and/or therapeutic tools in ASD management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism and autism spectrum disorders (ASDs) are heterogeneous neuro-developmental disorders characterized by dysfunctions in social interaction and communication skills, in addition to repetitive and stereotypic verbal and non-verbal behaviours (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR), American Psychiatric Association 2000; Toro et al. 2010; Williams et al. 2010). They are complex conditions that have their origins in the interaction of genes and environmental factors. The pathophysiology of autism is still unclear and no defined mechanisms of pathogenesis or effective treatments are currently available (Levy et al. 2009). Several biochemical and cellular events have been reported to be linked to ASDs: oxidative stress, decreased methylation capacity, limited production of glutathione, mitochondrial dysfunction, intestinal dysbiosis, increased toxic metal burden, and immune dysregulation and immune activation of neuroglial cells (Bradstreet et al. 2010).
Recent studies suggest that peripheral blood mononuclear cells (PBMCs) may represent a useful tool to investigate systemic neurochemical changes in neurodegenerative diseases (Buttarelli et al. 2006). Moreover, dysfunction in PBMC response could result in long-term immune alterations also in ASDs (Stubbs and Crawford 1977; Ashwood et al. 2006; Enstrom et al. 2010). Indeed, ASD children show an altered immune cell ratio and a decreased number of T lymphocytes, as well as altered gene expression in peripheral blood leucocytes (Warren et al. 1986; Denney et al. 1996; Gregg et al. 2008). In addition, PBMCs from ASD children present elevated levels of pro-inflammatory cytokines and interleukins (Molloy et al. 2006; Onore et al. 2009).
Caspases are cysteinyl aspartate-specific proteases that play a critical role in the regulatory and execution phases of apoptosis (Salvesen and Riedl 2008; Siniscalco et al., 2008). An increasing number of studies highlight non-apoptotic functions mediated by caspases (Launay et al. 2005; Yi and Yuan 2009; Bergmann and Steller 2010). Indeed, caspases play a critical role by acting as regulators of monocyte cell fate (Parihar et al. 2010) as well as by mediating both innate and adaptive immune responses (van de Veerdonk et al. 2011; Yazdi et al. 2010). Both these immune responses are of great interest in ASDs in light of the fact that immune abnormalities in autism pathogenesis have been reported (Gupta et al. 2010). In addition, caspases are related to several pro-inflammatory processes (Martinon and Tschopp 2007). These inflammatory responses are altered in ASDs. Moreover, pro-inflammatory cytokines could induce some of the behavioural symptoms of autism (Croonenberghs et al. 2002). Therefore, caspases could be an interesting target to better define the immune and inflammatory molecular pathways involved in autism pathophysiology.
To our knowledge there are no studies aimed at identifying possible changes in the expression of caspases in ASDs patients. In this study, we have addressed the issue of whether these disorders might modify over-expression and over-activation of several caspases in PBMCs from ASD patients.
Materials and Methods
Subjects
An informed consent was obtained from all subjects enrolled in this study in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
Autistic (n = 15) and healthy control (n = 10) children of both sexes age ranging 3–9 years old were enrolled into the study from the outpatient Centre for Autism of La Forza del Silenzio, Naples-Caserta, Italy. The diagnosis of autism was made using a clinical evaluation according the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria, along with the Childhood Autism Rating Scale (Schopler et al. 1993). The autistic children had to fulfil DSM IV criteria for autistic disorder, and score at least 30 points in the CARS scale. Participants were excluded if they had: a neurological and psychiatric disorder other than autism and comorbid disorders, such as epilepsy; history of liver, renal or endocrine disorder; current infection of respiratory tract or fever state of any origin; and mental retardation. Mental retardation or behavioural disorders, including hyperkinetic disorder or significant symptoms of hyperactivity, impulsiveness or restlessness were exclusion criteria only for the group of healthy control children, but were allowed as comorbid condition in the autistic cohort. Children diagnosed with Asperger’s syndrome were excluded from the study.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Fresh peripheral blood samples from ASD patients and healthy donors were drawn and collected in sterile EDTA tubes (Becton–Dickinson, Franklin Lakes, NJ). Peripheral blood mononuclear cells (PMBCs) were isolated by centrifugation over Histopaque 1077 density gradient (Sigma Chemical, St Louis, MO). Briefly, blood was diluted 1:1 in PBS, overlaid onto lymphocyte separation media (Lymphocyte Separation Medium—Lonza, Walkersville, MD), centrifuged at 2,200 rpm for 30 min at room temperature and plasma was removed. Mononuclear cell fraction was harvested and washed twice in phosphate buffer saline (PBS) (Sigma, St. Louis, MO). Final pellet was re-suspended in Tri-Reagent solution (Molecular Research Center Inc., Cincinnati, OH) or protein lysis buffer for further molecular analysis.
RNA Extraction and RT–PCR
Total RNA was extracted from PBMCs using a RNA Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH) according to the manufacturer’s protocol. The total RNA concentration and integrity were determined by Nanodrop ND-1000 UV spectrophotometer (Nano-Drop® Technologies, Thermo Scientific, Wilmington, DE). The mRNA levels of the caspase genes under analysis were measured by RT–PCR amplification, as previously reported (Siniscalco et al. 2007). Reverse Transcriptase from Avian Myeloblastosis Virus (AMV-RT; Promega, Madison, WI) was used. Briefly, for first-strand cDNA synthesis 200 ng total RNA, random examers, dNTPs (Promega, Madison, WI), AMV buffer, AMV-RT and recombinant RNasin ribonuclease inhibitor (Promega, Madison, WI) were assembled in diethyl-pyrocarbonate-treated water to a 20 μl final volume and incubated for 10 min at 65°C and 1 h at 42°C. RT minus controls were carried out to check potential genomic DNA contamination. These RT minus controls were performed without using the reverse transcriptase enzyme in the reaction mix. Aliquots of 2 μl cDNA were transferred into a 25 μl PCR reaction mixture containing dNTPs, MgCl2, reaction buffer, specific primers and GoTaq Flexi DNA polymerase (Promega, Madison, WI), and amplification reactions using specific primers and conditions for human caspase genes cDNA were carried out. Sequences for the human mRNAs from GeneBank (DNASTAR INC., Madison, WI) were used to design specific primer pairs for RT-PCRs (OLIGO 4.05 software, National Biosciences Inc., Plymouth, MN) (primers available on request). Each RT–PCR was repeated at least three times to achieve the best reproducibility data. The mean of the inter-assay variability of each RT-PCR assay was 0.05. The levels of mRNA measured were normalized with respect to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was chosen as the housekeeping gene. Indeed GAPDH is one of the most stably expressed genes in human peripheral blood (Stamova et al. 2009) To our knowledge, there is no molecular evidence of variation in GAPDH mRNA-levels in ASDs. The gene expression values were expressed as arbitrary units ± S.E.M. Amplification of the genes of interest and GAPDH was performed simultaneously. PCR products were resolved into 2.0% agarose gel. A semi-quantitative analysis of mRNA levels was carried out by the “Gel Doc 2000 UV System” (Bio-Rad, Hercules, CA).
Protein Extraction and Western Blot Analysis
For protein extraction, PBMCs were suspended in protein lysis buffer [HEPES 25 mM; EDTA 5 mM; SDS 1%; Triton X-100 1%; PMSF 1 mM; MgCl2 5 mM; Protease Inhibitor Cocktail (Roche, Mannheim, Germany); Phospahatase Inhibitor Cocktail (Roche, Mannheim, Germany)]. Protein concentration was determined using the method described by Bradford (1976). Each sample was loaded, electrophoresed in a 15% SDS–polyacrylamide gel and electroblotted onto a nitrocellulose membrane. The membrane was blocked in 5% milk, 1× Tris-buffered saline and 0.05% Tween-20. Primary antibodies to detect Caspase-7 (StressGen, Victoria, Canada) and Caspase-12 (Chemicon-Millipore, Temecula, CA) were used according to the manufacturer’s instructions at 1:1000 dilutions. Immunoreactive signals were detected with a horseradish peroxidase-conjugated secondary antibody and reacted with an ECL system (Amersham Pharmacia, Uppsala, Sweden). Protein levels were normalized with respect to the signal obtained with anti-β-actin monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000 dilution). The semi-quantitative analysis of protein levels was carried out by the Gel Doc 2000 UV System and the Gel Doc EZ Imager, using Image Lab 3.0 software (Bio-Rad, Hercules, CA).
Immunocytochemistry
For immunocytochemical analysis, mononuclear cells were re-suspended at 1 × 106 cell/ml in RPMI 1640 complete medium (Lonza, Verviers, Belgium) containing 10% fetal bovine serum (FBS) (EuroClone-Celbio, Milan, Italy), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (all Lonza), were plated in slides into a 12-well plate and incubated for 4 days at 37°C with 5% CO2. Cells were then fixed with 4% paraformaldheyde fixative. After washing in PBS, non-specific antibody binding was inhibited by incubation for 30 min in blocking solution (1% BSA in PBS). Primary antibodies were diluted in PBS blocking buffer and slides were incubated overnight at 4°C in primary antibodies to human polyclonal anti-active (cleaved) form Caspase-3 (1:200; Chemicon-Millipore, Temecula, CA). Fluorescent-labelled secondary antibodies (1:1000; Alexa Fluor 488, Molecular Probe; Invitrogen, Carlsbad, CA) specific to the IgG species used as a primary antibody were used to locate the specific antigens in each slide. Cells were counterstained with bisbenzimide (Hoechst 33258; Hoechst, Frankfurt, Germany) and mounted with mounting medium (90% glycerol in PBS). Fluorescently labelled slides were viewed with a fluorescence microscope (Leica, Wetzlar, Germany). Immunofluorescence images were analyzed with Leica FW4000 software (Leica, Wetzlar, Germany). Only bisbenzimide counterstained cells were considered as positive profiles so as to avoid over counting cells.
Statistical Analysis
Biomolecular data are expressed as means ± SEM. ANOVA, followed by Student–Neuman–Keuls post hoc test used to determine the statistical significance among groups. p < 0.05 was considered statistically significant.
Results
ASDs Trigger Over-Expression of Caspase Genes
We examined caspase expression mainly by RT–PCR, since this technique is a far more sensitive method for the detection of gene expression than immunocytochemistry (Giordano et al. 2011; Dickel et al. 2010). In addition, caspases under analysis here also show a transcriptional regulative mechanism (Aitken et al. 2011; Giordano et al. 2011; Salskov-Iversen et al. 2011; Andrianjafiniony et al. 2010; Banerjee et al. 2010; Fang et al. 2010; Heikaus et al. 2010; Lisi et al. 2010; Lakshmanan and Porter 2007; Dai and Krantz 1999). The semiquantitative analysis of PBMC-extracted mRNA levels, measured by RT-PCR amplification, showed an increase in the pro-inflammatory caspase-1, -4, -5 and in the cellular stress-related caspase-2 gene in PBMCs of ASD patients, whereas mRNA levels of caspase-8 gene did not change (Fig. 1; Table 1).
Over-expression of caspase-1, -2, -4, and -5, but not of caspase-8 in ASD-PBMCs. The measured mRNA levels were normalized with respect to GAPDH (housekeeping gene) and gene expression values were expressed as a percentage of arbitrary units ± SEM. Open circle indicates significant difference versus healthy controls. p values <0.05 were considered statistically significant. Legend: CTL healthy control subjects, ASD autism spectrum disorder patients
ASDs Increase Caspase-3, Caspase-7 and Caspase-12 Protein Levels
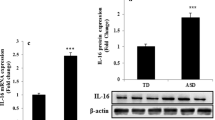
As principal executioner caspases, caspase-3, -7 and -12 show post-translational regulation (Martinez et al., 2010; Nakatsumi and Yonehara, 2010). We therefore determined the activation profiles of caspase-7 and caspase-12 by analysis of protein levels, as well as the activation of caspase-3 by immunocytochemistry. Western blot analysis showed a remarkable increase in caspase-7 and caspase-12 protein levels in ASD patients as compared to healthy controls (Fig. 2). Protein level analysis in ASD patients and control groups was performed simultaneously and in the same way. Caspase protein level was enhanced in all the ASD patients evaluated. Statistically significant differences were not observed in the control group.
Representative western blot analysis of caspase-7 and caspase-12 protein levels in the PBMCs obtained from the ASD patients and the healthy controls, respectively. Legend: CTL healthy control subjects; ASD autism spectrum disorder patients. The histograms indicate percentage variations in caspase-7 and -12 protein levels normalized with respect to the signal obtained for β-actin housekeeping protein in the PBMCs of ASD patients compared to the healthy controls (CTL). Open circle indicates significant differences versus healthy subjects. p < 0.05 was considered as the level of significance
To visualize caspase activation, we also investigated the protein levels of the active form of executioner caspase-3 in the PBMCs using fluorescence immunocytochemistry. Immunofluorescence analysis carried out using an antibody able to detect endogenous levels of the large fragments of activated caspase-3 without recognizing full-length caspase-3 or other cleaved caspases, confirmed that caspase-3 was over-activated in the PBMCs in ASD patients as compared to healthy controls (Fig. 3).
Representative fluorescent photomicrograph of PBMCs showing immunocytochemistry for active caspase-3. Arrows indicate active form Caspase-3 positive staining (green fluorescent). Cell nuclei were counterstained with bisbenzimide (blue fluorescence). a healthy control subjects; b autism spectrum disorder patients. Scale bar: 15 μm
Discussion
In this study we demonstrated for the first time the up-regulation and activation of several caspases in PBMCs from ASDs subjects.
It is well known that caspase activity is essential for several cellular processes other than apoptosis, including differentiation, activation and nuclear reprogramming pathways (Li et al. 2010; Lamkanfi et al. 2007; Algeciras-Schimnich et al. 2002).
Indeed, it is noteworthy that caspases are involved in the activation-induced proliferation of T lymphocytes (Paulsen et al. 2008), as well as in the activation of various hematopoietic cells, including the activation and differentiation of monocytes (Adam-Klages et al. 2005).
Caspase-1 is essential for inflammasome protein scaffold formation and for the subsequent cleavage and activation of the potent pro-inflammatory interleukin-1beta (IL-1β) and IL-18 (Bauernfeind et al. 2011; Yazdi et al. 2010). In this way, caspase-1 triggers innate immune responses; in addition IL-1β and IL-18 release initiates Th1- and Th17-mediated adaptive immune responses (van de Veerdonk et al. 2011). Recently, immunological abnormalities and changes in innate and adaptive immunity in autism pathogenesis have been reported (Gupta et al. 2010). Interestingly, the brain of ASD patients shows an increased innate and adaptive immune response through the Th1 pathway (Li et al. 2009). Innate and adaptive immune responses are also excessive in ASD-PBMCs (Jyonouchi et al. 2001). PBMCs from children with ASDs show increased activation of both Th1- and Th2- mediated immune response; however, it is not yet clear if these immune alterations in ASD-PBMCs correlate with immune changes within the central nervous system (Li et al. 2009). Imbalance in the critical components of innate immunity, i.e. CD3+ , CD4+ and CD8+ T cells, as well as in natural killer (NK) and in antigen-presenting (APC) cells, has been also demonstrated in ASDs (Gupta et al. 2010). In addition, ASD-PBMCs are able to over-produce IL-1β, resulting in long-term immune alterations (Enstrom et al. 2010).
The fact that we found caspase-1 gene over-expression could indicate that this caspase could play a key role in triggering immune response changes in ASD-PBMCs.
Besides caspase-1, the inflammatory caspase family includes both caspase-4 and -5, also involved in cytokine maturation (Lakshmanan and Porter 2007; Martinon and Tschopp 2007; Agard et al. 2010). Our data showing increased mRNAs for these pro-inflammatory caspases in ASD-PBMCs suggest that these proteolytic enzymes may act as inducers of altered immune responses in autistic children.
Caspase-3 and -7 belong to the executioner family and are closely related to the apoptotic process. However, they could also play physiological roles (Siniscalco et al. 2008). Caspase-7 is a direct substrate of caspase-1 in inflammatory processes (Lamkanfi et al. 2008) and has a different enzymatic site to caspase-3 (Riedl et al. 2001). Very interestingly, localized cytoplasm and activated caspase-3 and -7 are required for the proliferation of T lymphocytes, indicating that these two caspases could be involved in the activation of survival pathways, as well as a different pattern of cleaved substrates (McComb et al. 2010; Paulsen et al. 2008). Hence our results could indicate the participation of these two caspases in T lymphocyte activation in ASDs.
In some cases, caspases could act as cytoprotective rather than cytotoxic agents (Launay et al. 2005); indeed, we cannot exclude that also in our system they exert a protective role in response to ASD-mediated inflammatory stimuli.
However, due to the strong activation of caspase-3 found here, we cannot exclude an apoptotic process active in ASD-PBMCs.
Beside its role in apoptosis, caspase-8 also plays a role in T cell activation (Oberst et al. 2011). We did not find any difference in caspase-8 gene expression in ASD children compared to healthy matched controls. However, post-transcriptional mechanisms of activation could be taken into account. In addition, Lakshmanan and Porter report that in monocytic cells it is unlikely that caspase-8 is involved together with caspase-4 in cytokine production (Lakshmanan and Porter 2007). Indeed, these authors demonstrated that caspase-4 knock down monocytic cells show decreased cytokine production, although normally synthesizing procaspase-8.
A recent study reports that ASDs are related to endoplasmic reticulum (ER) stress (Fujita et al. 2010). ER imbalance in Ca2+ homeostasis and/or in the protein-folding machinery triggers activation of caspase-12 (Momoi 2004), whereas prolonged ER stress, such as sustained accumulation of unfolded proteins, triggers the activation of caspase-2 and ultimately results in caspase-mediated cell death (Murakami et al. 2007). However, unlike other caspases, caspase-2 is also constitutively localized in the nucleus, where it is involved in the G2/M DNA damage checkpoint maintenance (Shi et al. 2009). These two caspases are mainly involved in cellular stress pathways (i.e. oxidative stress, apoptosis). Indeed, activated caspase-2 and caspase-12 can act as sensors and/or effectors of cellular damage (Ruiz-Vela et al. 2005). On these issues and on the basis of our results, the activation of these two proteases could indicate possible cellular stress events in ASD-PBMCs.
Further experiments are required in order to better characterize caspase involvement in ASDs, as well as the exact molecular pathways activated by these proteolytic enzymes. Recently, Voineagu observed upregulation of immune response genes in the autistic brain. These immune changes have no evidence of a common genetic component and could be most likely secondary phenomena or caused by environmental factors (Voineagu et al. 2011). The caspase dysregulation in ASD-PBMCs we found here could be due to secondary phenomena. Indeed, a more recent study indicates that the influence of genetic factors on the susceptibility to develop autism could be overestimated, highlighting the potential role of environmental triggers in causing ASDs (Hallmayer et al. 2011). However, we cannot exclude that caspases may be a key mechanism linking primary and downstream processes.
Several studies indicate caspases as a therapeutic target for apoptosis- and inflammatory-related diseases (MacKenzie et al. 2010); this study provides evidence that caspases are activated in ASD-PBMCs, providing potential targets for the development of diagnostic and/or therapeutic strategies for ASDs. Diagnostic value of caspases in ASDs could derive from their unique and specific over-expression and over-activation pattern. Indeed, while several other diseases are characterized by activation of only specific caspases, ASDs seems to be associated to generalized activation of many caspase family members. Validation of caspases as a diagnostic tools and possible therapeutic target in clinical setting are necessary to confirm our hypothesis and are currently explored in our laboratories.
References
Adam-Klages, S., Adam, D., Janssen, O., & Kabelitz, D. (2005). Death receptors and caspases: Role in lymphocyte proliferation, cell death, and autoimmunity. Immunologic Research, 33(2), 149–166.
Agard, N. J., Maltby, D., & Wells, J. A. (2010). Inflammatory stimuli regulate caspase substrate profiles. Molecular Cell Proteomics, 9(5), 880–893.
Aitken, S. L., Corl, C. M., & Sordillo, L. M. (2011). Pro-inflammatory and pro-apoptotic responses of TNF-α stimulated bovine mammary endothelial cells. Veterinary Immunology and Immunopathology, 140(3–4), 282–290.
Algeciras-Schimnich, A., Barnhart, B. C., & Peter, M. E. (2002). Apoptosis independent functions of killer caspases. Current Opinion in Cell Biology, 14, 721–726.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, text revision, 4th ed. Washington, DC: American Psychiatric Press.
Andrianjafiniony, T., Dupré-Aucouturier, S., Letexier, D., Couchoux, H., & Desplanches, D. (2010). Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. American Journal of Physiology-Cell Physiology, 299, C307–C315.
Ashwood, P., Wills, S., & Van de Water, J. (2006). The immune response in autism: A new frontier for autism research. Journal of Leukocyte Biology, 80(1), 1–15.
Banerjee, M., Datta, M., Majumder, P., Mukhopadhyay, D., & Bhattacharyya, N. P. (2010). Transcription regulation of caspase-1 by R393 of HIPPI and its molecular partner HIP-1. Nucleic Acids Research, 38(3), 878–892.
Bauernfeind, F., Ablasser, A., Bartok, E., Kim, S., Schmid-Burgk, J., Cavlar, T., et al. (2011). Inflammasomes: Current understanding and open questions. Cellular and Molecular Life Sciences, 68(5), 765–783.
Bergmann, A., & Steller, H. (2010). Apoptosis, stem cells, and tissue regeneration. Science Signal, 3(145), re8.
Bradford, M. M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bradstreet, J. J., Smith, S., Baral, M., & Rossignol, D. A. (2010). Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Alternative Medicine Review, 15(1), 15–32.
Buttarelli, F. R., Circella, A., Pellicano, C., & Pontieri, F. E. (2006). Dopamine transporter immunoreactivity in peripheral blood mononuclear cells in amyotrophic lateral sclerosis. European Journal of Neurology, 13(4), 416–418.
Croonenberghs, J., Bosmans, E., Deboutte, D., Kenis, G., & Maes, M. (2002). Activation of the inflammatory response system in autism. Neuropsychobiology, 45, 1–6.
Dai, C., & Krantz, S. B. (1999). Interferon gamma induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood, 93(10), 3309–3316.
Denney, D. R., Frei, B. W., & Gaffney, G. R. (1996). Lymphocyte subsets and interleukin-2 receptors in autistic children. Journal of Autism and Developmental Disorders, 26, 87–97.
Dickel, H., Gambichler, T., Kamphowe, J., Altmeyer, P., & Skrygan, M. (2010). Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: New insights into the involvement of ‘alarmins’. Contact Dermatitis, 63(4), 215–222.
Enstrom, A. M., Onore, C. E., Van de Water, J. A., & Ashwood, P. (2010). Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain, Behavior, and Immunity, 24(1), 64–71.
Fang, H. Y., Lin, C. Y., Chow, K. C., Huang, H. C., & Ko, W. J. (2010). Microarray detection of gene overexpression in primary spontaneous pneumothorax. Experimental Lung Research, 36, 323–330.
Fujita, E., Dai, H., Tanabe, Y., Zhiling, Y., Yamagata, T., Miyakawa, T., et al. (2010). Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Disorder, 1(6), e47.
Giordano, C., Siniscalco, D., Melisi, D., Luongo, L., Curcio, A., Soukupova, M., et al. (2011). The galactosylation of N(ω)-nitro-l-arginine enhances its anti-nocifensive or anti-allodynic effects by targeting glia in healthy and neuropathic mice. European Journal of Pharmacology, 656(1–3), 52–62.
Gregg, J. P., Lit, L., Baron, C. A., Hertz-Picciotto, I., Walker, W., Davis, R. A., et al. (2008). Gene expression changes in children with autism. Genomics, 91(1), 22–29.
Gupta, S., Samra, D., & Agrawal, S. (2010). Adaptive and innate immune responses in Autism: Rationale for therapeutic use of intravenous immunoglobulin. Journal of Clinical Immunology, 30, S90–S96.
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with Autism. Archives of General Psychiatry (in press).
Heikaus, S., Pejin, I., Gabbert, H. E., Ramp, U., & Mahotka, C. (2010). PIDDosome expression and the role of caspase-2 activation for chemotherapy-induced apoptosis in RCCs. Cell Oncol, 32(1–2), 29–42.
Jyonouchi, H., Sun, S., & Le, H. (2001). Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. Journal of Neuroimmunology, 120(1–2), 170–179.
Lakshmanan, U., & Porter, A. G. (2007). Caspase-4 interacts with TNF receptor-associated factor 6 and mediates lipopolysaccharide-induced NF-kappaB-dependent production of IL-8 and CC chemokine ligand 4 (macrophage-inflammatory protein-1). Journal of Immunology, 179(12), 8480–8490.
Lamkanfi, M., Festjens, N., Declercq, W., Vanden Berghe, T., & Vandenabeele, P. (2007). Caspases in cell survival, proliferation and differentiation. Cell Death Differentiation, 14, 44–55.
Lamkanfi, M., Kanneganti, T. D., Van Damme, P., Vanden Berghe, T., Vanoverberghe, I., Vandekerckhove, J., et al. (2008). Targeted peptide-centric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Molecular Cell Proteomics, 7(12), 2350–2363.
Launay, S., Hermine, O., Fontenay, M., Kroemer, G., Solary, E., & Garrido, C. (2005). Vital functions for lethal caspases. Oncogene, 24(33), 5137–5148.
Levy, S. E., Mandell, D. S., & Schultz, R. T. (2009). Autism. Lancet, 374(9701), 1627–1638.
Li, X., Chauhan, A., Sheikh, A. M., Patil, S., Chauhan, V., Li, X. M., et al. (2009). Elevated immune response in the brain of autistic patients. Journal of Neuroimmunology, 207(1–2), 111–116.
Li, F., He, Z., Shen, J., Huang, Q., Li, W., Liu, X., et al. (2010). Apoptotic caspases regulate induction of iPSCs from human fibroblasts. Cell & Stem Cell Engineering, 7(4), 508–520.
Lisi, S., Sisto, M., Lofrumento, D., Frassanito, M. A., Caprio, S., Romano, M. L., et al. (2010). Regulation of mRNA caspase-8 levels by anti-nuclear autoantibodies. International Journal of Clinical & Experimental Medicine, 10, 199–203.
MacKenzie, S. H., Schipper, J. L., & Clark, A. C. (2010). The potential for caspases in drug discovery. Current Opinion in Drug Discovery & Development, 13(5), 568–576.
Martinez, J. A., Zhang, Z., Svetlov, S. I., Hayes, R. L., Wang, K. K., & Larner, S. F. (2010). Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis, 15(12), 1480–1493.
Martinon, F., & Tschopp, J. (2007). Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death and Differentiation, 14(1), 10–22.
McComb, S., Mulligan, R., & Sad, S. (2010). Caspase-3 is transiently activated without cell death during early antigen driven expansion of CD8(+) T cells in vivo. PLoS One, 5(12), e15328.
Molloy, C. A., Morrow, A. L., Meinzen-Derr, J., Schleifer, K., Dienger, K., Manning-Courtney, P., et al. (2006). Elevated cytokine levels in children with autism spectrum disorder. Journal of Neuroimmunology, 172(1–2), 198–205.
Momoi, T. (2004). Caspases involved in ER stress-mediated cell death. Journal of Chemical Neuroanatomy, 28(1–2), 101–105.
Murakami, Y., Aizu-Yokota, E., Sonoda, Y., Ohta, S., & Kasahara, T. (2007). Suppression of endoplasmic reticulum stress-induced caspase activation and cell death by the overexpression of Bcl-xL or Bcl-2. Journal of Biochemistry, 141(3), 401–410.
Nakatsumi, H., & Yonehara, S. (2010). Identification of functional regions defining different activity in caspase-3 and caspase-7 within cells. World Journal of Biological Chemistry, 285(33), 25418–25425.
Oberst, A., Dillon, C. P., Weinlich, R., McCormick, L. L., Fitzgerald, P., Pop, C., et al. (2011). Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature, 471(7338), 363–367.
Onore, C., Enstrom, A., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Van de Water, J., et al. (2009). Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. Journal of Neuroimmunology, 216(1–2), 126–129.
Parihar, A., Eubank, T. D., & Doseff, A. I. (2010). Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. Journal of Innate Immunity, 2(3), 204–215.
Paulsen, M., Ussat, S., Jakob, M., Scherer, G., Lepenies, I., Schütze, S., et al. (2008). Interaction with XIAP prevents full caspase-3/-7 activation in proliferating human T lymphocytes. European Journal of Immunology, 38(7), 1979–1987.
Riedl, S. J., Fuentes-Prior, P., Renatus, M., Kairies, N., Krapp, S., Huber, R., et al. (2001). Structural basis for the activation of human procaspase-7. Proceedings of the National Academy of Sciences of the United States of America, 98, 14790–14795.
Ruiz-Vela, A., Opferman, J. T., Cheng, E. H., & Korsmeyer, S. J. (2005). Proapoptotic BAX and BAK control multiple initiator caspases. European Molecular Biology Organization Reports, 6(4), 379–385.
Salskov-Iversen, M. L., Johansen, C., Kragballe, K., & Iversen, L. (2011). Caspase-5 expression is upregulated in lesional psoriatic skin. Journal of Investigative Dermatology, 131(3), 670–676.
Salvesen, G. S., & Riedl, S. J. (2008). Caspase mechanisms. Advances in Experimental Medicine and Biology, 615, 13–23.
Schopler, E., Reichler, R. J., & Renner, B. R. (1993). The childhood autism rating scale (CARS). Los Angeles: Western Psychological Services.
Shi, M., Vivian, C. J., Lee, K. J., Ge, C., Morotomi-Yano, K., Manzl, C., et al. (2009). DNA-PKcs-PIDDosome: a nuclear caspase-2-activating complex with role in G2/M checkpoint maintenance. Cell, 136(3), 508–520.
Siniscalco, D., Fuccio, C., Giordano, C., Ferraraccio, F., Palazzo, E., Luongo, L., et al. (2007). Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacological Research, 55(2), 158–166.
Siniscalco, D., Giordano, C., Fuccio, C., Luongo, L., Ferraraccio, F., Rossi, F., et al. (2008). Involvement of subtype 1 metabotropic glutamate receptors in apoptosis and caspase-7 over-expression in spinal cord of neuropathic rats. Pharmacological Research, 57(3), 223–233.
Stamova, B. S., Apperson, M., Walker, W. L., Tian, Y., Xu, H., Adamczy, P., et al. (2009). Identification and validation of suitable endogenous reference genes for gene expression studies in human peripheral blood. BMC Medical Genomics, 2, 49.
Stubbs, E. G., & Crawford, M. L. (1977). Depressed lymphocyte responsiveness in autistic children. Journal of Autism and Childhood Schizophrenia, 7, 49–55.
Toro, R., Konyukh, M., Delorme, R., Leblond, C., Chaste, P., Fauchereau, F., et al. (2010). Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends in Genetics, 26(8), 363–372.
van de Veerdonk, F. L., Netea, M. G., Dinarello, C. A., & Joosten, L. A. (2011). Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunology, 32(3), 110–116.
Voineagu, I., Wang, X., Johnston, P., Lowe, J. K., Tian, Y., Horvath, S., et al. (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature, 474(7351), 380–384.
Warren, R. P., Margaretten, N. C., Pace, N. C., & Foster, A. (1986). Immune abnormalities in patients with autism. Journal of Autism and Developmental Disorders, 16, 189–197.
Williams, K., Wheeler, D. M., Silove, N., & Hazell, P. (2010). Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). The Cochrane Database of Systematic Reviews, 8, CD004677.
Yazdi, A. S., Guarda, G., D’Ombrain, M. C., & Drexler, S. K. (2010). Inflammatory caspases in innate immunity and inflammation. Journal of Innate Immunity, 2(3), 228–237.
Yi, C. H., & Yuan, J. (2009). The Jekyll and Hyde functions of caspases. Stem Cells and Development, 16(1), 21–34.
Acknowledgments
First and foremost, we thank the many autism families who volunteered as participants in this research study. The authors gratefully thank Mr. Enzo Abate and no-profit organization “La Forza del Silenzio”- Italy for their useful assistance. We thank the Autism Research Institute, USA (ARI grant “Research that makes a difference” 2010) for financial support of this study.
Conflict of interest
Dr. Anna Sapone is scientific consultant of Schaer Italy Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siniscalco, D., Sapone, A., Giordano, C. et al. The Expression of Caspases is Enhanced in Peripheral Blood Mononuclear Cells of Autism Spectrum Disorder Patients. J Autism Dev Disord 42, 1403–1410 (2012). https://doi.org/10.1007/s10803-011-1373-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-011-1373-z