Abstract
Purpose
The patterns of uveitis in Tokyo have recently changed due to advances in examination tools. We aimed to investigate the changes in the patterns of uveitis between 2004–2015 and 2016–2018.
Methods
We retrospectively reviewed the data of 732 patients who visited the Uveitis Clinic at the University of Tokyo Hospital between January 2016 and December 2018. Background characteristics, laboratory results, and imaging findings were analysed. We compared the incidences of uveitis in 2016–2018 and 2004–2015 to identify changes in the patterns.
Results
The most frequent diagnoses were sarcoidosis (8.9%), herpetic iridocyclitis (6.7%), intraocular lymphoma (5.5%), Vogt–Koyanagi–Harada disease (4.8%), unclassified acute anterior uveitis (4.6%), Behçet’s disease (4.5%), bacterial endophthalmitis (2.9%), and Posner-Schlossman syndrome (2.6%). Suspected sarcoidosis (20.9%) was the most common cause of unclassified uveitis. The incidence of intraocular lymphoma was significantly higher in 2016–2018 than in 2004–2015. Between 2004 and 2018, herpetic iridocyclitis, bacterial endophthalmitis, and juvenile chronic iridocyclitis exhibited an increasing trend, and the incidences of Posner-Schlossman syndrome, unclassified acute anterior uveitis, Behçet’s disease, and Vogt–Koyanagi–Harada disease exhibited a decreasing trend.
Conclusion
The changing patterns of uveitis were characterised by increases in the incidence of intraocular lymphoma. This may be attributed to recent advances in examination tools, the changes in the referred patient population, and the aging Japanese population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Uveitis is a major cause of vision loss in developed countries [1]. It is one of the major causes of preventable blindness [2, 3] and severely affects the quality of life [4]. The distributions of the types and aetiologies of uveitis vary worldwide and are believed to be affected by genetic, ethnic, geographic, environmental, and lifestyle factors [5,6,7,8,9,10]. As a result, the patterns of uveitis vary greatly according to the population and the time of research. For example, one report from Taiwan revealed that the incidences of herpetic anterior uveitis, acute retinal necrosis, and cytomegalovirus (CMV) retinitis have recently increased, while those of toxoplasmosis and tuberculosis have decreased [11], compared to the results of a previous study [12]. Therefore, it is vital to analyse the epidemiology of this disease in various regions over a long period of time.
Currently, new examination tools for the diagnosis of uveitis, including interferon-gamma release assays; polymerase chain reaction (PCR) analysis for infectious agents using aqueous samples [13]; and multimodal imaging using optical coherence tomography (OCT), OCT angiography, and fundus autofluorescence [7, 14] have been developed to aid clinicians in the differential diagnosis of uveitis. Consequently, the number of definitive diagnoses of uveitis has been steadily increasing in Japan [15, 16]. However, approximately 30–40% of new patients with uveitis did not receive a definite diagnosis in a recent study [16].
Region-specific information regarding the patterns of uveitis is useful when diagnosing new patients, and there have been many reports on this topic from Japan as well as various other countries [2, 3, 8, 11, 12, 15,16,17,18,19,20,21,22,23,24,25,26]. Previously, we examined the long-term patterns of uveitis in new patients at the University of Tokyo Hospital between 1963 and 2015 [17,18,19] and concluded that the patterns of uveitis in Tokyo were changing rapidly in a short period of time because of advances in the examination tools for diagnosing uveitis. Therefore, up-to-date information regarding the patterns of uveitis in new patients is vital, and we believe that the data from our hospital are representative of the changing patterns of uveitis in Japan.
In the current study, we aimed to determine the incidence of uveitis from 2016–2018 and identify recent changes in the patterns of uveitis.
Methods
Patients and data collection
This study aimed to determine the incidence of uveitis between 2016 and 2018 and identify changes in its patterns. We retrospectively investigated the clinical records of 732 patients (333 men, 399 women) with uveitis who first visited the Uveitis Clinic of the University of Tokyo Hospital, a tertiary referral centre located in central Tokyo, between January 2016 and December 2018. Patients who had been diagnosed prior to 2016 but did not have active intraocular inflammation during 2016–2018 were excluded.
We extracted clinicodemographic data, including age, sex, diagnosis, anatomic location of inflammation, laboratory test results of blood and urine, and chest X-ray and fluorescein fundus angiography findings from the patients’ medical records.
Diagnosis of uveitis
We adopted the classification of uveitis used in a nationwide survey of uveitis conducted in 2009 in Japan [16]. The anatomic diagnosis was assessed according to the classification of the Standardization of Uveitis Working Group as anterior uveitis, intermediate uveitis, posterior uveitis, or panuveitis [27]. Our diagnostic methods for uveitis have been described previously [19]. Briefly, patients with uveitis underwent screening blood tests, urine tests, chest X-ray examination, and the Mantoux reaction test at the initial presentation. Furthermore, when infectious origin was suspected, an aqueous tap or vitrectomy was performed to collect a vitreous sample. Then, quantitative PCR analysis for uveitis-causing infectious agents, including herpes simplex virus (HSV), varicella zoster virus (VZV), and CMV [14], and microscopic examinations and cultures for bacteria were performed using ocular samples. Unilateral, granulomatous inflammation, iris atrophy, increased intraocular pressure during inflammation, and decreased number of corneal endothelial cell compared to that in the opposite eye were considered indicative of herpetic iridocyclitis. When any of these clinical features were present, anterior chamber tap for PCR test was recommended and performed when the patient agreed. When obvious skin lesions characteristic of herpes zoster ophthalmicus were observed, a diagnosis of VZV iridocyclitis was made without performing a PCR test. The diagnostic criteria for Posner-Schlossman syndrome were ‘repeated high intraocular pressure, mild anterior ocular inflammation, neither skin or corneal lesions characteristic of HSV or VZV, and at least one negative result for PCR test for herpesviruses, including CMV’. We diagnosed Fuchs heterochromic iridocyclitis according to the clinical diagnostic criteria reported in a previous study [28].
Blood cultures, serum β-d-glucan, interferon-gamma release assays, and human leukocyte antigen (HLA) typing were performed for suspected bacterial/fungal endophthalmitis, tuberculous uveitis, acute anterior uveitis, or Behçet’s disease. Moreover, vitreous fluid investigations were carried out in cases of suspected intraocular lymphoma. We diagnosed Behçet’s disease [29], sarcoidosis [30], Vogt–Koyanagi–Harada disease (VKH) [31], and presumed tuberculous uveitis [32] according to the diagnostic criteria for each disease. The diagnostic criteria of definite and presumed ocular sarcoidosis reported by the International Workshop on Ocular Sarcoidosis (IWOS) [30] were used for the definitive diagnosis of ocular sarcoidosis in this study. Conversely, the patients who did not meet the IWOS criteria for definite or presumed ocular sarcoidosis but met those of ocular clinical signs for probable ocular sarcoidosis (3 or more out of 7) [30] were suspected of having sarcoidosis in this study. Intraocular lymphoma was diagnosed when at least two of the following four criteria were met [19]: (1) cytology grade > 3; (2) interleukin (IL)-10/IL-6 concentration ratio > 1 or IL-10 level > 50 pg/mL in the intraocular fluid [33]; (3) light chain restriction by flow cytometry [34]; and (4) positive PCR results for immunoglobulin heavy chain gene rearrangement [34]. Regarding acute anterior uveitis, patients with unique systemic symptoms of ankylosing spondylitis, ulcerative colitis, or psoriasis were diagnosed with systemic disease-associated uveitis. Those with HLA-B27 were diagnosed with HLA-B27-associated acute anterior uveitis. Patients who were negative for HLA-B27 and developed acute onset iridocyclitis accompanied with highly viscous fibrin in the anterior chamber during an attack [35] were diagnosed as having unclassified acute anterior uveitis. Human T lymphotropic virus type-1 [36], diabetic iritis [37], acute zonal occult outer retinopathy [38], and juvenile chronic iridocyclitis [39] were diagnosed based on the typical ocular findings and clinical courses, as described in the respective previous reports.
Patterns in uveitis
We calculated the incidences of causative diseases for uveitis in new patients between 2016 and 2018 and compared the results with our previously reported findings (2004–2015) [17,18,19].
Statistical analysis
The proportions of patients with uveitic diseases in previous studies (2004–2015) and those of the current study (2016–2018) were compared statistically and are presented in Table 1. A significant increase in the incidence of a particular uveitic disease was determined by the chi-square test, by comparing the proportion of one uveitic disease with those of all other uveitic diseases over two study periods (between 2016–2018 and 2012–2015, 2016–2018, and 2004–2015). Statistical analyses were performed using GraphPad Prism for Windows (version 8; GraphPad Software Inc., La Jolla, CA, USA). A p-value < 0.0019 was considered statistically significant by Bonferroni correction.
Results
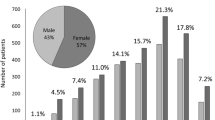
A total of 732 new patients (men: n = 333, women: n = 399) with uveitis were treated at our institution between 2016 and 2018. Their mean age was 56.4 ± 19.0 years (men: 57.2 ± 17.6 years; women: 55.7 ± 20.0 years). The most frequent age range was 60–69 years in both men and women (Fig. 1). Among these 732 patients, 449 (61.3%) received a definitive diagnosis of uveitis (Table 2). The most frequent diagnosis was sarcoidosis (n = 65, 8.9%), followed by herpetic iridocyclitis because of HSV, VZV, and CMV (n = 49, 6.7%) and intraocular lymphoma (n = 40, 5.5%). Six of the 16 cases of HLA-B27-associated acute anterior uveitis had ankylosing spondylitis.
Patients with herpetic iridocyclitis were categorised into five groups according to the reason for diagnosis, as previously described [19]. CMV-DNA positivity was the most common reason (n = 29, 59.2%), followed by skin lesions of herpes zoster ophthalmicus (n = 10, 20.4%), HSV-DNA positivity (n = 5, 10.2%), VZV-DNA positivity (n = 4, 8.2%), and HHV7-DNA positivity (n = 1, 2.0%) (Table 3).
Seventeen cases met the criteria for clinical diagnosis of Fuchs heterochromic iridocyclitis. We measured the Goldmann–Witmer coefficient for antibody against rubella virus (Q values) [40] in 12 cases, of which values of Q > 3 were found in four cases. In addition, we performed herpes virus PCR examination in five of the 17 cases, which were all negative.
Table 4 indicates the distributions of patients with uveitis across three age groups (< 20 years, 20–59 years, and ≥ 60 years). In these patients, juvenile chronic iridocyclitis, Behçet’s disease, and sarcoidosis were the most frequent diagnoses, respectively. Definitive diagnoses were not made for the remaining 283 patients (38.7%). Among these patients, suspected sarcoidosis was the most common diagnosis (n = 153, 20.9%), which accounted for 54.1% of all suspected diagnoses.
Table 5 presents the distribution of uveitis according to the anatomic classification (anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis). In this study, 242 of 732 patients (33.1%) had anterior uveitis, 11 (1.5%) had intermediate uveitis, 52 (7.1%) had posterior uveitis, and 427 (58.3%) had panuveitis. The distribution of uveitis according to the anatomic site did not markedly differ from that observed in our previous studies [17,18,19].
Table 1 indicates the shifts in the numbers and distributions of cases of uveitis diagnosed at the University of Tokyo Hospital over a 15-year period [17,18,19]. Compared to the findings of our previous study on the patterns of uveitis between 2013 and 2015 [19], there was no significant difference in the numbers of cases of specific uveitis diseases. However, we observed increasing trends in the incidences of sarcoidosis, VKH, and intraocular lymphoma in the past 3 years. In contrast, herpetic iridocyclitis, Posner-Schlossman syndrome, bacterial endophthalmitis, Fuchs heterochromatic iridocyclitis, unclassified acute anterior uveitis, and juvenile chronic iridocyclitis exhibited a decreasing trend. Compared to the findings of our previous studies on the uveitis patterns between 2004 and 2015 [17,18,19], the present analysis indicated that intraocular lymphoma (p = 0.001) and unclassified uveitis (p = 0.0003) significantly increased in the past 3 years. There were no significant differences in the patterns of other uveitis diseases, but we observed that the incidences of herpetic iridocyclitis, bacterial endophthalmitis, and juvenile chronic iridocyclitis exhibited an increasing trend throughout the 15-year period (2004–2018). Further, the incidences of Posner-Schlossman syndrome, unclassified acute anterior uveitis, Behçet’s disease, and VKH exhibited a decreasing trend during this period.
Table 6 presents the distributions of age at the first visit in this study (2016–2018) and in previous studies (2004–2006, 2007–2009, 2010–2012, and 2013–2015). The average age exhibited a gradual increasing trend.
Discussion
The current study demonstrated that the incidences of intraocular lymphoma in new patients with uveitis significantly increased in 2016–2018 compared to 2004–2015 [17,18,19]. Moreover, the incidences of herpetic iridocyclitis, bacterial endophthalmitis, and juvenile chronic iridocyclitis gradually increased over a 15-year period (2004–2018). In contrast, the incidences of Posner-Schlossman syndrome, unclassified acute anterior uveitis, Behçet’s disease, and VKH decreased during this period. One possible reason for the increasing trend in herpetic iridocyclitis and intraocular lymphoma is the aging Japanese population [41]. Indeed, the average age of new patients with uveitis at our hospital is gradually increasing: the average age of patients with uveitis in the current study (2016–2018) was 3.2 years higher than that in 2004–2006 [17]. Further, the average ages of patients with herpetic iridocyclitis and intraocular lymphoma in the current study were 62.0 ± 15.3 years and 67.1 ± 12.7 years, respectively. However, the mean age at the onset of anterior uveitis associated with herpes virus was > 55 years in a Japanese study [42]; these findings suggested that herpetic iridocyclitis is common in older individuals. Thus, we expect that the incidences of herpetic iridocyclitis and intraocular lymphoma will further increase in Japan.
Some causative diseases of uveitis tend to occur more frequently in a particular age range. For example, herpes iritis and intraocular malignant lymphoma are considered to be common in the elderly [42, 43]. To clarify these trends in our study population, we divided the patients into children to young (< 20 years), young to middle-aged (20–59 years), and elderly (≥ 60 years) individuals (Table 4). Our results revealed that the most frequent uveitis causes in patients aged ≥ 60 years were sarcoidosis, intraocular lymphoma, and herpetic iridocyclitis. The incidences of these top three diseases remain high compared to those reported in a study conducted 3 years prior [19].
We speculated that the long-term increase in the number of patients with herpetic iridocyclitis and the decrease in the number of patients with Posner-Schlossman syndrome and unclassified acute anterior uveitis may be attributed to the recurrent use of PCR assays for HSV-, VZV-, and CMV-DNA using anterior chamber fluid. Since 2012, we have performed these PCR assays routinely in cases of suspected herpetic iridocyclitis. This might lead to increases in referrals of patients with suspected herpetic iridocyclitis. Regarding the increase in the incidence of intraocular lymphoma, there are three potential reasons. First, we actively conducted diagnostic vitrectomies for patients with suspected intraocular lymphoma, which increased the rate of diagnosis. Second, there has been an increase in the number of patients with primary central nervous system lymphoma [44]; intraocular lymphoma is classified as central nervous system lymphoma, and we predict that a greater increase in the number of patients with central nervous lymphoma will correspond to a greater increase in the number of patients with intraocular lymphoma. Third, as one-arm prospective clinical trials for primary intraocular lymphoma that aim to suppress brain seeding have been carried out since 2008 [34], referrals of patients with suspected intraocular lymphoma might be increasing in our hospital.
The percentage of patients aged < 20 years increased from 2004 to 2018 (2.4%, 4.1%, 4.2%, 4.1%, and 4.8% in 2004–2006, 2007–2009, 2010–2012, 2013–2015, and 2016–2018, respectively). This may have contributed to the increase of patients with JCI. The actual reasons for increasing incidence of childhood uveitis in our hospital remain unknown, but we speculate that the chances of referral to our facility for highly specialised pediatric uveitis may have increased. In addition, among the JCI patients, there were no patients with juvenile idiopathic arthritis, in line with previous reports from Japan [45].
In contrast, the incidences of sarcoidosis, Behçet’s disease, and VKH gradually decreased at our institution. We cannot provide adequate reasons as to why the frequencies of these diseases decreased, but it might be attributed to the fact that the severity of Behçet’s uveitis is milder in Japan and can be treated without referrals [46].
This study had several limitations. First, it was a retrospective study conducted in a tertiary referral university hospital. Most of the patients were referred to our hospital. Thus, there may be a selection bias towards patients with severe disease. Second, the study period was only 3 years. However, the data of > 700 patients were analysed in this study, and thus, we believe that the results of this study are reflective of the current patterns of uveitis in Japan. Further multicentre studies comparing different regions of Japan would be beneficial for characterising recent trends of uveitis in Japan.
Conclusions
The recent patterns of uveitis in the central Tokyo area have indicated increasing incidences of herpetic iridocyclitis, bacterial endophthalmitis, intraocular lymphoma, and juvenile chronic iridocyclitis. This trend may be associated with the recent advances in the examination tools used for the diagnosis of uveitis, particularly PCR, the change in population of the referred patients, such as increase in number of children presenting with uveitis, and the aging Japanese population. As the incidences of these diseases are increasing in Japan, ophthalmologists should focus on these diseases and perform tests, such as PCR and vitreous biopsy, for definitive diagnoses without delay.
Availability of data and materials
The datasets used and analysed in the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI (2004) Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 88:1159–1162. https://doi.org/10.1136/bjo.2003.037226
Gritz DC, Wong IG (2004) Incidence and prevalence of uveitis in Northern California. The Northern California epidemiology of uveitis study. Ophthalmology 111:491–500. https://doi.org/10.1016/j.ophtha.2003.06.014
Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF (2008) Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol 146:890–896. https://doi.org/10.1016/j.ajo.2008.09.014
Hui MM, Wakefield D, Patel I, Cvejic E, McCluskey PJ, Chang JH (2017) Visual functioning and health-related quality-of-life are compromised in patients with uveitis. Ocul Immunol Inflamm 25:486–491. https://doi.org/10.3109/09273948.2016.1139734
Miserocchi E, Fogliato G, Modorati G, Bandello F (2013) Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol 23:705–717. https://doi.org/10.5301/ejo.5000278
Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S (2018) A focus on the epidemiology of uveitis. Ocul Immunol Inflamm 26:2–16. https://doi.org/10.1080/09273948.2016.1196713
Agarwal A, Aggarwal K, Gupta V (2019) Infectious uveitis: an Asian perspective Eye 33:50–65. https://doi.org/10.1038/s41433-018-0224-y
Hsu YR, Huang JC, Tao Y et al (2019) Noninfectious uveitis in the Asia-Pacific region. Eye 33:66–77. https://doi.org/10.1038/s41433-018-0223-z
Rosenbaum JT (2009) Uveitis: contrasting the approaches in Japan and the United States. Jpn J Ophthalmol 63:1–6. https://doi.org/10.1007/s10384-018-0633-2
Llorenç V, Mesquida M, Sainz de la Maza M et al (2015) Epidemiology of uveitis in a Western urban multiethnic population. The challenge of globalization Acta Ophthalmol 93:561–567. https://doi.org/10.1111/aos.12675
Chen SC, Chuang CT, Chu MY, Sheu SJ (2017) Patterns and aetiologies of uveitis at a tertiary referral centre in Taiwan. Ocul Immunol Inflamm 25:S31-38. https://doi.org/10.1080/09273948.2016.1189577
Chou LC, Sheu SJ, Hong MC, Hsiao YC, Wu TT, Chuang CT (2003) Endogenous uveitis: experiences in Kaohsiung Veterans General Hospital. J Chin Med Assoc 66:46–50
Mochizuki M, Sugita S, Kamoi K, Takase H (2017) A new era of uveitis: impact of polymerase chain reaction in intraocular inflammatory diseases. Jpn J Ophthalmol 61:1–20. https://doi.org/10.1007/s10384-016-0474-9
Deák GG, Zhou M, Sporysheva A, Goldstein DA (2020) Novel imaging modalities in patients with uveitis. Can J Ophthalmol 55:20–29. https://doi.org/10.1016/j.jcjo.2019.06.005
Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S (2007) Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol 51:41–44. https://doi.org/10.1007/s10384-006-0383-4
Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M (2012) The 2009 prospective multi-centre epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol 56:432–435. https://doi.org/10.1007/s10384-012-0158-z
Nakahara H, Kaburaki T, Takamoto M et al (2015) Statistical analyses of endogenous uveitis patients (2007–2009) in central Tokyo area and comparison with previous studies (1963–2006). Ocul Immunol Inflamm 23:291–296. https://doi.org/10.3109/09273948.2014.920036
Nakahara H, Kaburaki T, Tanaka R et al (2017) Frequency of uveitis in the central Tokyo area (2010–2012). Ocul Immunol Inflamm 25:S8-14. https://doi.org/10.3109/09273948.2015.1133840
Shirahama S, Kaburaki T, Nakahara H, Tanaka R, Takamoto M, Fujino Y (2018) Epidemiology of uveitis (2013–2015) and changes in the patterns of uveitis (2004–2015) in the central Tokyo area: a retrospective study. BMC Ophthalmol 18:189. https://doi.org/10.1186/s12886-018-0871-6
Nguyen M, Siak J, Chee SP, Diem VQH (2017) The spectrum of uveitis in Southern Vietnam. Ocul Immunol Inflamm 25:S100-106. https://doi.org/10.1080/09273948.2016.1231826
Abaño JM, Galvante PR, Siopongco P, Dans K, Lopez J (2017) Review of epidemiology of uveitis in Asia: pattern of uveitis in a tertiary hospital in the Philippines. Ocul Immunol Inflamm 25:S75-80. https://doi.org/10.1080/09273948.2017.1335755
Siak J, Jansen A, Waduthantri S, Teoh CS, Jap A, Chee SP (2017) The pattern of uveitis among Chinese, Malays, and Indians in Singapore. Ocul Immunol Inflamm 25:S81-93. https://doi.org/10.1080/09273948.2016.1188968
Baarsma GS (1992) The epidemiology and genetics of endogenous uveitis: a review. Curr Eye Res 11:1–9. https://doi.org/10.3109/02713689208999505
Hart CT, Zhu EY, Crock C, Rogers SL, Lim LL (2019) Epidemiology of uveitis in urban Australia. Clin Exp Ophthalmol 47:733–740. https://doi.org/10.1111/ceo.13517
Al-Shakarchi FI (2014) Pattern of uveitis at a referral centre in Iraq. Middle East Afr J Ophthalmol 21:291–295. https://doi.org/10.4103/0974-9233.142263
Al Dhibi HA, Al Shamsi HN, Al-Mahmood AM et al (2017) Patterns of uveitis in a tertiary care referral institute in Saudi Arabia. Ocul Immunol Inflamm 25:388–395. https://doi.org/10.3109/09273948.2015.1133836
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 140:509–516. https://doi.org/10.1016/j.ajo.2005.03.057
La Hey E, Baarsma GS, De Vries J, Kijlstra A (1991) Clinical analysis of Fuchs’ heterochromic cyclitis. Doc Ophthalmol 78:225–235. https://doi.org/10.1007/BF00165685
Namba K, Goto H, Kaburaki T et al (2015) A major review: Current aspects of ocular Behçet’s disease in Japan. Ocul Immunol Inflamm 23:S1-23. https://doi.org/10.3109/09273948.2014.981547
Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA (2019) Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol 103:1418–1422. https://doi.org/10.1136/bjophthalmol-2018-313356
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, Pivetti-Pezzi P, Tessler HH, Usui M (2001) Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 131:647–652. https://doi.org/10.1016/s0002-9394(01)00925-4
Testi I, Agrawal R, Mahajan S et al. (2019) Tubercular uveitis: nuggets from collaborative ocular tuberculosis study (COTS)-1. Ocul Immunol Inflamm, 1–9. https://doi.org/https://doi.org/10.1080/09273948.2019.1646774
Cassoux N, Giron A, Bodaghi B, Tran TH, Baudet S, Davy F, Chan CC, Lehoang P, Merle-Béral H (2007) IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci 48:3253–3259. https://doi.org/10.1167/iovs.06-0031
Kaburaki T, Taoka K, Matsuda J et al (2017) Combined intravitreal methotrexate and immunochemotherapy followed by reduced-dose whole-brain radiotherapy for newly diagnosed B-cell primary intraocular lymphoma. Br J Haematol 179:246–255. https://doi.org/10.1111/bjh.14848
Sonoda KH, et al. (2021) Jpn J Ophthalmol in press
Mochizuki M, Tajima K, Watanabe T, Yamaguchi K (1994) Human T lymphotropic virus type 1 uveitis. Br J Ophthalmol 78:149–154. https://doi.org/10.1089/jop.2016.0124
Oswal KS, Sivaraj RR, Murray PI, Stavrou P (2013) Clinical course and visual outcome in patients with diabetes mellitus and uveitis. BMC Res Notes 6:167. https://doi.org/10.1186/1756-0500-6-167
Francis PJ, Marinescu A, Fitzke FW, Bird AC, Holder GE (2005) Acute zonal occult outer retinopathy: towards a set of diagnostic criteria. Br J Ophthalmol 89:70–73. https://doi.org/10.1136/bjo.2004.042416
Ohno S, Char DH, Kimura SJ, O’Connor GR (1997) HLA antigens and antinuclear antibody titres in juvenile chronic iridocyclitis. Br J Ophthalmol 61:59–61. https://doi.org/10.1136/bjo.61.1.59
Suzuki J, Goto H, Komase K, Abo H, Fujii K, Otsuki N, Okamoto K (2010) Rubella virus as a possible etiological agent of Fuchs heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol 248:1487–1491. https://doi.org/10.1007/s00417-010-1434-6
Nishi N, Yoshizawa T, Okuda N (2017) Effects of rapid aging and lower participation rate among younger adults on the short-term trend of physical activity in the National Health and Nutrition Survey, Japan. Geriatr Gerontol Int 17:1677–1682. https://doi.org/10.1111/ggi.12956
Takase H, Kubono R, Terada Y et al (2014) Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol 58:473–482. https://doi.org/10.1007/s10384-014-0340-6
Abu Samra K, Oray M, Ebrahimiadib N, Lee S, Anesi S, Foster CS (2018) Intraocular Lymphoma: descriptive data of 26 patients including clinico-pathologic features, vitreous findings, and treatment outcomes. Ocul Immunol Inflamm 26:347–352. https://doi.org/10.1080/09273948.2016.1193206
Citterio G, Reni M, Gatta G, Ferreri AJM (2017) Primary central nervous system lymphoma. Crit Rev Oncol Hematol 113:97–110. https://doi.org/10.1016/j.critrevonc.2017.03.019
Keino H, Watanabe T, Taki W, Nakayama M, Nakamura T, Yan K, Okada AA (2017) Clinical features of uveitis in children and adolescents at a tertiary referral centre in Tokyo. Br J Ophthalmol 10:406–410. https://doi.org/10.1136/bjophthalmol-2015-308194
Nakahara H, Kaburaki T, Tanaka R, Yoshida A, Takamoto M, Kawata M, Fujino Y, Kawashima H, Aihara M (2020) Comparisons of clinical features in Japanese patients with Behçet’s uveitis treated in the 1990s and the 2000s. Ocul Immunol Inflamm 28:262–269. https://doi.org/10.1080/09273948.2018.1559928
Acknowledgements
The manuscript was edited for English grammar by a native writer at Editage.
Funding
This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI No. 18K09398, No. 19K09986), a Grant-in-Aid for Research on Refractory Diseases of the Health Sciences Research from the Ministry of Health, Labour, and Welfare (#20FC1012), and a Grant-in-Aid for Eisai Japan (2019), Alcon, Novartis Pharma (2019), and Santen Pharmaceutical (2019).
Author information
Authors and Affiliations
Contributions
TS was involved in the study design, data collection, analysis of the results, and drafting of the manuscript. TK and SS participated in the study design, data collection, and reviewing and editing of the manuscript. RT, KK, HN, MT, HK, and MA participated in the data collection and reviewing and editing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
This retrospective study was approved by the research ethics committee of the Graduate School of Medicine and Faculty of Medicine at The University of Tokyo. This study was conducted in compliance with the Declaration of Helsinki and the ethical guidelines for medical and health research involving human subjects.
Consent to participate
We used an opt-out approach, which is a substitute for consent, by informing participants of the purpose and conduct of the study and guaranteeing them the opportunity to be excluded, rather than obtaining direct consent from each patient.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, T., Kaburaki, T., Tanaka, R. et al. Incidence and changing patterns of uveitis in Central Tokyo. Int Ophthalmol 41, 2377–2388 (2021). https://doi.org/10.1007/s10792-021-01791-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01791-4