Abstract
Purpose
To investigate the epidemiology of uveitis in Japan and assess its changes over time.
Study design
Retrospective multicenter study
Methods
Sixty-six hospitals in Japan with uveitis specialty clinics participated in this retrospective nationwide survey. A questionnaire was sent to each hospital to survey the total number of patients who made a first visit to the outpatient uveitis clinic of each hospital between 1 April 2016 and 31 March 2017. The diagnosis of uveitis was based on guidelines when available or on commonly used diagnostic criteria.
Results
In 2016, new patients with uveitis accounted for 3.2% of the total number of new patients with ophthalmic diseases. A total of 5378 patients were enrolled in the survey; 3408 cases could be classified with a specific uveitis entity, and 1970 cases were described as unclassified intraocular inflammation. Among the classified cases, the most frequent disease was sarcoidosis (10.6%), followed by Vogt–Koyanagi–Harada disease (8.1%), herpetic iritis (6.5%), acute anterior uveitis (5.5%), sclerouveitis (4.4%), Behçet’s disease (4.2%), malignant disease (2.6%), acute retinal necrosis (1.7%), Posner–Schlossman syndrome (1.7%), and diabetic iritis (1.4%). The rates of sarcoidosis, Vogt–Koyanagi–Harada disease, and Behçet’s disease were similar; however, the rate of herpes iritis increased (4.2–6.5%) when compared with the 2009 survey.
Conclusions
Some changes were observed between the previous nationwide surveys (2002 and 2009) and the present survey. It must be valuable to continue such nationwide epidemiologic surveys at regular intervals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis, i.e., inflammation of the intraocular tissues including the iris, ciliary body, and choroid tissues, sometimes causes severe intraocular inflammation and leads to irreversible loss of vision. The various causes of uveitis are generally classified into infectious and noninfectious diseases, and this classification is important when selecting the treatment [1]. The treatment in infectious cases is antimicrobial agents, whereas in noninfectious cases, it is corticosteroids and other immunosuppressants.
The rate of the primary disease of uveitis varies greatly from region to region, and thus, epidemiologic surveys are being conducted in each country [2]. The Japanese Ocular Inflammation Society (JOIS) performed nationwide epidemiologic surveys of uveitis in 2002 and 2009 [3, 4]. Those surveys revealed that sarcoidosis was the most common cause of uveitis, followed by Vogt–Koyanagi–Harada disease. They also revealed that in 2002 the third-most frequent disease was Behçet’s disease, and in 2009 the third rank was replaced by acute anterior uveitis (AAU). Since the rate of uveitis changes over time owing to environmental and hereditary factors, periodic updates of the epidemiologic data are required. We conducted the present study to determine the most recent epidemiology findings for uveitis and its changes in Japan since 2002 and 2009.
Methods
Sixty-six hospitals providing uveitis consultations participated in this retrospective survey. The ethics review committee of each facility approved the study protocol, and the study was conducted in accordance with the tenets of the Declaration of Helsinki. A questionnaire was sent to each of the hospitals to survey the total number of patients who made a first visit to the outpatient uveitis clinic of each hospital during the 1-year period from 1 April 2016 to 31 March 2017. The questionnaire was sent to each hospital on 1 October 2018 with the request to return it by the end of June 2019; thus, each hospital had a term of ≥18 months (from March 2017 to June 2019) to achieve a concrete diagnosis even for the difficult cases.
We collected the following information from the patients’ medical records for the present retrospective analyses: clinical data including sex, age at the first visit, diagnosis, anatomic type of uveitis, methods used for the diagnosis, pathogen(s) of infectious uveitis, and information such as the HLA type for acute anterior uveitis. Each case of uveitis was diagnosed on the basis of international guidelines [5,6,7,8], Japanese guidelines [9], or common diagnostic criteria [10,11,12,13], as in the previous surveys by the JOIS. Patients who had not been diagnosed with a specific uveitis entity were designated as “unclassified intraocular inflammation.”
After a discussion among the members of the JOIS Uveitis Survey Working Group, several items from the 2002 and 2009 surveys were modified. The anatomic location of uveitis was surveyed and classified as anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis as per International Uveitis Study Group definitions [7] since this classification is very helpful in determining the etiology of uveitis. “Pure scleritis” without intraocular inflammation was excluded. We focused on “sclerouveitis,” which currently indicates “scleritis with intraocular inflammation.” We only included cases of endophthalmitis that were “metastatic endogenous endophthalmitis,” excluding both postoperative endophthalmitis and traumatic endophthalmitis. Although AAU was the third-rank uveitis in the 2009 survey, there are no international diagnostic criteria. In this 2016 survey, we thus distributed the “appendix criteria for AAU” with the questionnaire as follows: “Acute anterior uveitis is acute onset iridocyclitis, accompanied with highly viscous fibrin in the anterior chamber during an attack.” “Retinal vasculitis” did appear in the 2009 survey, but its definition was obscure. We thus defined “retinal vasculitis” as “retinal vasculitis in young people with mild anterior chamber inflammation and vitreous opacification, including frosted branch angiitis.” We divided cases of “white-dot syndromes” with ocular inflammation into the following 5 categories: multiple evanescent white-dot syndrome (MEWDS), acute posterior multifocal placoid pigment epitheliopathy (APMPPE), serpiginous choroiditis, multifocal choroiditis, and “others.”
Results
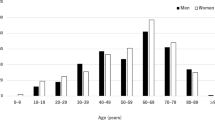
A total of 5378 patients were enrolled in this survey; 3408 patients were diagnosed as having a specific uveitis entity, and 1970 patients (36.6%) were designated as having unclassified intraocular inflammation. As a reference, the total number of new patients with ophthalmic diseases at the 66 hospitals during the same study period was 167,981, and thus, the percentage of uveitis cases among the total population was 3.2% (Fig. 1, upper column).
The lower column in Fig. 1 provides the distributions of the age and sex of the 5378 uveitis patients. Uveitis was most commonly observed among individuals in their 40s to 70s, with the 60s being the most frequent age group of occurrence. Females had a higher prevalence of uveitis than did males at any age. Figure 2 gives the distributions of the anatomic types and specific diagnoses. When compared with previous reports [1, 2], the present data revealed a lower rate of intermediate uveitis (2.5%) and a higher rate of panuveitis (44.9%). Specific diagnoses were divided into infectious versus noninfectious etiology. Among all cases, the rate of infectious uveitis was 15.4%, that of noninfectious uveitis was 47.2%, and that of unclassified uveitis was 37.3%.
Table 1 shows the distribution of specific uveitic diseases in this survey. Sarcoidosis (n = 570, 10.6%) was the most frequent cause of uveitis; Vogt–Koyanagi–Harada disease (n = 435, 8.1%), the second-most frequent; and herpetic iritis (n = 352, 6.5%), the third-most frequent. The fourth-most frequent diagnosis was acute anterior uveitis (n = 298), of which 59 patients (19.7%) were HLA-B27 positive, 71 patients (23.8%) were HLA-B27 negative, and the remaining 168 patients were not examined for HLA typing. The rate of unclassified uveitis was 36.6% (n = 1970), which was similar to the corresponding rate in the 2009 survey (33.5%).
Table 2 summarizes the top 6 uveitis diagnoses in the 3 nationwide surveys (2002, 2009, and 2016). Sarcoidosis and VKH disease remained the first- and second-most common specific diagnosis over the 3 surveys. Behçet’s disease was the third major cause of uveitis in the 2002 survey at 6.2%; its rate decreased to 3.9% in 2009 and remained low at 4.2% in 2016.
The 2016 rate of herpetic iritis (6.5%) was increased when compared with the last 2 surveys. Possibly, the greater use of polymerase chain reaction (PCR) testing provided a more definitive diagnosis and pushed up the rate of herpetic iritis. In contrast, the rate of scleritis (which was included in the 2009 survey) was decreased. This is probably because we picked up only “sclerouveitis” cases in this most recent 2016 survey.
Table 3 summarizes the types of virus in herpetic iritis and acute retinal necrosis. For herpetic anterior uveitis, we accepted both clinical diagnoses and test-proven diagnoses. The clinical diagnoses were obtained by the treating physicians on the basis of typical anterior findings, e.g., mutton-fat keratic precipitates and a unilateral depigmented atrophic iris with high intraocular pressure (IOP) for varicella zoster iritis, and a corneal coin lesion with low-density corneal endothelial cells for cytomegalovirus (CMV) iritis. The test-proven diagnoses were based on a positive PCR result and/or a high titer of virus-specific antibody in the ocular fluid. Although our survey revealed a 44.3% rate of subjective clinical diagnoses, we also observed that the ratio of objective test-proven iritis had increased over the previous surveys. In addition, it is notable that CMV infections accounted for nearly one-half of the cases of test-proven iritis. Regarding acute retinal necrosis, which was diagnosed on the basis of the Japanese criteria [9], the results of our survey demonstrated that varicella zoster virus was the major cause of the disease.
Table 4 summarizes the causes of sclerouveitis, which was the fifth-most frequent disease in the current survey. Approximately one-third of these cases (33.6%) were associated with collagen-vascular disease, 4.2% were associated with infectious disease, and 62.2% were idiopathic. Among the collagen-vascular diseases, the rate of rheumatoid arthritis-associated sclerouveitis was 10.5%. Of the idiopathic cases, 51.3% were anterior and 10.9% were posterior sclerouveitis.
Table 5 summarizes the malignant diseases associated with uveitis in the total population. More than 90% of the uveitis cases were caused by lymphoma, consisting of primary intraocular lymphoma, primary central nervous system (CNS) lymphoma, and systemic lymphoma.
Discussion
The JOIS conducted nationwide uveitis surveys in 2002 and 2009 [3, 4]. The 2002 survey was retrospective, and the 2009 survey, prospective. For this most recent (2016) survey, we chose a retrospective design rather than a prospective study. With this design, the treating physicians had at the very least ≥18 months to follow up their patients, and therefore, our design provided a long period to reach definite diagnoses. The JOIS committee members decided to use the most commonly used diagnostic criteria for this survey, taking into account those used in the previous 2 nationwide surveys. Since we examined the age, sex, and anatomic type of uveitis for the first time, we could not compare these factors with those of the previous surveys. The aging of Japanese society and other factors may influence the distribution of uveitis; thus, we should keep these items in future questionnaires.
In comparison with those 2 previous surveys, we were able to increase the number of participating hospitals and patients. The 66 participating hospitals were tertiary referral medical centers in their respective regions. The advantage of conducting a survey at medical centers is that the diagnoses may be more reliable; however, the disadvantage is that the distribution of uveitis etiology may differ somewhat from the true distribution. It is possible that since many private hospitals tend to take care of mild uveitis, only severe cases may be referred to medical centers.
We would like to emphasize that sarcoidosis is the most prominent cause of uveitis in Japan. The Japanese diagnostic criteria [14] for sarcoidosis in general divide sarcoidosis into cases with a “biopsy-proven diagnosis” and those with a “clinical diagnosis.” In our 2016 survey, the proportion of histology-proven diagnoses was 48.6% and that of clinical diagnoses was 51.4%. In Japan’s medical insurance system, the diagnosis of sarcoidosis must comply with the Japanese diagnostic criteria for this disease. Patients with suspected sarcoidosis but who did not meet the Japanese diagnostic criteria were strictly excluded from the current study. Mochizuki et al. recently provided the revised international diagnostic criteria for “ocular sarcoidosis” proposed by consensus at the International Workshop on Ocular Sarcoidosis [15]. Those criteria are well organized, but we did not follow them in our present nationwide survey. The diagnostic criteria for sarcoidosis in Japan were established and revised in 1989, 2006, and 2015. We need to bear in mind that our 3 nationwide surveys (2002, 2009, and 2016) might reflect on the “sarcoidosis criteria at the time” and be biased in comparison.
Among cases of herpetic anterior uveitis, cytomegalovirus (CMV) anterior uveitis was found to be the most common disease as a cause in the current survey. This disease is thought to be due to local reactivation of latent CMV and is usually unilateral. The acute form may present as Posner–Schlossman syndrome, consisting of recurrent hypertensive anterior uveitis with few granulomatous keratic precipitates. In the past decade, more cases of CMV anterior uveitis have been identified, probably owing to the increase in the availability of specific PCR testing. The establishment and increasing use of a multiplex PCR test for several common ocular infectious disease pathogens in Japan may also have helped to increase the detection rate [16, 17].
The number of patients with Behçet’s disease in Japan has been reported to be decreasing [4], and our present survey indicates that this trend has not changed. This decrease in Japan over nearly a decade suggests that Behçet’s disease might be correlated with exogenous factors such as the climate, public health, and dietary habits rather than with endogenous factors, such as age, sex, ethnicity, and immunogenetic background.
Our analyses revealed that most of the uveitis associated with malignant diseases was caused by lymphoma (93.6%). Although primary intraocular lymphoma is the main cause of uveitis, primary CNS lymphoma and systemic lymphoma also cause uveitis. Grange et al. examined 853 patients presenting with uveitis and observed that twenty-one (2.5%) were diagnosed with neoplastic masquerade syndromes [18], which is very similar to our present finding. They demonstrated that patients with neoplastic masquerade syndromes were more likely to be older, male, or non-African American and to have posterior segment inflammation and unilateral disease [19]. We should bear in mind that these life-threatening syndromes are now the seventh-most frequent cause of uveitis in Japan, and ophthalmologists play an important role in their diagnoses because in almost 60% of uveitis cases, the cause is primary intraocular lymphoma.
Our study has several limitations. We chose a retrospective design, and although retrospective studies can be completed within a relatively short time, the potential bias cannot be controlled by the identification of confounding factors in advance. The most reliable survey will be “prospective” and simultaneously designed to guarantee a certain “patient follow-up duration” to achieve definite diagnoses. Another limitation is the diagnostic method. In our questionnaire-based survey, diagnosis was fully dependent on each physician; therefore, some differences in the diagnostic method might have occurred. For instance, for herpetic iritis, we included even those diagnosed according to the clinical opinion of the physician even if PCR or antibody tests were not performed (clinical diagnosis group, 44.3%; Table 3).
Investigations of epidemiologic changes over time require comparisons of periodically acquired etiologic data from the same diagnostic categories from the same institutions. In addition, to standardize the diagnosis in all participating institutions in a survey, easily understandable diagnostic guidelines for intraocular inflammation are needed. A national epidemiologic survey should include not only university hospitals but also general clinics. In the next survey, we must consider these factors and establish a well-designed format for a periodic epidemiologic national survey.
References
Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–79.
Hsu YR, Huang JC, Tao Y, Kaburaki T, Lee CS, Lin TC, et al. Noninfectious uveitis in the Asia-Pacific region. Eye (Lond). 2019;33:66–77.
Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51:41–4.
Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. 2012;56:432–5.
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt–Koyanagi–Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–52.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Deschenes J, Murray PI, Rao NA, Nussenblatt RB. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16:1–2.
Chen EJ, Bin Ismail MA, Mi H, Ho SL, Lim WK, Teoh SC, et al. Ocular Autoimmune Systemic Inflammatory Infectious Study (OASIS)—report 1: epidemiology and classification. Ocul Immunol Inflamm. 2018;26:732–46.
Takase H, Okada AA, Goto H, Mizuki N, Namba K, Ohguro N, et al. Development and validation of new diagnostic criteria for acute retinal necrosis. Jpn J Ophthalmol. 2015;59:14–20.
Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117:663–7.
Petty RE, Southwood TR. Classification of childhood arthritis: divide and conquer. J Rheumatol. 1998;25:1869–70.
Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208.
Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26:2–16.
Ishihara M. Validity, usefulness and difficulties of the diagnosis of sarcoidosis by new diagnostic criteria and guidelines for sarcoidosis. Article in Japanese. Nippon Ganka Gakkai Zasshi. 2010;114:665–7.
Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103:1418–22.
Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120:1761–8.
Nakano S, Sugita S, Tomaru Y, Hono A, Nakamuro T, Kubota T, et al. Establishment of multiplex solid-phase strip PCR test for detection of 24 ocular infectious disease pathogens. Investig Ophthalmol Vis Sci. 2017;58:1553–9.
Grange LK, Kouchouk A, Dalal MD, Vitale S, Nussenblatt RB, Chan CC, et al. Neoplastic masquerade syndromes in patients with uveitis. Am J Ophthalmol. 2014;157:526–31.
Touhami S, Audo I, Terrada C, Gaudric A, LeHoang P, Touitou V, et al. Neoplasia and intraocular inflammation: from masquerade syndromes to immunotherapy-induced uveitis. Prog Retin Eye Res. 2019;72:100761.
Acknowledgements
We thank the institutions that participated in this survey: Hokkaido University, Health Sciences University of Hokkaido, Sapporo Medical University, Hirosaki University, Yamagata University, Tohoku University, Tsukuba University, National Defense Medical College, JCHO Tokyo Shinjuku Medical Center, Jikei University School of Medicine, Juntendo University, Juntendo University Urayasu Hospital, Juntendo University Nerima Hospital, Kyorin University, National Center for Global Health and Medicine, Nihon University, Nippon Medical School, Showa University, Tokyo Medical and Dental University, Tokyo Medical University, Tokyo Women’s Medical University, Tokyo Women’s Medical University Yachiyo Medical Center, Tokyo Metropolitan Geriatric Hospital, Teikyo University School of Medicine (University Hospital Mizonokuchi), The University of Tokyo, Yokohama City University, Niigata University, Shinshu University, Matsumoto Dental University, Gifu University, Gunma University, Jichi Medical University, Dokkyo Medical University, Nippon Medical School Tama Nagayama Hospital, Nagoya University, Nagoya City University, University of Toyama, Kanazawa University, University of Fukui, Shiga University of Medical Science, Kyoto Prefectural University of Medicine, Osaka Medical College, Osaka City University, Japan Community Health Care Organization Osaka Hospital, Yodogawa Christian Hospital, Wakayama Medical University, Kobe University, Hyogo College of Medicine, Okayama University, Kawasaki Medical School, Tottori University, Shimane University, Hiroshima University, Yamaguchi University, Tokushima University, Ehime University, Kochi University, Kagawa University, Kyushu University, Fukuoka University, Fukuoka Dental College, Kurume University, Saga University, Nagasaki University, Oita University, and Kagoshima University.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
K. Sonoda, none; E. Hasegawa, none; K. Namba, grant (Mitsubishi Tanabe, AbbVie, Eisai, EP-CRSU), speaker fee (HOYA, Alcon, Pfizer, AstraZeneca, Novartis, Kowa, Chugai, Senju, Mitsubishi Tanabe, Eisai, AbbVie, Santen); A. A. Okada, consultant fee (Bayer, AbbVie, Astellas, Daiichi-Sankyo, Allergan, Chugai), research grant, lecture fee (Bayer, Novartis, Santen, Mitsubishi Tanabe, Alcon), lecture fee (Senju, Otsuka); N. Ohguro, none; H. Goto, none.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Koh-Hei Sonoda
About this article
Cite this article
Sonoda, KH., Hasegawa, E., Namba, K. et al. Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol 65, 184–190 (2021). https://doi.org/10.1007/s10384-020-00809-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00809-1