Abstract
Purpose
To compare the clinical characteristics of anterior uveitis (AU) caused by herpes simplex virus (HSV), varicella-zoster virus (VZV), or cytomegalovirus (CMV).
Methods
The medical records were reviewed of 46 patients whose diagnoses were based on their clinical characteristics [e.g., unilateral involvement, presence of keratic precipitates (KPs), and elevation of intraocular pressure (IOP)] and on PCR detection of herpes virus DNA in the aqueous humor. The demographics, chief complaints, and clinical characteristics of the three types of herpetic AU were compared.
Results
Of the 46 patients with AU, eight had HSV-AU, 20 had VZV-AU, and 18 had CMV-AU. HSV-AU and VZV-AU shared common features, i.e., a relatively acute disease process and the presence of large KPs. Among the three groups of patients, the characteristic features of those with VZV-AU were severe intraocular inflammation, as shown by severe aqueous flare, highest viral load in the aqueous humor, and presence of segmental iris atrophy. In comparison, patients with CMV-AU had the mildest intraocular inflammation, lowest corneal endothelial cell density, and highest IOP.
Conclusions
Although the AU caused by each of the three types of herpes viruses has a number of common features, each disease also has distinct features that should facilitate an accurate diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpetic anterior uveitis (AU), caused by herpes simplex virus (HSV) or varicella-zoster virus (VZV), is one of the most common types of infectious uveitis in many countries, including Japan [1–3]. Cytomegalovirus (CMV) is also known to cause AU in immunocompetent individuals [4–12]. The diagnosis of AU caused by these viruses (HSV-AU, VZV-AU, CMV-AU, respectively) has benefited from the use of PCR technology to detect the genomic DNA of the respective herpes virus in small amounts of ocular fluids [11, 13, 14].
HSV-AU, VZV-AU, and CMV-AU share several common clinical features, such as unilaterality, presence of keratic precipitates (KPs), and elevated intraocular pressure (IOP) [3]. However, they are also characterized by different clinical findings, and a detailed account of the differences that can be used to differentiate them has not been published.
The aim of our study was to compare the clinical features of AU caused by HSV, VZV, and CMV, with a focus on their similarities and differences.
Patients and methods
This study involved a retrospective review of the medical charts of patients with herpetic AU who were followed at the Uveitis Clinic of the Tokyo Medical and Dental University (TMDU) Hospital or the Miyata Eye Hospital between June 2006 and February 2012. The study was approved by the internal ethics committees of the TMDU Hospital and the Miyata Eye Hospital. The procedures used conformed to the tenets of the Declaration of Helsinki, and informed consent was obtained from each patient for the procedures performed and for review of their records.
Patients with herpetic AU during the study period were eligible for entry. Herpetic AU was initially suspected on the basis of the clinical manifestations, such as unilateral involvement, presence of KPs, and elevated IOP. Once informed consent had been obtained from these patients, an anterior chamber (AC) tap was performed; the aqueous humor thus obtained was used for multiplex PCR [11], and the viral loads were measured by real-time PCR [11] in most cases. Only patients in whom herpes viral DNA was detected by PCR were included in the study. Those patients with the typical ocular manifestations of herpetic AU but for whom no results of the viral examination of the aqueous humor were available or, alternatively, the results of the viral examination were negative were excluded from the study.
The final patient cohort comprised 46 patients (26 men, 20 women) with a mean age of 56 ± 16 (range 15–80) years. Each patient’s demographics, chief complaint, medical treatment, and clinical manifestations were recorded. Data regarding the medical treatment were collected at the initial presentation to our clinics or when diagnosis was made by PCR. Most of the data, including the chief complaint, visual acuity, and ocular manifestations, were obtained at the initial examination. To evaluate the long-term effects of herpes virus infection, the corneal endothelial cell density (CECD) and presence of iris atrophy were determined at the final visit. The aqueous flare count was determined at the time of aqueous humor sampling, whereas the IOP was determined at the time when inflammation was most severe. Patients were defined as being in the “acute phase” if the disease duration from onset to diagnosis by PCR was <1 month, in the “subacute phase” when the disease duration was >1 month and <1 year, and in the “chronic phase” when the disease duration was >1 year.
Statistical analyses were performed using the Fisher exact test and the Mann–Whitney test. Regression analyses were used to determine the significance of the associations between different characteristics and the different types of herpetic AU. A P value of <0.05 was considered to be significant.
Results
Patient demographics
Eight patients had HSV-AU, 20 patients had VZV-AU, and 18 patients had CMV-AU (Table 1). The percentage of men with HSV-AU was 12.5 %, with VZV-AU, 55.0 %, and with CMV-AU, 77.8 %. All patients had unilateral disease, as defined in the inclusion criteria. The mean duration of symptoms from onset to diagnosis by PCR was 78 days for HSV-AU patients, 498 days for VZV-AU patients, and 2,375 days for CMV-AU patients. The duration was significantly longer in patients with CMV-AU than in those with VZV-AU (P < 1 × 10−4) or HSV-AU (P = 8 × 10−4; Fig. 1). In one case of VZV-AU, Epstein–Barr virus DNA was also detected in the aqueous humor. One of the patients with VZV-AU (5 %), but none of the patients with HSV-AU or CMV-AU, exhibited underlying immunocompromising conditions, such as those resulting from the presence of a malignant tumor and/or the effects of systemic chemotherapy/long-term use of immunosuppressants. One patient with VZV-AU was treated with long-term massive corticosteroid and cyclophosphamide therapy for antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. Most of the patients were not examined for the presence of human immunodeficiency virus infection (Table 1).
Comparison of disease duration in patients with anterior uveitis (AU) caused by herpes simplex virus (HSV-AU), varicella-zoster virus (VZV-AU), or cytomegalovirus (CMV-AU). The respective disease durations from onset to detection of viral DNA in the aqueous humor were compared, revealing that disease duration was longer in patients with CMV-AU than in those with HSV-AU and VZV-AU. Horizontal bars Median value. *P = 8 × 10−4, **P < 1 × 10−4
Chief complaints
The chief complaints at the onset of the disease are listed in Table 2. Because patients with long disease duration were included in the study, especially those with CMV-AU, data were collected from the referring letter to our clinic or by asking patients who were referred from other clinics. Conjunctival redness was reported by three patients with HSV-AU (43 %) and by 11 patients with VZV-AU (61 %), but not by any patients with CMV-AU. The number of patients with conjunctival redness differed significantly between VZV-AU and CMV-AU patients (P = 0.001) and between HSV-AU and CMV-AU patients (P = 0.043). Ocular pain was reported by four patients with HSV-AU (57 %), by five patients with VZV-AU (28 %), but by no patients with CMV-AU. The number of patients with ocular pain was significantly higher in patients with HSV-AU than in patients with CMV-AU (P = 0.011).
Medical therapies for herpetic AU
The medical therapies given to patients with herpetic AU when they were referred to our clinic or when a definitive diagnosis was made based on PCR findings are summarized in Table 3. Briefly, most of the patients with one of the three types of herpetic AU were given corticosteroid eye drops, and two patients with VZV-AU (10 %) received oral corticosteroid therapy. Fewer than 30 % of the patients were given antiviral agents. After the definite diagnosis based on PCR findings had been made, corticosteroid eye drops were given to all patients with HSV-AU, to 16 patients with VZV-AU (80 %), and to 17 patients with CMV-AU (94 %). The corticosteroid eye drop treatment was subsequently terminated in four patients with VZV-AU (20 %) and three patients with CMV-AU (6 %) due to suspected steroid-induced glaucoma. A number of patients had received topical aciclovir or oral valaciclovir previously in the referring clinic because of clinically suspected herpetic AU. After the PCR-based definitive diagnosis had been made in our clinics, most of the patients with HSV-AU or VZV-AU received topical aciclovir or oral valaciclovir, and most of those with CMV-AU received oral valganciclovir, with seven patients (39 %) receiving topical ganciclovir. The frequency of patients who received IOP-reducing therapy was <30 % in the HSV-AU patients; however, the frequency was higher in the VZV-AU and CMV-AU patients when they were referred to our clinics or when the diagnosis was made by PCR, as shown in Table 3.
Comparison of clinical manifestations of herpetic AU
We compared the clinical manifestations of the three types of herpetic AU. Most of the data were collected at the initial examination in our clinics, but the CECD and presence of iris atrophy were determined at the final examination. The aqueous flare count and IOP were determined at the time of aqueous humor sampling.
Dermal manifestations at the onset of AU were seen only in patients with VZV-AU, in four patients (20 %) with herpes zoster ophthalmicus with painful blisters and one patient (5 %) with zoster sine herpete with dermal pain but no blisters (Table 4).
The CECD at the final examination was measured in three of the eight patients with HSV-AU, six of the 20 patients with VZV-AU, and 15 of the 18 patients with CMV-AU (Fig. 2a). The mean CECD was approximately 2,400–3,200 cells/mm2 in normal eyes, 2,743 (range 2,525–2,994) cells/mm2 in HSV-AU eyes, 2,362 (range 1,538–2,915) cells/mm2 in VZV-AU eyes, and 1,599 (range 480–2,688) cells/mm2 in CMV-AU eyes. The density was significantly lower in CMV-AU eyes than in VZV-AU eyes (P = 0.014). Because the HSV-AU sample size was small, statistical analyses were performed only for comparisons between VZV-AU and CMV-AU eyes. We also determined whether a significant correlation existed between CECD and disease duration from disease onset until measurement of CECD in patients with CMV-AU, but we found no correlation (r 2 = 0.030, P = 0.611). To determine whether previous treatment affected CECD, we divided the patients into two groups according to a CECD of ≥1,000 and a CECD of <1000, respectively, and analyzed correlations between the previous treatment and the CECD of the two groups. No effect of corticosteroids (P = 0.523) or antiviral agents (P = 0.1548) was found. Four patients with CMV-AU (23 %) developed bullous keratopathy, of whom three (17 %) underwent corneal surgery, including one patient who underwent penetrating keratoplasty and two patients who underwent Descemet’s stripping automated endothelial keratoplasty (Fig. 2a).
Comparison of ocular findings in patients with HSV-AU, VZV-AU, or CMV-AU. Horizontal bars Median value. a Comparisons of corneal endothelial cell densities (CECD) of the three groups. Patients with CMV-AU had significantly lower CECD than did patients with VZV-AU (P = 0.014). Data were collected from three of the eight patients with HSV-AU, six of the 20 patients with VZV-AU, and 15 of the 18 patients with CMV-AU. The data obtained from patients with HSV-AU were not used for the statistical analyses owing to the small number of patients in the sample. Solid circles Patients who did not develope bullous keratopathy, Open squares Patients who underwent penetrating keratoplasty, open triangles patients who received Descemet’s stripping automated endothelial keratoplasty, cross (x) patient who developed bullous keratopathy but did not receive corneal surgery. b Viral loads in the aqueous humor measured by real-time PCR. The viral load in eyes with CMV-AU was the lowest for the three types of herpetic AU (P = 0.001). c The aqueous flare counts in eyes with CMV-AU were significantly lower than those in eyes with VZV-AU (P = 0.009). pc/ms Photon count per millisecond. d Maximum intraocular pressure (IOP) at the inflammatory phase in eyes with CMV-AU was significantly higher than that in eyes with HSV-AU (P = 0.022). Solid circles Patients who did not receive surgery, open squares patients who underwent trabeculectomy before antiviral agents were given, open triangles patients who underwent trabeculectomy after antiviral therapy was performed, cross (x) patient who was transferred to another hospital and for whom, therefore, the prognosis of IOP was not known. For all figure parts: *P < 0.05, **P < 0.01
Pseudodendritic keratitis at the initial presentation was seen in only one patient with VZV-AU (5 %) and in none with HSV-AU or CMV-AU (Table 4). The disease duration of the patient who showed pseudodendritic keratitis from disease onset was 79 days; this patient had been treated with corticosteroid eye drops and antiviral agents. Coin-shaped lesions, the typical sign of corneal endotheliitis due to CMV infection [9], were seen in two patients with CMV-AU (11 %); these two patients were not those described above who developed bullous keratopathy. Endotheliitis was seen in two patients with HSV-AU (25 %), four patients with VZV-AU (20 %), and one patient with CMV-AU (6 %) despite the finding of decreased CECD in eyes with CMV-AU. We compared the disease durations of patients with endotheliitis with those of patients without it but found no difference between the two groups (P = 0.198). We also analyzed whether the previous treatment affected the presence of corneal endotheliitis, but no effects were found for previous treatment with corticosteroids (P = 0.496) or antiviral agents (P = 0.089).
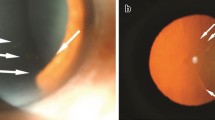
Medium-to-large KPs were seen in all eight eyes with HSV-AU (Fig. 3a), in 17 eyes with VZV-AU (85 %; Fig. 3b), and in seven eyes with CMV-AU (39 %). The incidence of eyes with medium-to-large KPs was significantly higher in patients with VZV-AU or HSV-AU than in eyes with CMV-AU (P = 0.004). Small KPs were seen in none of the eyes with HSV-AU, in two eyes with VZV-AU (10 %), and in eight eyes with CMV-AU (44 %; Fig. 3c). The incidence of eyes with small-to-fine KPs was significantly higher in eyes with CMV-AU than in those with VZV-AU (P = 0.020) or HSV-AU (P = 0.028). We also analyzed whether the disease duration or previous treatment affected the presence or size of KPs. We compared the patients with medium-to-large KPs to those with small-to-fine KPs or without KPs but found no difference in the disease duration (P = 0.122) and no effect for corticosteroids (P = 0.342) or antiviral agents (P = 0.266).
Representative images of keratic precipitates (KPs) of herpetic AU. a Photoslit image of a patient with HSV-AU; large, mutton-fat KPs were mostly observed. b Photoslit image of a patient with VZV-AU; medium-to-large KPs were observed. c Photoslit image of a patient with VZV-AU; small-to-fine KPs were observed
The viral load in the aqueous humor was measured in all eyes with HSV-AU or CMV-AU and in 18 of the eyes with VZV-AU (Fig. 2b). The mean viral load was 1.0 × 106 (range 5.0 × 103 to 7.0 × 106) copies/mL for HSV-AU, 1.0 × 108 (range 6.6 × 103 to 9.5 × 108) copies/mL for VZV-AU, and 1.1 × 105 (range 2.2 × 102 to 1.0 × 106) copies/mL for CMV-AU. The load was significantly higher in eyes with VZV-AU than in eyes with CMV-AU (P = 0.001). We also analyzed whether the disease duration or previous treatment correlated with the viral load. Regression analysis did not show any correlation between disease duration and viral load in the aqueous humor of patients with HSV-AU (r 2 = 0.022, P = 0.728), VZV-AU (r 2 = 0.040, P = 0.581), or CMV-AU (r 2 = 0.093, P = 0.219), or in all herpetic AU (r 2 = 0.012, P = 0.671). We then compared the patients whose viral load was more or less than 1 × 106 copies/mL and analyzed whether the previous treatment correlated with the viral load. Previous usage of corticosteroids (P = 0.304) or antiviral agents (P = 0.349) did not show any correlation between the two groups.
The aqueous flare photon count was determined in five of the eight patients with HSV-AU, 17 of the 20 patients with VZV-AU, and 15 of the 18 patients with CMV-AU at the time of aqueous humor sampling (Fig. 2c). The mean photon count was 33 (range 12–78) photons/ms for HSV-AU, 97 (range 9–501) photons/ms for VZV-AU, and 17 (range 4–38) photons/ms for CMV-AU. The photon count was significantly higher for VZV-AU than for CMV-AU (P = 0.009). We also analyzed whether disease duration or previous treatment correlated with the aqueous flare. Regression analysis did not show any correlation between disease duration and the photon count in patients with HSV-AU (r 2 = 0.021, P = 0.443), VZV-AU (r 2 = 0.003, P = 0.836), or CMV-AU (r 2 = 0.001, P = 0.930) or in all herpetic AU (r 2 = 0.016, P = 0.460). We then compared the patients whose photon count was more or less than 50 photons/ms and analyzed whether the previous treatment correlated with the viral load. Previous usage of corticosteroids (P = 0.347) or antiviral agents (P = 0.475) did not show any correlation between the two groups.
We then determined whether the viral load and aqueous flare count for each type of AU were significantly correlated. We found a significant positive correlation between the viral load and aqueous flare count in eyes with VZV-AU (r 2 = 0.699, P = 1 × 10−4), but not in eyes with HSV-AU (r 2 = 0.657, P = 0.096) or CMV-AU (r 2 = 0.020, P = 0.619; Fig. 4).
Correlation between aqueous flare count and viral load in the aqueous humor. The aqueous flare count in eyes with VZV-AU was significantly correlated with the viral load (r 2 = 0.699, P = 1 × 10−4), but the correlation was not significant in eyes with HSV-AU (r 2 = 0.657, P = 0.096) or CMV-AU (r 2 = 0.020, P = 0.619)
At the final examination, segmental iris atrophy was seen in one eye with HSV-AU (13 %), in eight eyes with VZV-AU (40 %), but in none of the eyes with CMV-AU (Table 4). Diffuse iris atrophy was seen in one eye with HSV-AU (13 %), one eye with VZV-AU (5 %), and six eyes with CMV-AU (33 %). Segmental iris atrophy was seen more frequently in eyes with VZV-AU than in eyes with CMV-AU (P = 0.003), whereas diffuse iris atrophy was seen more frequently in eyes with CMV-AU than in eyes with VZV-AU (P = 0.032). To determine whether disease duration affected the development of iris atrophy, we compared disease duration among the patients who developed segmental iris atrophy (n = 9) with those who developed diffuse iris atrophy (n = 8) or patients who did not develop iris atrophy (n = 29). We found a significant difference between the patients who developed segmental iris atrophy (mean = 6 days) and those who developed diffuse iris atrophy (mean = 398 days; P = 0.0213). No significant difference was found between patients who developed iris atrophies and patients who did not (mean = 510 days).
The gonioscopic findings or the number of anterior vitreous cells did not differ significantly among the three groups of herpetic AU.
Finally, we examined the maximum IOP recorded at the severest inflammatory phase (Fig. 2d). The average maximum IOP was 30 (range 18–42) mmHg for eyes with HSV-AU, 35 (range 17–60) mmHg for eyes with VZV-AU, and 41 (range 14–70) mmHg for eyes with CMV-AU. The maximum IOP was significantly higher in eyes with CMV-AU than in eyes with HSV-AU (P = 0.022). The incidence of an IOP > 25 mmHg was 63 % in eyes with HSV-AU, 85 % in eyes with VZV-AU, and 78 % in eyes with CMV-AU. We then compared the patients whose maximum IOP was >25 mmHg and those whose maximum IOP was ≤21 mmHg to determine whether the previous treatment differed between two groups. This comparison revealed that previous use of corticosteroids (P = 0.202) or antiviral agents (P = 0.436) did not differ between the two groups. In most cases of herpetic AU, IOP decreased after initiation of antiviral therapy, with some exceptions for patients who eventually underwent trabeculectomy. Trabeculectomy was performed for three patients with CMV-AU (17 %) before diagnosis by PCR and also for three patients with VZV-AU (15 %) and four patients with CMV-AU (23 %) whose IOP did not decrease after antiviral therapy was performed (Fig. 2d).
Differences in VZV-AU between eyes with and without dermal manifestations
Although dermal signs, such as blisters or dermal pain, are important features of VZV infection [15], only four patients (20 %) had herpes zoster ophthalmicus and one patient (5 %) had zoster sine herpete. To investigate the differences between the two types of VZV-AU, i.e., VZV-AU with and without dermal signs, we compared the VZV viral load, aqueous flare count, and maximum IOP and found no significant differences between the two patient groups (Table 5). These data suggest that intraocular inflammation in eyes with VZV-AU is not significantly associated with the presence of dermal signs.
Discussion
Our analysis identified clinical characteristics that were common to all three types of herpetic AU studied, as well as those which significantly among these types of herpetic AU. Although all patients had unilateral disease, there were no significant differences in the affected eye.
HSV-AU shared a number of common features with VZV-AU, such as relatively acute disease process and presence of large KPs, but the degree of intraocular inflammation in eyes with HSV-AU was less severe than that in eyes with VZV-AU. None of our patients with HSV-AU had typical dendritic keratitis [16], indicating that HSV infection in patients with unilateral KPs, even if the intraocular inflammation is not severe or the IOP is not elevated, should be considered suggestive of HSV.
The characteristic features of VZV-AU were severe intraocular inflammation, high viral load in the aqueous humor, high aqueous flare count, segmental iris atrophy, and elevated IOP. However, the severity of VZV-AU was not correlated with the presence or absence of dermal manifestations.
Our data show that the VZV viral load and maximum IOP were significantly correlated. We speculate that VZV in the aqueous humor may have increased the resistance of aqueous outflow because of direct VZV infection of the trabecular meshwork. Marsh and colleagues reported fluorescein iris angiography findings suggestive of segmental iris atrophy and sphincter atrophy in VZV-AU being caused by localized ischemia of the iris pigment epithelial cells [15]. Such ischemia was not observed in eyes with HSV-AU, possibly suggesting that VZV—but not HSV—invades the root of the iris epithelium and destroys the vascular structure. Because the iris root and the trabecular meshwork are adjoining tissues, we suggest that the ocular manifestations of VZV-AU are characterized primarily by the infection of VZV at the iris root, followed by the spread of VZV to the adjoining trabecular meshwork, which may lead to an increase in the resistance of aqueous outflow. This notion may be supported by our data, in which the duration of segmental iris atrophy was significantly shorter than that of diffuse iris atrophy, suggesting different mechanisms in the formation of iris atrophy.
CMV-AU exhibited some unique characteristics when compared with those of HSV-AU or VZV-AU. The chief complaints at disease onset was milder in patients with CMV-AU than in those with HSV-AU or VZV-AU because of the absence of conjunctival redness and ocular pain in the former. However, a recall bias should be considered because the duration of CMV-AU is longer than that of the other types of herpetic AU. KPs were found in all three types of herpetic AU, but their sizes differed significantly, with eyes with CMV-AU having both medium-to-large (39 %) and small-to-fine (44 %) KPs, and all of the eyes with HSV-AU and most of those with VZV-AU having medium-to-large KPs. Therefore, the size of the KPs can be considered to be an important clinical sign for differentiating CMV-AU from the other types of herpetic AU. The decrease in the CECD was significant in CMV-AU, and it is well known that CMV is associated with corneal endotheliitis [9, 10]. Because only two of our patients (11 %) showed coin-shaped lesions, which are typically seen in corneal endotheliitis due to CMV infection, as reported by Koizumi and colleagues [9], in which there was only one case (6 %) of corneal endotheliitis accompanied by coin-shaped lesions, whether CMV-AU and endotheliitis belong to the same spectrum of diseases remains inconclusive. However, our data suggest that even if the typical manifestations of corneal endotheliitis are not found, we should nevertheless monitor CECD because some of our patients with CMV-AU eventually developed bullous keratopathy. We therefore recommend that the CECD be determined during the diagnosis and follow-up of eyes with CMV-AU. At the initial presentation, in our patients anterior segment inflammation of the eye was less severe in eyes with CMV-AU than in those with HSV-AU or VZV-AU, as shown by the lower viral load in the aqueous humor and the lower aqueous flare counts. With respect to maximum IOP, eyes with CMV-AU showed a higher IOP than did those with HSV-AU.
Taken together, we suggest that the characteristic features of CMV-AU are mild ocular symptoms, mild anterior segment inflammation, as shown by small KPs, lower viral load in the aqueous humor, lower aqueous flare counts, decreased CECD, and elevated IOP.
On the other hand, disease duration from the onset to the detection of viral DNA in the aqueous humor was significantly longer in eyes with CMV-AU, and nearly one-half of our patients with HSV-AU or VZV-AU were examined in the acute phase, whereas only one of 18 patients with CMV-AU was examined in the acute phase (Table 1). Because most of the clinical data on CMV-AU other than the ocular symptoms were obtained in the chronic state, it is possible that the clinical features of CMV-AU, such as decreased CECD or mild anterior segment inflammation, had been severely altered by the effect of long-term intraocular inflammation or previous nonspecific treatment, such as topical steroids or IOP-reducing treatments. Our analysis did not show any correlation between CECD and disease duration or treatment profiles, but it should be noted that our analysis was performed using cross-sectional data. Therefore, a longitudinal study should be performed to verify our findings.
This study has a number of limitations. First, the study was conducted in only two clinics in Japan, and the number of patients was small. Secondly, it was a retrospective study, and as such it lacks important information such as that from a negative control group to calculate the sensitivity and specificity of the clinical signs. Thirdly, in most of the CMV cases, the disease was chronic, whereas in most of the VZV cases, the disease was acute or subacute, and more than half of the HSV cases were acute cases. Although no correlation was found between disease duration and CECD, corneal endotheliitis, KPs, viral load, or aqueous flare count, it must be considered that the difference in disease duration may have severely affected the characteristic features of CMV-AU. To overcome these limitations and determine the exact features of herpetic AU, a multicenter prospective study should be performed.
In conclusion, the three types of herpetic AU studied here had a number of features in common, but each disease also had its own distinct features. To make an accurate diagnosis and initiate proper treatment promptly, clinicians are encouraged to make an accurate diagnosis before initiating corticosteroid therapy.
References
Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51:41–4.
Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. 2012;56:432–5.
Jap A, Chee SP. Viral anterior uveitis. Curr Opin Ophthalmol. 2011;22:483–8.
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–40.
Chee SP, Jap A. Presumed fuchs heterochromic iridocyclitis and Posner–Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol. 2008;146:883.e1–889.e1.
de Schryver I, Rozenberg F, Cassoux N, Michelson S, Kestelyn P, Lehoang P, et al. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br J Ophthalmol. 2006;90:852–5.
Hwang YS, Lin KK, Lee JS, Chang SH, Chen KJ, Lai CC, et al. Intravitreal loading injection of ganciclovir with or without adjunctive oral valganciclovir for cytomegalovirus anterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2010;248:263–9.
Kawaguchi T, Sugita S, Shimizu N, Mochizuki M. Kinetics of aqueous flare, intraocular pressure and virus-DNA copies in a patient with cytomegalovirus iridocyclitis without retinitis. Int Ophthalmol. 2007;27:383–6.
Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology. 2008;115:292.e3–297.e3.
Miyanaga M, Sugita S, Shimizu N, Morio T, Miyata K, Maruyama K, et al. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol. 2010;94:336–40.
Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92:928–32.
Kongyai N, Pathanapitoon K, Sirirungsi W, Kunavisarut P, de Groot-Mijnes JD, Rothova A. Infectious causes of posterior uveitis and panuveitis in Thailand. Jpn J Ophthalmol. 2012;56:390–5.
De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, et al. Polymerase chain reaction and Goldmann–Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–8.
Zhang Y, Kimura T, Fujiki K, Sakuma H, Murakami A, Kanai A. Multiplex polymerase chain reaction for detection of herpes simplex virus type 1, type 2, cytomegalovirus, and varicella-zoster virus in ocular viral infections. Jpn J Ophthalmol. 2003;47:260–4.
Marsh RJ, Easty DL, Jones BR. Iritis and iris atrophy in Herpes zoster ophthalmicus. Am J Ophthalmol. 1974;78:255–61.
Hogan MJ, Kimura SJ, Thygeson P. Pathology of Herpes simplex kerato-iritis. Trans Am Ophthalmol Soc. 1963;61:75–99.
Acknowledgments
We thank Professor Duco Hamasaki for his critical review of the manuscript. We also thank Dr. Takashi Komizo for his assistance in collecting the data.
Conflicts of interest
H. Takase, None; R. Kubono, None; Y. Terada, None; A. Imai, None; S. Fukuda, None; M. Tomita, None; M. Miyanaga, None; K. Kamoi, None; S. Sugita, None; K. Miyata, None; M. Mochizuki, None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takase, H., Kubono, R., Terada, Y. et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol 58, 473–482 (2014). https://doi.org/10.1007/s10384-014-0340-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-014-0340-6