Abstract
Understanding the ecological and behavioral variation of primates is central to improving conservation strategies. Studies of chimpanzees (Pan troglodytes) have shown marked behavioral variability across a wide range of habitats. By comparison, bonobos (Pan paniscus) occur mainly in continuous forest sites, and socioecological models explaining behavioral differences between the two species are based on the assumption that bonobo habitat is richer and more stable than chimpanzee habitats. The objective of this study was to assess bonobo ecology in a forest–savanna mosaic. We specifically explored 1) bonobo home range size, habitat use, and dietary composition; 2) forest fragmentation in a bonobo community’s home range; and 3) seasonality of dietary diversity, habitat use, and spatial distribution. Using 2 years of observational data on location, habitat type, and dietary composition, we found that the bonobo community's home range size, density, and dietary diversity were similar to those observed in continuous forest sites. Bonobos used areas with lower forest fragmentation than the surrounding areas and preferentially spent time in forested habitats. During the dry season, bonobos consumed a lower diversity of fruit species, spent more time in sparse forest with Marantaceae understory, and were closer to the seasonal center of their home range than during the rainy season. Our results contribute to our understanding of behavioral variation in bonobos and suggest that forest–savanna mosaic is valuable to bonobo conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A better understanding of the extent of behavioral variation in threatened species is of critical importance to tailor conservation action to ecological need (Strier 2017). Multiple field studies are therefore needed to understand the ecology of species whose range encompasses diverse environments. This approach is particularly relevant for primates, as most primates are threatened and inhabit various environments (Estrada et al. 2017), including mosaic vegetation types (forest–savanna, forest–agriculture mosaic); fragmented and continuous forests; and with variation in the extent of human proximity, density, and practices resulting in diverse levels of anthropogenic pressure.
Field studies of primates have highlighted many intraspecific differences in ecological strategies. Intraspecific differences are particularly evident in home range size, daily path length, diet, and habitat use (e.g., guerezas, Colobus guereza: Harris and Chapman 2007; diademed sifakas, Propithecus diadema: Irwin 2008; blue monkeys, Cercopithecus mitis: Tesfaye et al. 2013; crab-eating macaques, Macaca fascicularis: Sha and Hanya 2013; northern pig-tailed macaques, Macaca leonina: Gazagne et al. 2020; chimpanzees, Pan troglodytes: Potts et al. 2011). Many taxa exhibit seasonal variation in ranging patterns, habitat use, and diet within the same groups (e.g., olive baboons, Papio anubis: Barton et al. 1992; white-headed langur, Trachypithecus poliocephalus: Hendershott et al. 2018; northern pig-tailed macaques: Gazagne et al. 2020; chimpanzees: Chancellor et al. 2012). The spatiotemporal availability of food resources influences feeding strategies and habitat use (Fan and Jiang 2008; Tutin et al. 1997; Wallace 2008). Feeding strategies and habitat use are closely related to each other, and during periods of fruit scarcity, a group’s home range may be smaller and primates may consume fallback foods. Alternatively, a group may extend its home range to meet resource needs (Hendershott et al. 2018; Schoener 1971; van Schaik and Brockman 2005). In fragmented environments, forest patch size can positively influence home range size, but the effect of habitat fragmentation on home range size differs greatly according to the matrix configuration and a species’ ability to travel between forest patches (e.g., Bicca-Marques 2003; Boyle et al. 2013; Irwin 2008; Onderdonk and Chapman 2000; Tutin 1999). Other factors affecting home range and feeding strategies include intraspecific competition, intergroup dynamics, and interspecific competition (Grant et al. 1992; Mitani and Rodman 1979). The lack of comparability between studies, often due to the use of different methods, limits our understanding of factors underlying these different strategies (Boyle et al. 2009; Pearce et al. 2013).
Chimpanzees are the most widely distributed great apes (Humle et al. 2016) and live in a variety of habitats, from open mosaic environments to continuous tropical forest, that differ in vegetation, climate, elevation, topography, and human sociocultural context. Within their range of habitats, studies of chimpanzees in savanna mosaic habitats have been particularly fruitful and relevant to our understanding of their behavioral and ecological variation (Moore 1996; Lindshield et al. in prep.). Along the continuum of habitats inhabited by chimpanzees, savanna habitats are particularly dry (annual rainfall <1360 mm/yr) and sparsely forested (forest cover <12.5%) compared to other chimpanzee habitats (van Leeuwen et al. 2020). Chimpanzees inhabiting savanna habitats have a larger home range size, a lower density (2–50 times), and a lower dietary diversity than those inhabiting more forested environments, probably because of lower availability and higher spatial dispersion of food resources and water (Baldwin et al. 1982; McGrew et al. 1988; Ogawa et al. 2007; Piel et al. 2017; Pruetz 2006; Pruetz and Bertolani 2009; Skinner and Pruetz 2012; van Leeuwen et al. 2020; cf. Basabose 2005). Chimpanzees in savanna mosaic environments use forest preferentially relative to its availability but also rely frequently on open woodland habitats, especially for foraging and nesting (Hernandez-Aguilar et al. 2013; McGrew et al. 1988; Ndiaye et al. 2018; Piel et al. 2017; Pruetz and Bertolani 2009). The high seasonality of rainfall and water availability, temperature, and food availability in these environments likely influences seasonal variation in chimpanzee habitat use (Baldwin et al. 1982; Wessling et al. 2018), but seasonal patterns of savanna chimpanzee space use have not yet been directly investigated.
The other Pan species, the bonobo (Pan paniscus), ranges only within the Democratic Republic of the Congo (DRC) (Fruth et al. 2016). Its geographic range is smaller than that of chimpanzees and has little variation in habitat types across its range compared with chimpanzees (mainly equatorial forest, and forest–savanna mosaic at the southern periphery; Gruber and Clay 2016; Pennec et al. 2016; Thompson 2002; White and Wrangham 1988). This apparent ecological stability has led to a hypothesis to explain behavioral differences between the two Pan species (Boesch et al. 2002; Furuichi 2009), namely that richer and less variable food resource availability (especially fruits and high-quality terrestrial herbs) in bonobo habitats led to more tolerant relationships, more stable diets, and smaller home ranges in bonobos than in chimpanzees (Hohmann et al. 2006; Kano 1992; Wrangham et al. 1996; cf. Georgiev et al. 2011).
Understanding intraspecific differences in ecological strategies will further elucidate the boundaries of bonobo behavioral variation, which we can then compare to variation observed in chimpanzees. Information about bonobo ranging patterns and habitat use is available for some forest field sites in DRC. At Lomako, two studies found substantial differences in home range size for the same bonobo communities (2 and 22 km2; Badrian and Badrian 1984; Waller 2011), without clear explanations for these differences. At Wamba, three studies found different home range sizes and densities for the same bonobo communities, and seasonal changes in habitat preference (Hashimoto et al. 1998; Hashimoto and Furuichi 2015; Terada et al. 2015). This seasonality in habitat use is probably linked to spatiotemporal variability in fruit availability, because fruit is the preferred food of bonobos (Conklin-Brittain et al. 2001). For example, when fruits of Uapaca sp. are available in swamp forest at Wamba, bonobos spend more time in this forest type (Terada et al. 2015). Terrestrial herbaceous vegetation (THV) also plays a major role in the bonobo diet (Badrian and Malenky 1984; Conklin-Brittain et al. 2001; Malenky and Stiles 1991) and when fruit availability is lower, bonobos may eat more THV and spend more time in THV-rich forest types (Kano and Mulavwa 1984; Terada et al. 2015). However, other studies in bonobos have found no effect of fruit availability on THV consumption (Malenky and Wrangham 1994; White and Wrangham 1988). Finally, bonobos have been described as nesting mostly in mixed terra firma forest, also called primary forest or dry forest, with a predominance of mixed forest with Marantaceae understory (Hashimoto et al. 1998; Idani et al. 1994; Kano 1992; Reinartz et al. 2006; Terada et al. 2015).
We have a poor understanding of bonobo ecology in a mosaic environment in contrast to forest field sites. Broad-scale models that predict suitable habitats for bonobos have found negative influences of forest fragmentation and human proximity, except at the west of their range (Hickey et al. 2012, 2013), making this region interesting to assess ecological and behavioral variation in bonobos. Moreover, a better understanding of the temporal variation in habitat types used by bonobos is important when developing conservation strategies, especially in landscape mosaics where forest patch composition and forest connectivity can limit migration between communities (Serckx et al. 2014; Terada et al. 2015).
The Manzano study site is at the southwestern periphery of the bonobos’ geographic range (Bolobo Territory, DRC) and consists of a forest–savanna mosaic (Inogwabini et al. 2008; Pennec et al. 2016). Located at the edge of the Congo Basin, this mosaic probably originates from both natural and anthropogenic influences, including seasonal fires in savannas and slash-and-burn agriculture (Demichelis et al. 2020; Namur 1990). In this forest–savanna mosaic environment, bonobo density and dietary diversity falls within the range of the more forested bonobo study sites (Hashimoto et al. 1998; Inogwabini et al. 2008; Mohneke and Fruth 2008; Narat 2014; Sabater Pi and Vea 1990; Serckx et al. 2014, 2015; Uehara 1988; Van Krunkelsven 2001). Additionally, a previous study in the same region found high seasonal variation of fruit availability and, based on a multi-year transect survey, suggested that bonobos adapt their ranging patterns to local fruit availability (Serckx et al. 2014). While it appears that dietary diversity and bonobo density in this forest–savanna mosaic do not follow the pattern observed in savanna mosaic chimpanzees (lower density and lower dietary diversity) data are lacking on their home range, habitat use, and the potential seasonality of these parameters.
The objective of this study was to assess aspects of bonobo ecology in a forest–savanna mosaic. We specifically explored 1) bonobo home range size, habitat use, and dietary composition; 2) forest patch fragmentation level in the community home range relative to the broader landscape; and 3) seasonal variation in dietary diversity, habitat use, and spatial distribution. To do this, we analyzed 2 years of observational data on bonobo location, habitat type, and dietary composition to determine bonobo home range and seasonal variations in dietary diversity, habitat use, and spatial distribution. We used satellite images to characterize edge density and determine if bonobos selected areas with relatively lower fragmentation compared with what is available in this region. The bonobo community we studied lives in a forest–savanna mosaic, and the proportion of forest landcover in this area is lower compared with continuous forest sites. We hypothesized that food resources determine home range size. If resources are more dispersed than at sites with more continuous forest cover, as observed for chimpanzees in savanna habitats, we predicted that the bonobos’ home range would be larger than in sites with more continuous forest cover (Baldwin et al. 1982; McGrew et al. 1988; Ogawa et al. 2007; Pruetz and Bertolani 2009; Pruetz 2006; Skinner and Pruetz 2012). Second, we hypothesized that the level of fragmentation influences bonobo distribution locally. Because forest fragmentation is a negative predictor of bonobo occurrence at a broad scale (Hickey et al. 2013), we predicted that the level of forest fragmentation would be lower in the bonobo home range than in the surrounding area. Finally, although habitats inhabited by bonobos are considered more stable and less seasonal compared to those inhabited by chimpanzees (Oelze et al. 2016; White 1998), fruit availability shows high temporal variation in this forest–savanna mosaic (Serckx et al. 2015). We hypothesized that this temporal variation in food availability would be linked to variation in the bonobo dietary diversity. Because fruit availability is lower in the dry season (Malenky and Wrangham 1994; cf. van Schaik and Pfannes 2005), we predicted that we would observe lower fruit dietary diversity during the dry season than the wet season. As bonobos consume fallback foods during periods of low preferred food availability (Serckx et al. 2015), we predicted that bonobos would shift their habitat use and space use to feed on these fallback foods during the dry season.
Methods
Study Site
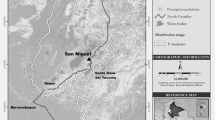
We conducted our study in Manzano forest near Embirima Village (2°34′S 16°22′E), Bolobo Territory, DRC (Fig. 1). Located in the southwestern part of the Congo Basin, the local climate features tropical characteristics with an alternation of dry and rainy seasons and the mean annual rainfall is 1625 mm. The vegetation is 58% forest and 42% savanna with an elevation of 332–557 m (Pennec et al. 2016). Thus, this area is less forested than the other bonobo study sites in forest–savanna mosaic (e.g., Lukuru: 80% forest, 20% savanna, Thompson 1997). Although it can be characterized as a mosaic habitat, this study site is not comparable to the most extreme savanna habitats inhabited by chimpanzees, typically characterized as <12.5% forest cover and <1360 mm of annual rainfall (van Leeuwen et al. 2020). Forest patches are mainly composed of terra firma forest and seasonally inundated forests. Two main types of savannas are present: seasonally inundated herbaceous savannas and shrub savannas. The two most frequently encountered primate species are bonobos and red-tailed monkeys (Cercopithecus ascanius). The human population of the nearby Embirima village is ca. 800 people and the land managed by the village occupies an area of 135 km2, resulting in a human density of 6 inhabitants/km2 (Mbou-Mon-Tour NGO, unpubl. data). Local people, the Bateke, respect a taboo against eating bonobos and use the forest daily for activities such as slash and burn agriculture, hunting, fishing, and gathering (Narat et al. 2015a). The local people participate in a community-based conservation project (Forest Concession of Local Communities of the Mbali River) led by the Congolese NGO Mbou-Mon-Tour and initiated in 2001 in the Bolobo Territory and in 2006 for this village (Narat et al. 2015a). As part of this project, villagers have designated areas dedicated to conservation and rules of use in these areas. Among the 82.6 km2 of forest managed by this village, 35.9 km2 are community conservation forests for bonobos and monkeys where some activities are forbidden (hunting, fishing with poison, agriculture), whereas others are permitted (e.g., caterpillar, fruit and leaf gathering, and fishing). This conservation strategy aims to maintain spatial overlap between humans and primates. Habituation of the Manzano bonobo community began in 2012, and long-term protocols have been established to study bonobo ecology, sociality, and health since 2015 (Narat et al. 2015b).
Data Collection
From February 2016 to January 2018, we followed the Manzano bonobo community for 205 days and conducted 469 h of direct observation (days per month with bonobo observations: mean = 8.5 ± SD 3.7, range = 1–17). The community was composed of 22–24 individuals (3 adult males and 6 or 7 adult females) at time of this study. We collected data using ad libitum sampling (Altmann 1974). When we were with bonobos, we recorded geographical coordinates (using a Garmin GPSMAP 64) every 50 m, and recorded the following data for each set of geographical coordinates (defining an observation point): start and end time; habitat type; and feeding events, defined as the consumption of one item of one species at this observation point. We also recorded fresh (estimated to <1 h) food remains of bonobos, even if we did not directly observe its consumption, as supplemental data to improve the description of diet at each observation point.
We recorded all signs of human presence when searching for or following bonobos to describe human activities (hunting, fishing, gathering, agriculture, moving, fire, other) in areas used by bonobos. However, because of the strong bias in these data, which we collected only when searching for or following bonobos, we did not analyze these data as factors that could influence bonobo presence.
At each observation point, we recorded the main habitat types based on descriptions by Pennec et al. (2016). Mixed forest with open understory (MFO) had a high density of trees (426 trees/ha) and lianas (136 lianas/ha) among terra firma forests, with Pentaclethra eetveldeana, Plagiostyles africana, Anonidium manii, and Staudtia kamerunensis as the most important species, as determined by the Importance Value Index (Curtis 1959) calculated for each species. The understory had abundant woody regeneration with low herbaceous plant density. Mixed forest with Marantaceae understory (MFM) had a similar structure to MFO, with Plagiostyles africana, Sorindeia juglandifolia, Chaetocarpus africanus, and Piptadeniastrum africanum as the important tree species. The understory had a low density of woody regeneration but high Marantaceae density (Sarcophrynium schweinfurthianum dominating Haumania liebrechtsiana). Sparse forest with Marantaceae understory (SFM) had the highest diversity index and tree composition was similar to MFM. However, the structure was different with lower density of trees, an underrepresentation of trees with diameter at breast height (DBH) < 30 cm, and with important large tree species including Celtis tessmannii, Klainedoxa gabonensis, and Millettia laurentii. Pentaclethra eetveldeana was the most frequently represented species in SFM. The understory had the poorest woody regeneration but high Marantaceae density (H. liebrechtsiana dominating S. schweinfurthianum). Seasonally inundated forest (SIF) had high tree and liana densities (343 trees/ha and 235 lianas/ha) and 80% of trees with DBH ≤ 30 cm. The understory was seasonally inundated with a moderate density of woody regeneration and Marantaceae species dominated by other herbaceous species. Hymenocardia acida, Maprounea africana, and Annona senegalensis generally dominated shrub savannas, but some savannas were herbaceous and swampy. Other habitat types were field and fallow (resulting from slash and burn agriculture technique maintaining a canopy cover dense enough to consider these vegetation types as forested), edge, and road (dirt).
We collected daily rainfall with a rain gauge from 2012 to 2018 and calculated the mean monthly rainfall over this period. We defined dry and rainy seasons using a threshold of 100 mm/month (Dupain et al. 2002; Hunt and McGrew 2002) (Fig. 2). According to this definition, the dry season lasts from June to September and the rainy season from October to May (Fig. 2). The bonobo observation time was distributed between seasons as follows: 87 days (221 h) during the dry season and 118 days (248 h) during the rainy season.
Boxplot of monthly rainfall recorded near Manzano forest from 2012 to 2018. Triangles represent the mean, black bars the median, and white boxes the interquartile range (25th–75th percentile). The lower limits of the vertical lines represent the smallest value within 1.5 times the interquartile range below the 25th percentile, and the upper limits represent the largest value within 1.5 times the interquartile range above the 75th percentile for that month. Black dots are values outside of these limits. The horizontal dashed line shows the threshold between dry and rainy seasons.
Data Analyses
Home Range Size, Habitat Use, and Dietary Composition
We determined home range using three complementary methods: minimum convex polygons (MCPs), grid cell adjustment, and kernel density estimation. First, we determined bonobo home range size with MCPs (Mohr 1947; Southwood 1978) of all observation points collected during the study period. This method overestimates the home range size but the data are easily comparable with those of previous studies (e.g., Terada et al. 2015; Waller 2011) (see Table II). To assess the robustness of our dataset, we used the following resampling strategy: for each number of data points (N) in increments of 10, we randomly selected N observation points from our full data set (N = 794) with replacement 1000 times, and computed the MCP home range estimate for each iteration. Second, to exclude the areas from the polygon where bonobos were never observed, we adjusted the polygon by superimposing a 500-m grid and clipping out cells that were never visited (Grueter et al. 2009; Hashimoto et al. 1998; Vieira et al. 2019). We also used this grid cell method to facilitate comparisons with some existing data on bonobo home range size (Hashimoto et al. 1998). Third, we weighted each location observation by the time spent at each point and employed the QGIS HEATMAP plugin to generate a kernel density map (radius = 200 m, pixel size = 25 m, quartic kernel shape) (Ferreguetti et al. 2018; Katsis et al. 2018; Kshettry et al. 2017). This analysis generates a map of intensity of use and allows identification of the areas of highest use by bonobos within their home range. Based on this layer, we defined hotspots as those pixels with the highest 10% of values from the map of intensity of use (kernel analyses with a threshold of 10%). To describe habitat use, we determined the percentage of time spent in each habitat type based on the data collected at each observation point. We included all species consumed by bonobos (from direct observation or fresh food remains) at least once during the study period in the dietary composition.
Forest Fragmentation and Edge Density
To evaluate forest fragmentation, we performed an unsupervised classification (Landsat 8, August 14, 2016) using ENVI 4.5 (K-means method, 10 iterations, four classes) to determine both the forest cover and edge. We used the normalized difference vegetation index (NDVI) obtained from the red and near infrared bands of the Landsat 8 image to calculate the mean NDVI of the four spectral classes (NDVI value increases with photosynthetic activity, Myneni et al. 1995). Then, based on these values and our knowledge of the area, we assigned vegetation types to the spectral classes: continuous canopy forest (mean NDVI = 0.27 ± SD 0.02), open areas (degraded forests and swampy savanna, mean = 0.21 ± SD 0.03), savanna type 1 (mean = 0.14 ± SD 0.02), and savanna type 2 (mean = 0.10 ± SD 0.03). We used the continuous canopy forest class to determine the forest cover, and its contour to determine the edge. To estimate forest fragmentation, we calculated the edge density (m/ha) of the home range (Hickey et al. 2012) for the MCP and grid adjustment (a single value for each home range assessment method) as follows:
ED = D ÷ A, where ED is the edge density, D is the linear distance of edge (meters) in the area considered and A is the area (ha).
To assess if bonobos home ranges have lower edge density than the surrounding area, we also determined the edge density in two larger circles. For the immediate vicinity of the home range, we determined the radius of a circle using the maximum distance between the weighted home range center—defined as the average geographic coordinates weighted by the duration at each point—and the limit of the MCP. We then defined a circle with three times this radius, excluding the first circle, to characterize the edge density of the surrounding region.
Seasonal Variation in Dietary Diversity, Habitat Use, and Spatial Distribution
To analyze seasonal patterns in space use, we performed the same analysis as with the full dataset (kernel density maps: radius = 200 m, pixel size = 25 m, quartic kernel shape) on points collected during the dry season and those collected during the rainy season, weighted by the time spent at each point. We defined seasonal hotspots as the highest 10% of values.
To explore seasonal differences in plant dietary diversity, we first calculated an index of plant taxa diversity consumed in seasonal hotspots, accounting for hotspot size and time spent in hotspots, as follows:
\( {Dt}_i=\raisebox{1ex}{${Nt}_i$}\!\left/ \!\raisebox{-1ex}{${At}_i\ast {Tt}_i$}\right. \) where Dti is the diversity of taxa consumed in hotspot i, Nti is the number of taxa consumed in hotspot i, Ati is the area of hotspot i (ha), and Tti is the time spent in hotspot i (hours). Then, we used a Wilcoxon test to compare the diversity index in hotspots between seasons (both for all plant items and only for fruits).
To assess seasonal differences in habitat use, we calculated the percentage of time spent in each habitat type for all points recorded during the dry season and the rainy season, and for those located only in seasonal hotspots. Then, we compared these percentages between the dry and rainy seasons, both for all points and for those located in seasonal hotspots with a chi-squared test. To simplify the analyses to conform to a chi-squared test structure, we grouped all habitats with a percentage value lower than 5% as “other.”
To examine seasonal variation in the spatial distribution of bonobos, we calculated the average geographic coordinates weighted by the duration at each point for each season to represent the seasonal weighted home range center. Then, we used a Wilcoxon test to compare the mean distance of observation points to the seasonal weighted home range center between seasons.
We used QGIS software (version 3.4.1-Madeira, QGIS Development Team 2018) for all spatial analyses. We used R (version 3.5.1, R Core Team 2018) for statistical analyses.
Ethical Note
Our study adhered to all research requirements of the DRC. We obtained research authorization through our local collaborator, the NGO Mbou-Mon-Tour. The authors have no conflict of interest or competing financial interests to declare.
Data availability
The datasets generated and analyed during the current study are available from the corresponding author on reasonable request.
Results
Home Range Size, Habitat Use, and Dietary Diversity
Using the MCP method, the bonobo home range was 20.3 km2 during the study period (N=794 points; 365 for the dry season and 429 for the rainy season) and included 72% forest cover (Fig. 3). The maximum distance between the weighted home range center and the limit of the MCP was 4 km. When we resampled observation points with replacement and recomputed home range area, increasing sample size reduced estimate variance and estimates converged toward the value obtained in our complete dataset (Fig. 4). Bonobos occurred in 13.0 km2 with a 500-m grid superimposed on the MCP (83% forest cover), and 9.4 km2 with the map of intensity of use (86% forest cover; Fig. 3). Based on the Manzano community composition during the study period (10 adults, 5 subadults, and 9 juveniles), we calculated densities of 0.5 mature individuals/km2 and 1.2 individuals/km2 with the MCP method, and 0.8 mature individuals/km2 and 1.8 individuals/km2 with the 500-m grid cell method.
(a) Spatial distribution of bonobo observation points in Manzano forest, DRC, from February 2016 to January 2018. (b) Home range estimated by the minimum convex polygon and 500-m grid cell methods. (c) Signs of human presence. (d) Intensity of space use by bonobos (kernel 100% and hotspots based on kernel 10%).
Estimates of home range area for bonobos in Manzano forest, DRC, between February 2016 and January 2018 for 1000 resamples with replacement per sample size. Horizontal dashed line represents the home range estimate in our complete dataset. Black bars represent the median, and white boxes the interquartile range (25th–75th percentile). The lower limits of the vertical lines represent the smallest value within 1.5 times the interquartile range below the 25th percentile, and the upper limits represent the largest value within 1.5 times the interquartile range above the 75th percentile for that month. Black dots are values outside of these limits. N = 794 data points for the full data set.
Three hotspots were located in the northeastern part of the home range (67 ha, 16 ha, 6 ha) and represented 58.3% of the total observation time. Two smaller hotspots were in the southern part of the home range (5 ha, 4 ha) and represented 4.7% of the total observation time. Bonobo space use outside these hotspots accounted for the remaining 37.0% of total observation time.
We described habitats for more than 85% of the observation points over the study period. Observation points without habitat description are due to omission during data collection. We observed bonobos in forested habitat types 97% of observation time (37% in SFM, 28% in MFO, 24% in MFM, 5% in fallow, 2% in SIF, and 1% in field) and in savannas (1%), edge (1%), and road (1%). We observed the same general pattern in the percentage of habitat types in the hotspots during the overall study period: bonobos occurred principally in SFM (44%), MFO (24%), and MFM (21%).
We recorded 919 plant consumption events. Bonobos consumed 83 plant taxa (55 identified to the species level, 17 to the genus level, 2 to the family level, and 9 with only vernacular identification), including consumption of fruit from 69 taxa (Electronic Supplementary Material [ESM] Table SI).
Edge Density
We estimated an edge density of 16.7 m/ha using the MCP home range, 19.5 m/ha using the 500-m grid cell home range, and 19.9 m/ha using the kernel 100% home range. Edge density was higher in the immediate vicinity of the home range (24.9 m/ha) than in the home range, and even higher in the larger surrounding neighborhood (38.6 m/ha) (Table I).
Seasonal Variation in Dietary Diversity, Habitat Use, and Spatial Distribution
Bonobos consumed lower fruit diversity/ha/h in dry season hotspots than in rainy season hotspots (Nhotspots dry season = 4, Nhotspots rainy season = 14, Wilcoxon test, W = 7, P = 0.04) (ESM Fig. S1), but we found no significant seasonal difference when comparing the taxonomic diversity of all items (/ha/h) consumed in hotspots (Wilcoxon test, W = 11, P = 0.13).
Bonobos spent a higher percentage of time in SFM during the dry season and in MFO and MFM during the rainy season (224 h of observation with habitat description during the rainy season and 180 h during the dry season, chi-squared test: χ2 = 13.4, df = 3, P = 0.004; the “other” category includes SIF, fallow, field, savanna, edge and road; Fig. 5a). Seasonal hotspots showed the same difference in habitat use (147 h of observation with habitat description during the rainy season and 105 h during the dry season, chi-squared test: χ2 = 29.0, df = 3, P < 0.001; Fig. 5b).
During the dry season bonobos used areas significantly closer to the seasonal weighted home range center than in the rainy season (Ndry = 365, Nrainy = 429, Wilcoxon test: W = 126,540, P < 0.001). Based on kernel analyses, dry season hotspots were more aggregated and less numerous than those in the rainy season, but did not differ in size (dry season: N = 4, mean area = 14.1 ha ± SD 12.6, range: 2.4–29.8; rainy season: N = 14, mean area = 6.3 ha ± SD 10.4, range: 0.1–40.6; Wilcoxon test: W = 15, P = 0.19; Fig. 6).
Discussion
In this forest–savanna mosaic, bonobos predominantly used forested habitat and rarely used savannas in their home range. Furthermore, we found that the forest in their home range was substantially less fragmented than the surrounding area. We also identified seasonal differences in diet and space use: in the dry season bonobos consumed a lower diversity of fruit species, spent more time in sparse forest with Marantaceae understory, and had a more aggregated spatial distribution than in the rainy season.
Home Range Size, Habitat Use, and Dietary Diversity
The home range of the Manzano bonobo community encompassed 20 km2 based on the MCP method, 13 km2 using the 500-m grid cell method and 9.4 km2 based on kernel density analyses. The variability of home range size estimates decreased and estimates converged toward the estimated size obtained using our complete data set (Fig. 4). These results indicate that the inferred home range size was not underestimated despite our limited survey effort due to financial constraints. Contrary to our prediction, the Manzano bonobos did not have a larger home range than bonobos living in continuous forest sites, where estimated home range sizes range 2–84 km2 (Table II). The Manzano bonobos spent most of their time in forested habitats (97%), of which the composition is typical of Congo Basin forests (Pennec et al. 2016). A recent study on another bonobo community living 15 km from our study site found the same pattern with only 0.5% of observation points in savanna (Onishi et al. 2020).
Bonobos consumed 83 plant taxa during our study. This estimate is lower than the dietary diversity estimated in a previous study on the same bonobo community (107 plant taxa consumed; Narat 2014), probably due to differences in survey effort (two times more people dedicated to data collection in 2014) and interannual variation. Nonetheless, the dietary diversity is comparable with other more forested bonobo study sites (Table II) and more than double the dietary diversity reported at Lukuru, another bonobo study site described as a forest–savanna mosaic (38 plant taxa; Thompson 1997). This difference could be due to the habituation level of bonobos, the duration of the study, or to specific ecological characteristics of the study sites.
Although several primate species show intraspecific variation in home range size, habitat use, and dietary diversity according to the environment (Gazagne et al. 2020; Harris and Chapman 2007; Irwin 2008; Sha and Hanya 2013; Tesfaye et al. 2013), our results suggest that bonobos do not vary greatly in these parameters, even in the most fragmented environment known for bonobos. Chimpanzees living in savanna mosaic have larger home range (up to 10 times), lower densities (2–50 times), and a lower dietary diversity than those living in forested habitats (Baldwin et al. 1982; McGrew et al. 1988; Ogawa et al. 2007; Piel et al. 2017; Pruetz 2006; Pruetz and Bertolani 2009; Skinner and Pruetz 2012; van Leeuwen et al. 2020; cf. Basabose 2005). Moreover, although chimpanzees preferentially use forest patches in mosaic habitats, they also frequently use open habitats for foraging and nesting, unlike bonobos we studied (Hernandez-Aguilar et al. 2013; McGrew et al. 1988; Ndiaye et al. 2018; Piel et al. 2017; Pruetz and Bertolani 2009). As the main hypothesis to explain the pattern observed in chimpanzees is the high spatial dispersal of food resources, our results suggest that this forest–savanna mosaic has comparable food availability compared to continuous forest inhabited by bonobos. However, further comparisons of spatiotemporal variation in food availability and bonobo diet across study sites are necessary to further examine differences in food availability.
Edge Density and Forest Fragmentation
Because of differences in methodologies used to define the edge and calculate edge density, we cannot directly compare the edge density calculated for this single site to previous studies evaluating edge density at broader scales. However, the edge density within the bonobo home range at Manzano was higher than those previously described for a much larger area at Maringa–Lopori–Wamba (Hickey et al. 2012). As predicted, edge density within the Manzano bonobo home range was less than half of the edge density of the larger surrounding area around their home range (20× the home range size), showing that bonobos tend to use less fragmented areas in this landscape. The effect of fragmented habitat on primates varies with the species’ plasticity and with the matrix configuration (Bicca-Marques 2003; Boyle et al. 2013; Irwin 2008; Onderdonk and Chapman 2000; Tutin 1999). To explore the complex effect of fragmented landscape on bonobo ecology further, we should consider different scales and include parameters describing both the landscape (e.g., forest cover, number of patches, edge density) and the patches (e.g., size, shape, isolation), as highlighted previously for primates and other large terrestrial mammal species (Galan-Acedo et al. 2019; Garmendia et al. 2013). As we have observed another bonobo community (unhabituated but identifiable due to physical abnormalities) in the southwestern part of the same forest patch at least three times since 2012, we cannot exclude the presence of bonobos in more fragmented areas surrounding the habituated bonobo community. A regional investigation of bonobo presence would help to define which forest patches shelter bonobo communities and identify potential corridors and potential fragmentation thresholds that limit bonobo migration. As we found that bonobos rarely use savannas and we never observed nests in savannas, they may hinder bonobo migration. It is critical to assess migration patterns in such a fragmented forest region to complement previous genetic data, which indicated that the population in the western portion of the bonobo range is isolated from the population at the center of their range (Kawamoto et al. 2013).
Forest fragmentation is often due to human proximity, which increases human access to forest (Wade et al. 2003). In future studies, we need to independently assess human activities in this region to evaluate the direct influence of human proximity on bonobo spatial distribution at this site on the periphery of the bonobo home range. Previous studies demonstrate clear negative relationships between human presence and bonobo presence (Hickey et al. 2013; Reinartz et al. 2006). However, relatively high bonobo densities occur near villages for which eating bonobos is a taboo (Thompson et al. 2008), showing the need to consider the sociocultural context to assess the influence of humans on bonobo distribution, as shown for other primate species (Estrada et al. 2006; Riley and Fuentes 2011; Setchell et al. 2017; Strindberg et al. 2018).
Seasonality of Dietary Diversity, Habitat Use, and Spatial Distribution
During the dry season, bonobos consumed a lower diversity of fruit/ha/h, spent more time in SFM, and used fewer and more aggregated hotspots near the seasonal weighted home range center than during the rainy season. These results confirm our predictions about seasonal variation in bonobo diet, habitat use, and space use. These patterns may be in line with the consumption of fallback foods in SFM during the dry season. However, most of the fallback foods previously identified in neighboring bonobo communities in another forest patch of the region are not more abundant in SFM than in other forest types in the Manzano forest (Pennec et al. 2016; Serckx et al. 2015). Thus, bonobos may preferentially use SFM during the dry season to consume fallback foods other than those identified by Serckx et al. (2015) or this habitat preference could be due to other parameters (for example, preferred foods may be present in SFM during the dry season). To explore the influence of spatiotemporal variation of food availability on diet, habitat use and space use in this forest–savanna mosaic more deeply, we need to test for preferred foods and potential fallback foods in Manzano forest using relevant data including feeding time and phenology, then determine if preferred foods and fallback foods are consumed more in specific habitat types. At Wamba, a more forested site, seasonal variability of food availability probably influences habitat use but not ranging patterns (Furuichi et al. 2008; Hashimoto et al. 1998; Terada et al. 2015). Therefore, gaps remain in our understanding of bonobo ecology concerning the effect of food availability on habitat use and space use, and their variation in relation to the environmental context.
Conclusion
Our findings provide insights into the ecology of bonobos living in a forest–savanna mosaic. The Manzano bonobos inhabit the most fragmented and open conditions known for this species, but have a similar home range size, density, and dietary diversity to those in continuous forest sites. Bonobos spent almost all their time in forest patches and selected the least fragmented areas in this forest–savanna mosaic, highlighting the need to focus conservation effort on the largest forest patches. However, we need to further explore the complex effect of fragmented habitats on bonobos and include more comprehensive landscape metrics and patch metrics in future studies. The seasonal patterns we observed in habitat use, dietary composition and spatial dispersion may be due to spatiotemporal variation in food availability, as shown in chimpanzees and many other primates species (e.g., Albert et al. 2013; Fiore 2003; Hanya et al. 2006; Porter et al. 2007; Robbins and McNeilage 2003; Twinomugisha and Chapman 2008). However, we need to explore the potential influence of spatiotemporal variation in food availability on diet, habitat use, ranging pattern, and activity budget, for example, by testing for preferred foods, fallback foods, and their distribution in specific habitat types. Such data will fill gaps in our understanding of bonobo ecology and help to improve bonobo conservation strategies, especially in this region where the community-based conservation approach maintains spatial overlap between humans and bonobos. Moreover, we need cross-site comparisons at several scales (between groups from a same subpopulation and between populations, Strier 2017) to improve interspecific comparisons, both for the Pan genus and for primates in general, to contribute to socioecological models and a more accurate assessment of the conservation status of these species.
References
Albert, A., Huynen, M.-C., Savini, T., & Hambuckers, A. (2013). Influence of food resources on the ranging pattern of northern pig-tailed macaques (Macaca leonina). International Journal of Primatology, 34(4), 696–713. https://doi.org/10.1007/s10764-013-9690-z.
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3–4), 227–266.
Badrian, A., & Badrian, N. (1984). Social organization of Pan paniscus in the Lomako Forest, Zaire. In R. L. Susman (Ed.), The pygmy chimpanzee (pp. 325–346). New York: Springer-Verlag http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-1-4757-0082-4_13.
Badrian, N., & Malenky, R. K. (1984). Feeding ecology of Pan paniscus in the Lomako Forest, Zaire. In R. L. Susman (Ed.), The pygmy chimpanzee (pp. 275–299). New York: Springer-Verlag http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-1-4757-0082-4_11.
Baldwin, P. J., McGrew, W. C., & Tutin, C. E. (1982). Wide-ranging chimpanzees at Mt. Assirik, Senegal. International Journal of Primatology, 3(4), 367–385.
Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W., & Simpson, A. J. (1992). Habitat use and resource availability in baboons. Animal Behaviour, 43(5), 831–844. https://doi.org/10.1016/S0003-3472(05)80206-4.
Basabose, A. K. (2005). Ranging patterns of chimpanzees in a Montane Forest of Kahuzi, Democratic Republic of Congo. International Journal of Primatology, 26(1), 33. https://doi.org/10.1007/s10764-005-0722-1.
Beaune, D., Bretagnolle, F., Bollache, L., Bourson, C., Hohmann, G., & Fruth, B. (2013). Ecological services performed by the bonobo (Pan paniscus): Seed dispersal effectiveness in tropical forest. Journal of Tropical Ecology, 29(5), 367–380. https://doi.org/10.1017/S0266467413000515.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In L. K. Marsh (Ed.), Primates in fragments: Ecology and Conservation (pp. 283–303). New York: Springer Science+Business Media. https://doi.org/10.1007/978-1-4757-3770-7_18.
Boesch, C., Hohmann, G., & Marchant, L. F. (2002). Behavioural diversity in chimpanzees and bonobos. Cambridge: Cambridge University Press.
Boyle, S. A., Lenz, B. B., Gilbert, K. A., Sprionello, W. R., Gómez, M. S., et al. (2013). Primates of the Biological Dynamics of Forest Fragments Project: A history. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments: Complexity and resilience (pp. 57–74). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media. https://doi.org/10.1007/978-1-4614-8839-2_5
Boyle, S. A., Lourenço, W. C., da Silva, L. R., & Smith, A. T. (2009). Home range estimates vary with sample size and methods. Folia Primatologica, 80(1), 33–42. https://doi.org/10.1159/000201092.
Chancellor, R. L., Rundus, A. S., & Nyandwi, S. (2012). The influence of seasonal variation on chimpanzee (Pan troglodytes schweinfurthii) fallback food consumption, nest group size, and habitat use in Gishwati, a montane rain forest fragment in Rwanda. International Journal of Primatology, 33(1), 115–133. https://doi.org/10.1007/s10764-011-9561-4.
Conklin-Brittain, N. L., Knott, C. D., & Wrangham, R. W. (2001). The feeding ecology of apes. In The apes: Challenges for the 21st century (pp. 167–174). http://www.researchgate.net/publication/230823364_The_Feeding_Ecology_of_Apes/file/79e415053546b78615.pdf. Accessed 25 July 2014.
Curtis, J. T. (1959). The vegetation of Wisconsin: An ordination of plant communities. Madison: University of Wisconsin Press.
Demichelis, C., Oszwald, J., Gasquet-Blanchard, C., Narat, V., Bokika, J.-C., et al (2020). Multidimensional analysis of landscape dynamics in a Central African forest-savannah mosaic. African Journal of Ecology. https://doi.org/10.1111/aje.12750.
Dupain, J., Van Elsacker, L., Nell, C., Garcia, P., Ponce, F., & Huffman, M. A. (2002). New evidence for leaf swallowing and Oesophagostomum infection in bonobos (Pan paniscus). International Journal of Primatology, 23(5), 1053–1062.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., et al (2017). Impending extinction crisis of the world’s primates: Why primates matter. Science Advances, 3(1), e1600946. https://doi.org/10.1126/sciadv.1600946.
Estrada, A., Saenz, J., Harvey, C., Naranjo, E., Muñoz, D., & Rosales-Meda, M. (2006). Primates in agroecosystems: Conservation value of some agricultural practices in Mesoamerican landscapes. In A. Estrada, P. A. Garber, M. M. Pavelka, & L. Luecke (Eds.), New perspectives in the study of Mesoamerican primates (pp. 437–470). Developments of Primatology: Progress and Prospects. New York: Springer Science+Business Media. http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/0-387-25872-8_22.
Fan, P.-F., & Jiang, X.-L. (2008). Effects of food and topography on ranging behavior of black crested gibbon (Nomascus concolor jingdongensis) in Wuliang Mountain, Yunnan, China. American Journal of Primatology, 70(9), 871–878. https://doi.org/10.1002/ajp.20577.
Ferreguetti, Á. C., Pereira-Ribeiro, J., Prevedello, J. A., Tomás, W. M., Rocha, C. F. D., & Bergallo, H. G. (2018). One step ahead to predict potential poaching hotspots: Modeling occupancy and detectability of poachers in a neotropical rainforest. Biological Conservation, 227, 133–140. https://doi.org/10.1016/j.biocon.2018.09.009.
Fiore, A. D. (2003). Ranging behavior and foraging ecology of lowland woolly monkeys (Lagothrix lagotricha poeppigii) in Yasuní National Park, Ecuador: Lagothrix range use and foraging ecology. American Journal of Primatology, 59(2), 47–66. https://doi.org/10.1002/ajp.10065.
Fruth, B., Hickey, J. R., André, C., Furuichi, T., Hart, J., et al. (2016). Pan paniscus: The IUCN Red List of Threatened Species 2016: e.T15932A102331567. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T15932A17964305.en
Furuichi, T. (2009). Factors underlying party size differences between chimpanzees and bonobos: A review and hypotheses for future study. Primates, 50(3), 197–209. https://doi.org/10.1007/s10329-009-0141-6.
Furuichi, T., Mulavwa, M., Yangozene, K., Yamba-Yamba, M., Motema-Salo, B., et al. (2008). Relationships among fruit abundance, ranging rate, and party size and composition of bonobos at Wamba. In T. Furuichi & J. Thompson (Eds.), The bonobos: Behavior, ecology, and conservation (pp. 135–149). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media. https://doi.org/10.1007/978-0-387-74787-3_8
Galan-Acedo, C., Arroyo-Rodriguez, V., Andresen, E., & Arasa-Gisbert, R. (2019). Ecological traits of the world’s primates. Scientific Data, 6, 55. https://doi.org/10.1038/s41597-019-0059-9.
Garmendia, A., Arroyo-Rodríguez, V., Estrada, A., Naranjo, E. J., & Stoner, K. E. (2013). Landscape and patch attributes impacting medium- and large-sized terrestrial mammals in a fragmented rain forest. Journal of Tropical Ecology, 29(4), 331–344. https://doi.org/10.1017/S0266467413000370.
Gazagne, E., José-Domínguez, J. M., Huynen, M.-C., Hambuckers, A., Poncin, P., et al (2020). Northern pigtailed macaques rely on old growth plantations to offset low fruit availability in a degraded forest fragment. American Journal of Primatology, 82(5), e23117. https://doi.org/10.1002/ajp.23117.
Georgiev, A. V., Thompson, M. E., Lokasola, A. L., & Wrangham, R. W. (2011). Seed predation by bonobos (Pan paniscus) at Kokolopori, Democratic Republic of the Congo. Primates, 52, 309–314. https://doi.org/10.1007/s10329-011-0256-4.
Grant, J. W. A., Chapman, C. A., & Richardson, K. S. (1992). Defended versus undefended home range size of carnivores, ungulates and primates. Behavioral Ecology and Sociobiology, 31(3), 149–161. https://doi.org/10.1007/BF00168642.
Gruber, T., & Clay, Z. (2016). A comparison between bonobos and chimpanzees: A review and update. Evolutionary Anthropology: Issues, News, and Reviews, 25(5), 239–252. https://doi.org/10.1002/evan.21501.
Grueter, C. C., Li, D., Ren, B., & Wei, F. (2009). Choice of analytical method can have dramatic effects on primate home range estimates. Primates, 50(1), 81–84. https://doi.org/10.1007/s10329-008-0113-2.
Hanya, G., Kiyono, M., Yamada, A., Suzuki, K., Furukawa, M., et al (2006). Not only annual food abundance but also fallback food quality determines the Japanese macaque density: Evidence from seasonal variations in home range size. Primates, 47(3), 275–278. https://doi.org/10.1007/s10329-005-0176-2.
Harris, T. R., & Chapman, C. A. (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48(3), 208–221. https://doi.org/10.1007/s10329-006-0036-8.
Hashimoto, C., & Furuichi, T. (2015). Sex differences in ranging and association patterns in chimpanzees in comparison with bonobos. In T. Furuichi, J. Yamagiwa, & F. Aureli (Eds.), Dispersing primate females (pp. 105–126). Tokyo: Springer Japan. https://doi.org/10.1007/978-4-431-55480-6_5.
Hashimoto, C., Tashiro, Y., Kimura, D., Enomoto, T., Ingmanson, E. J., et al (1998). Habitat use and ranging of wild bonobos (Pan paniscus) at Wamba. International Journal of Primatology, 19(6), 1045–1060.
Hendershott, R., Rawson, B., & Behie, A. (2018). Home range size and habitat use by Cat Ba langurs (Trachypithecus poliocephalus) in a disturbed and fragmented habitat. International Journal of Primatology, 39(4), 547–566. https://doi.org/10.1007/s10764-018-0051-9.
Hernandez-Aguilar, R. A., Moore, J., & Stanford, C. B. (2013). Chimpanzee nesting patterns in savanna habitat: Environmental influences and preferences. American Journal of Primatology, 75(10), 979–994. https://doi.org/10.1002/ajp.22163.
Hickey, J. R., Carroll, J. P., & Nibbelink, N. P. (2012). Applying landscape metrics to characterize potential habitat of bonobos (Pan paniscus) in the Maringa-Lopori-Wamba landscape, Democratic Republic of Congo. International Journal of Primatology, 33(2), 381–400. https://doi.org/10.1007/s10764-012-9581-8.
Hickey, J. R., Nackoney, J., Nibbelink, N. P., Blake, S., Bonyenge, A., et al (2013). Human proximity and habitat fragmentation are key drivers of the rangewide bonobo distribution. Biodiversity and Conservation, 22(13–14), 3085–3104. https://doi.org/10.1007/s10531-013-0572-7.
Hohmann, G., Fowler, A., Sommer, V., & Ortmann, S. (2006). Frugivory and gregariousness of Salonga bonobos and Gashaka chimpanzees: The influence of abundance and nutritional quality of fruit. In G. Hohmann, M. M. Robins, & C. Boesch (Eds.), Feeding ecology in apes and other primates (pp. 123–159). Cambridge: Cambridge University Press.
Humle, T., Maisels, F., Oates, J. F., Plumptre, A., & Williamson, E. A. (2016). Pan troglodytes: The IUCN Red List of Threatened Species 2016: e.T159 33A129038584. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T15933A17964454.en
Hunt, K. D., & McGrew, W. C. (2002). Chimpanzees in the dry habitats at Assirik, Senegal, and at Semliki Wildlife Reserve, Uganda. In C. Boesch, G. Hohmann, & L. F. Marchant (Eds.), Behavioural diversity in chimpanzees and bonobos. Cambridge: Cambridge University Press http://public.eblib.com/choice/publicfullrecord.aspx?p=217791. Accessed July 2020.
Idani, G., Kuroda, S., Kano, T., & Asato, R. (1994). Flora and vegetation of Wamba Forest, Central Zaire with reference to Bonobo (Pan paniscus) foods. Tropics, 3(3/4), 309–332.
Inogwabini, B.-I., Bewa, M., Longwango, M., Abokome, M., & Vuvu, M. (2008). The bonobos of the Lake Tumba – Lake Maindombe Hinterland: Threats and opportunities for population conservation. In T. Furuichi & J. Thompson (Eds.), The bonobos: Behavior, ecology and conservation (pp. 273–290). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media. http://www.springerlink.com.gate1.inist.fr/content/l5731j1w944043t3/. Accessed 6 December 2011
Irwin, M. T. (2008). Diademed sifaka (Propithecus diadema) ranging and habitat use in continuous and fragmented forest: Higher density but lower viability in fragments? Biotropica, 40(2), 231–240. https://doi.org/10.1111/j.1744-7429.2007.00368.x.
IUCN, & ICCN. (2012). Bonobo (Pan paniscus) conservation strategy 2012–2022 (IUCN/SSC Primate.). Gland, Switzerland.
Kano, T. (1992). The last ape: Pygmy chimpanzee behavior and ecology. Stanford, CA: Stanford University Press.
Kano, T., & Mulavwa, M. (1984). Feeding ecology of the pygmy chimpanzees (Pan paniscus) of Wamba. In R. L. Susman (Ed.), The pygmy chimpanzee: Evolutionary biology and behavior (pp. 233–274). New York: Springer-Verlag.
Katsis, L., Cunneyworth, P. M. K., Turner, K. M. E., & Presotto, A. (2018). Spatial patterns of primate electrocutions in Diani, Kenya. International Journal of Primatology, 39(4), 493–510. https://doi.org/10.1007/s10764-018-0046-6.
Kawamoto, Y., Takemoto, H., Higuchi, S., Sakamaki, T., Hart, J. A., Hart, T. B., et al (2013). Genetic structure of wild bonobo populations: Diversity of mitochondrial DNA and geographical distribution. PLoS ONE, 8(3), e59660. https://doi.org/10.1371/journal.pone.0059660.
Kshettry, A., Vaidyanathan, S., & Athreya, V. (2017). Leopard in a tea-cup: A study of leopard habitat-use and human-leopard interactions in north-eastern India. PLoS ONE, 12(5), e0177013. https://doi.org/10.1371/journal.pone.0177013.
Lindshield S. R., Hernandez-Aguilar A., Korstjens A. H., Marchant L.F., Narat V., Ndiaye P. I., Ogawa H., Piel A. K., Pruetz J. D., Stewart F. A., van Leeuwen K. L., Wessling E. G., Yoshikawa M. (in prep). Chimpanzee (Pan troglodytes) in Savanna landscapes
Lucchesi, S., Cheng, L., Janmaat, K., Mundry, R., Pisor, A., & Surbeck, M. (2020). Beyond the group: How food, mates, and group size influence intergroup encounters in wild bonobos. Behavioral Ecology, 31(2), 519–532. https://doi.org/10.1093/beheco/arz214.
Malenky, R. K., & Stiles, E. W. (1991). Distribution of terrestrial herbaceous vegetation and its consumption by Pan paniscus in the Lomako Forest, Zaire. American Journal of Primatology, 23(3), 153–169.
Malenky, R. K., & Wrangham, R. (1994). A quantitative comparison of terrestrial herbaceous food consumption by Pan paniscus in the Lomako Forest, Zaire, and Pan troglodytes in the Kibale Forest, Uganda. American Journal of Primatology, 32(1), 1.
McGrew, W. C., Baldwin, P. J., & Tutin, C. E. G. (1988). Diet of wild chimpanzees (Pan troglodytes verus) at Mt. Assirik, Senegal: I. Composition. American Journal of Primatology, 16(3), 213–226. https://doi.org/10.1002/ajp.1350160304.
Mitani, J. C., & Rodman, P. S. (1979). Territoriality: The relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behavioral Ecology and Sociobiology, 5(3), 241–251. https://doi.org/10.1007/BF00293673.
Mohneke, M., & Fruth, B. (2008). Bonobo (Pan paniscus) density estimation in the SW-Salonga National Park, Democratic Republic of Congo: Common methodology revisited. In T. Furuichi & J. Thompson (Eds.), The bonobos: Behavior, ecology and conservation (pp. 151–166). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media. http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-0-387-74787-3_9.
Mohr, C. O. (1947). Table of equivalent populations of North American small mammals. American Midland Naturalist, 37(1), 223. https://doi.org/10.2307/2421652.
Moore, J. (1996). Savanna chimpanzees, referential models and the last common ancestor. In L. F. Marchant, T. Nishida, & W. C. McGrew (Eds.), Great ape societies (pp. 275–292). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511752414.022.
Myneni, R. B., Hall, F. G., Sellers, P. J., & Marshak, A. L. (1995). The interpretation of spectral vegetation indexes. IEEE Transactions on Geoscience and Remote Sensing, 33(2), 481–486.
Namur, C. de. (1990). Aperçu sur la végétation de l’Afrique centrale atlantique. In R. Lanfranchi & D. Schwartz (Eds.), Paysages quaternaires de l’Afrique Centrale atlantique (ORSTOM., pp. 60–67). Paris.
Narat, V. (2014). Interactions bonobos-habitats-humains: Habituation, écologie, santé et conservation. Thèse de Doctorat en Sciences de la Nature et de l’Homme, Spécialité Eco-éthologie. Muséum national d’Histoire naturelle, Paris. https://hal.archives-ouvertes.fr/tel-02159268/. Accessed July 2020
Narat, V., Pennec, F., Ledo-Bisson, L., Bokika Ngawolo, J. C., Dumez, R., & Krief, S. (2015a). Bonobo conservation as a means for local development: An innovative local initiative of community-based conservation in Democratic Republic of Congo. Journal of Primatology, 4(2). https://doi.org/10.4172/2167-6801.1000127.
Narat, V., Pennec, F., Simmen, B., Ngawolo, J. C. B., & Krief, S. (2015b). Bonobo habituation in a forest–savanna mosaic habitat: Influence of ape species, habitat type, and sociocultural context. Primates, 56(4), 339–349. https://doi.org/10.1007/s10329-015-0476-0.
Ndiaye, P. I., Badji, L., Lindshield, S. M., & Pruetz, J. D. (2018). Nest-building behaviour by chimpanzees (Pan troglodytes verus) in the non-protected area of Diaguiri (Kedougou, Senegal): Implications for conservation. Folia Primatologica, 89(5), 316–326. https://doi.org/10.1159/000490945.
Oelze, V. M., Douglas, P. H., Stephens, C. R., Surbeck, M., Behringer, V., et al (2016). The steady state great ape? Long term isotopic records reveal the effects of season, social rank and reproductive status on bonobo feeding behavior. PLoS ONE, 11(9), e0162091. https://doi.org/10.1371/journal.pone.0162091.
Ogawa, H., Idani, G., Moore, J., Pintea, L., & Hernandez-Aguilar, A. (2007). Sleeping parties and nest distribution of chimpanzees in the Savanna Woodland, Ugalla, Tanzania. International Journal of Primatology, 28(6), 1397–1412. https://doi.org/10.1007/s10764-007-9210-0.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology, 21(4), 587–611.
Onishi, E., Brooks, J., Leti, I., Monghiemo, C., Bokika, J. C., Shintaku, Y., Idani, G., Yamamoto, S. (2020). Nkala Forest: Introduction of a forest-savanna mosaic field site of wild bonobos and its future prospects. Pan Africa News, 27(1), 2–5.
Pearce, F., Carbone, C., Cowlishaw, G., & Isaac, N. J. B. (2013). Space-use scaling and home range overlap in primates. Proceedings of the Royal Society B: Biological Sciences, 280(1751). https://doi.org/10.1098/rspb.2012.2122.
Pennec, F., Krief, S., Hladik, A., Lubini Ayingweu, C., Bortolamiol, S., et al (2016). Floristic and structural vegetation typology of bonobo habitats in a forest-savanna mosaic (Bolobo Territory, D.R.Congo). Plant Ecology and Evolution, 149(2), 199–215. https://doi.org/10.5091/plecevo.2016.1157.
Piel, A. K., Strampelli, P., Greathead, E., Hernandez-Aguilar, R. A., Moore, J., & Stewart, F. A. (2017). The diet of open-habitat chimpanzees (Pan troglodytes schweinfurthii) in the Issa valley, western Tanzania. Journal of Human Evolution, 112, 57–69. https://doi.org/10.1016/j.jhevol.2017.08.016.
Porter, L. M., Sterr, S. M., & Garber, P. A. (2007). Habitat use and ranging behavior of Callimico goeldii. International Journal of Primatology, 28(5), 1035–1058. https://doi.org/10.1007/s10764-007-9205-x.
Potts, K. B., Watts, D. P., & Wrangham, R. W. (2011). Comparative feeding ecology of two communities of chimpanzees (Pan troglodytes) in Kibale National Park, Uganda. International Journal of Primatology, 32, 669–690. https://doi.org/10.1007/s10764-011-9494-y.
Pruetz, J. D. (2006). Feeding ecology of savanna chimpanzees at Fongoli, Senegal. In G. Hohmann, M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates (pp. 161–182). Cambridge: Cambridge University Press.
Pruetz, J. D., & Bertolani, P. (2009). Chimpanzee (Pan troglodytes verus) behavioral responses to stresses associated with living in a savanna-mosaic environment: implications for hominin adaptations to open habitats. PaleoAnthropology, 2009, 252–262.
QGIS Development Team. (2018). QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.org. Accessed July 2018
R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org
Reinartz, G. E., Isia, I. B., Ngamankosi, M., & Wema, L. W. (2006). Effects of forest type and human presence on bonobo (Pan paniscus) density in the Salonga National Park1. International Journal of Primatology, 27, 603–634. https://doi.org/10.1007/s10764-006-9020-9.
Riley, E. P., & Fuentes, A. (2011). Conserving social–ecological systems in Indonesia: Human–nonhuman primate interconnections in Bali and Sulawesi. American Journal of Primatology, 73(1), 62–74. https://doi.org/10.1002/ajp.20834.
Robbins, M. M., & McNeilage, A. (2003). Home range and frugivory patterns of mountain gorillas in Bwindi Impenetrable National Park, Uganda. International Journal of Primatology, 24(3), 467–491. https://doi.org/10.1023/A:1023741328338.
Sabater Pi, J., & Vea, J. (1990). Nest-building and population estimates of the bonobo from the Lokoe-Lilungu-Ikomaloki region of Zaire. Primate Conservation, 11, 43–48.
Sabater Pi, J., & Vea, J. J. (1994). Comparative inventory of foods consumed by the wild pygmy chimpanzee (Pan paniscus; Mammalia) in the Lilungu-Lokofe region of the Republic of Zaire. Journal of African Zoology, 108(4), 381–396.
Samuni, L., Wegdell, F., & Surbeck, M. (n.d. preprint). Behavioural diversity of bonobo prey preference as a potential cultural trait. bioRxiv, 2020.06.02.130245. https://doi.org/10.1101/2020.06.02.130245
Schoener, T. W. (1971). Theory of feeding strategies. Annual Review of Ecology and Systematics, 2(1), 369–404. https://doi.org/10.1146/annurev.es.02.110171.002101.
Serckx, A., Huynen, M.-C., Bastin, J.-F., Hambuckers, A., Beudels-Jamar, R. C., et al (2014). Nest grouping patterns of bonobos (Pan paniscus) in relation to fruit availability in a forest-savannah mosaic. PLoS ONE, 9(4), e93742. https://doi.org/10.1371/journal.pone.0093742.
Serckx, A., Kühl, H. S., Beudels-Jamar, R. C., Poncin, P., Bastin, J.-F., & Huynen, M.-C. (2015). Feeding ecology of bonobos living in forest-savannah mosaics: Diet seasonal variation and importance of fallback foods: Bonobo diet in forest-savannah mosaics. American Journal of Primatology, 77(9), 948–962. https://doi.org/10.1002/ajp.22425.
Setchell, J. M., Fairet, E., Shutt, K., Waters, S., & Bell, S. (2017). Biosocial conservation: Integrating biological and ethnographic methods to study human–primate interactions. International Journal of Primatology, 38(2), 401–426. https://doi.org/10.1007/s10764-016-9938-5.
Sha, J. C. M., & Hanya, G. (2013). Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed macaques (Macaca fascicularis). American Journal of Primatology, 75(6), 581–592. https://doi.org/10.1002/ajp.22137.
Skinner, M. F., & Pruetz, J. D. (2012). Reconstruction of periodicity of repetitive linear enamel hypoplasia from perikymata counts on imbricational enamel among dry-adapted chimpanzees (Pan troglodytes verus) from Fongoli, Senegal. American Journal of Physical Anthropology, 149(3), 468–482. https://doi.org/10.1002/ajpa.22145.
Southwood, T. R. (1978). Ecological methods: With particular reference to the study of insect populations, 2nd ed. Dordrecht: Springer. https://doi.org/10.1007/978-94-009-1225-0
Strier, K. B. (2017). What does variation in primate behavior mean? American Journal of Physical Anthropology, 162(S63), 4–14. https://doi.org/10.1002/ajpa.23143.
Strindberg, S., Maisels, F., Williamson, E. A., Blake, S., Stokes, E. J., et al (2018). Guns, germs, and trees determine density and distribution of gorillas and chimpanzees in Western Equatorial Africa. Science Advances, 4(4), eaar2964. https://doi.org/10.1126/sciadv.aar2964.
Terada, S., Nackoney, J., Sakamaki, T., Mulavwa, M. N., Yumoto, T., & Furuichi, T. (2015). Habitat use of bonobos (Pan paniscus) at Wamba: Selection of vegetation types for ranging, feeding, and night-sleeping: Habitat use of bonobos at Wamba. American Journal of Primatology, 77(6), 701–713. https://doi.org/10.1002/ajp.22392.
Tesfaye, D., Fashing, P. J., Bekele, A., Mekonnen, A., & Atickem, A. (2013). Ecological flexibility in Boutourlini’s blue monkeys (Cercopithecus mitis boutourlinii) in Jibat Forest, Ethiopia: A comparison of habitat use, ranging behavior, and diet in intact and fragmented forest. International Journal of Primatology, 34(3), 615–640. https://doi.org/10.1007/s10764-013-9684-x.
Thompson, J. A. M. (1997). The history, taxonomy and ecology of the bonobo (Pan paniscus Schwarz, 1929) with a first description of a wild population living in a forest/savanna mosaic habitat. Dissertation, Univeristy of Oxford.
Thompson, J. A. M. (2002). Bonobos of the Lukuru Wildlife Research Project. In Behavioural diversity in chimpanzees and bonobos. Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511606397.007.
Thompson, J. A. M., Nestor, L. M., & Kabanda, R. B. (2008). Traditional land-use practices for bonobo conservation. In T. Furuichi & J. Thompson (Eds.), The bonobos: Behavior, ecology, and conservation, Developments in Primatology: Progress and Prospects (pp. 227–244). New York: Springer Science+Business Media.
Tutin, C. E. (1999). Fragmented living: Behavioural ecology of primates in a forest fragment in the Lopé Reserve, Gabon. Primates, 40(1), 249–265.
Tutin, C. E., Ham, R. M., White, L. J., & Harrison, M. J. (1997). The primate community of the Lopé Reserve, Gabon: Diets, responses to fruit scarcity, and effects on biomass. American Journal of Primatology, 42(1), 1–24.
Twinomugisha, D., & Chapman, C. A. (2008). Golden monkey ranging in relation to spatial and temporal variation in food availability. African Journal of Ecology, 46(4), 585–593. https://doi.org/10.1111/j.1365-2028.2007.00905.x.
Uehara, S. (1988). Grouping patterns of wild pygmy chimpanzees (Pan paniscus) observed at a marsh grassland amidst the tropical rain forest of Yalosidi, Republic of Zaire. Primates, 29(1), 41–52.
Van Krunkelsven, E. (2001). Density estimation of bonobos (Pan paniscus) in Salonga National Park, Congo. Biological Conservation, 99(3), 387–391.
van Leeuwen, K. L., Hill, R. A., & Korstjens, A. H. (2020). Classifying chimpanzee (Pan troglodytes) landscapes across large-scale environmental gradients in Africa. International Journal of Primatology. https://doi.org/10.1007/s10764-020-00164-5.
van Schaik, C. P., & Brockman, D. K. (2005). Seasonality in primate ecology, reproduction, and life history: An overview. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates (pp. 3–20). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511542343.002.
van Schaik, C. P., & Pfannes, K. R. (2005). Tropical climates and phenology: A primate perspective. In D. K. Brockman & C. P. Van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 23–54). Cambridge: Cambridge University Press.
Vieira, W. F., Kerry, C., & Hockings, K. J. (2019). A comparison of methods to determine chimpanzee home-range size in a forest–farm mosaic at Madina in Cantanhez National Park, Guinea-Bissau. Primates, 1–11. https://doi.org/10.1007/s10329-019-00724-1
Wade, T. G., Riitters, K., Wickham, J. D., & Jones, K. B. (2003). Distribution and causes of global forest fragmentation. Conservation Ecology, 7(2), art7. https://doi.org/10.5751/ES-00530-070207.
Wallace, R. B. (2008). Factors influencing spider monkey habitat use and ranging patterns. In C. J. Campbell (Ed.), Spider monkeys (pp. 138–154). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511721915.005.
Waller, M. T. (2011). The ranging behavior of bonobos in the Lomako Forest. Dissertation, University of Oregon. Retrieved from https://scholarsbank.uoregon.edu/xmlui/handle/1794/11648. Accessed Sept 2014
Wessling, E. G., Kühl, H. S., Mundry, R., Deschner, T., & Pruetz, J. D. (2018). The costs of living at the edge: Seasonal stress in wild savanna-dwelling chimpanzees. Journal of Human Evolution, 121, 1–11. https://doi.org/10.1016/j.jhevol.2018.03.001.
White, F. (1998). Seasonality and socioecology: The importance of variation in fruit abundance to bonobo sociality. International Journal of Primatology, 19(6), 1013–1027.
White, F. J., & Wrangham, R. W. (1988). Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour, 148–164.
Wrangham, R. W., Chapman, C. A., Clark-Arcadi, A. P., & Isabirye-Basuta, G. (1996). Social ecology of Kanyawara chimpanzees: Implications for understanding the costs of great ape groups. In W. C. McGrew, L. F. Marchant, & T. Nishida (Eds.), Great ape societies (pp. 45–57). Cambridge: Cambridge University Press. http://books.google.fr/books?hl=fr&lr=&id=iGfjJ4lKb1IC&oi=fnd&pg=PA45&dq=Socioecology+of+kanyawara+chimpanzee&ots=6KZ4RDpHG2&sig=t4fkXenueN-yZF_THI_lxOV5A44. Accessed Nov 2011
Acknowledgements
Our sincere thanks to local and national authorities in DRC. We are grateful to Mbou-Mon-Tour NGO (DRC) and to the unit of Eco-anthropology, the Pôle Image and Bonobo ECO (France) for their support in this research both monetary and in kind. We are most indebted to the two main local field assistants, Osa Otsiu Epany and Muzungu Ngofuna, for their great job in this long-term project and to trackers of the Embirima village. We are deeply grateful to anonymous reviewers; Erin Wessling, guest editor; and Johanna Setchell, editor-in-chief for their very important comments and suggestions to improve this manuscript. We thank Mallory Eckstut, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
FP and VN conceived and designed the study, encoded data, analyzed data, and wrote the manuscript. CG participated in the writing of the manuscript. LM encoded and analyzed data. CM and JCB supported data collection and protocol design. RL participated in data analyses.
Corresponding author
Additional information
Handling Editor: Erin G. Wessling
Supplementary Information
ESM 1
(PDF 255 kb)
Rights and permissions
About this article
Cite this article
Pennec, F., Gérard, C., Meterreau, L. et al. Spatiotemporal Variation in Bonobo (Pan paniscus) Habitat Use in a Forest–Savanna Mosaic. Int J Primatol 41, 775–799 (2020). https://doi.org/10.1007/s10764-020-00180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-020-00180-5