Abstract
Comparisons of the behavior and ecology of primates living in intact and fragmented forest are critical to the development of conservation strategies for the many primate taxa threatened by habitat loss. From July 2009 to April 2010, we investigated the habitat use, ranging behavior, and diet of two groups of Boutourlini’s blue monkeys (Cercopithecus mitis boutourlinii), a subspecies endemic to western Ethiopia, whose habitats had experienced different levels of disturbance at Jibat Forest. Forest Group occupied primarily continuous tree-dominated forest with little human disturbance whereas Fragment Group inhabited a heavily degraded 2- to 3-km2 forest fragment nearly surrounded by farmland and connected tenuously to the continuous forest by a narrow corridor of riverine forest. Mean daily path lengths for both groups were nearly identical (Forest Group: 799 m; Fragment Group: 783 m) and exhibited little seasonal variability. The mean home range areas of Forest Group and Fragment Group were 72.0 and 61.2 ha, respectively. Forest Group (N = 2232 feeding records) fed mostly on fruits (52.5 %), though they also ate animal prey (14.7 %), young leaves (11.1 %), shoots (8.7 %), and flowers (7.3 %). In contrast, fruits accounted for only 17.0 % of Fragment Group’s diet (N = 2903 feeding records), with shoots (29.8 %), young leaves (17.1 %), animal prey (13.1 %), seeds (9.6 %), and flowers (6.8 %) also making substantial contributions to their diet. Only Fragment Group engaged in crop raiding, consuming seeds from barley and wheat extensively (33–41 % of diet) during 2 mo. Fragment Group (N = 33) ate more plant species than Forest Group (N = 24), though both groups exploited a small number of plant species relative to other subspecies of blue monkeys. Our study revealed that, like most other blue monkey subspecies, Boutourlini’s blue monkeys are quite flexible in the habitats they occupy as well as in their foraging habits. Despite this ecological flexibility, the long-term conservation of Boutourlini’s blue monkey is far from assured given its limited distribution, the rapidly growing human population, and the high rates of forest clearance in western Ethiopia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss is arguably the gravest threat facing primates inhabiting tropical forests today (Chapman et al. 2006a; IUCN 2012). The primate taxa with the greatest flexibility to cope with this threat will be the ones most likely to persist through the 21st century and beyond (Chapman et al. 2006a; Marsh 2003; Onderdonk and Chapman 2000). One of the most common outcomes of human-induced habitat loss in tropical forest ecosystems is forest fragmentation (Harrison and Bruna 1999; Laurance et al. 1998; Riitters et al. 2000). Many forest-dwelling animals, including some primates, are adversely affected by the fragmentation of their habitat (Andren 1994; Isabirye-Basuta and Lwanga 2008; Marsh 2003; Turner 1996). Forest fragmentation results in population declines in some primates and complete extirpation in others (Boyle 2008; Chapman et al. 2007; Estrada and Coates-Estrada 1996). In general, flexibility in dietary and ranging patterns and the ability to use matrix environments appear to be crucial to the survival of primates in fragments (Anderson et al. 2007; Onderdonk and Chapman 2000).
Owing to their importance to assessing the long-term conservation prospects of many of the world’s primate taxa, studies of the impact of forest fragmentation on the behavior and ecology of primates have become a major focus of research (Marsh 2003; Tutin 1999). A growing body of evidence suggests that fragmentation influences the lives of primates in many arenas, including impacting their daily path lengths, home range sizes, dietary compositions, physiological stress levels, gastrointestinal parasite loads, and opportunities for dispersal (Bicca-Marques 2003; Boyle 2012; Chapman et al. 2006b; Cristóbal-Azkarate and Arroyo-Rodríguez 2007; Eniang 2003; Martinez-Mota et al. 2007).
Blue monkeys (Cercopithecus mitis) are among the most widely distributed of Africa’s arboreal primate species and inhabit a variety of forest types (tropical moist forest: Butynski 1990; Cords 1987; tropical montane forest: Beeson et al. 1996; Kaplin 2001; coastal dune forest: Lawes 1990, 1991), including forest fragments across much of their range (Lawes 2002). Intensive studies of blue monkey feeding ecology across their range show that they also exhibit extremely variable diets, with their top food item ranging from fruit (45.8–57.1 %: Cords 1987; Kaplin et al. 1998; Schlichte 1978) to insects (35.9–39.4 %: Butynski 1990) to leaves (44.1–46.9 %: Fairgrieve and Muhumuza 2003; Plumptre 2006; Twinomugisha and Chapman 2008) depending on location. Given their wide distribution and flexibility for a forest primate, blue monkeys as a species are considered to be at low risk of extinction (Kingdon et al. 2008). However, there are several blue monkey subspecies with highly localized distributions whose basic biology and conservation requirements remain virtually unknown (Kingdon et al. 2008). For example, Boutourlini’s blue monkey (Cercopithecus mitis boutourlini), a subspecies endemic to the western side of the Ethiopian Rift Valley between Lake Tana and Lake Turkana (Butynski and Gippoliti 2008; Yalden et al. 1977), has never before been the subject of field study. This subspecies was recently listed as Vulnerable by IUCN because of extensive and uncontrolled destruction of the forests it occupies for both timber and agricultural production (Butynski and Gippoliti 2008). However, we know nothing about its natural history, including its habitat requirements, dietary needs, and ranging behavior, or its ability to respond to habitat fragmentation.
To learn more about Boutourlini’s blue monkey, we conducted a 10-mo field study of the subspecies in Jibat Forest, western Ethiopia from July 2009 to April 2010. We sought to determine the extent to which the behavior and ecology of these monkeys is influenced by fragmentation and other forms of human disturbance to their habitat. We therefore selected two focal groups, a relatively undisturbed contiguous forest-dwelling group (Forest Group) and a group occupying a heavily disturbed forest fragment (Fragment Group) surrounded by farmland except for a narrow strip of riparian forest connecting it to the contiguous forest, and compared their patterns of habitat use, ranging behavior, and diet. Because the disturbance level, vegetational composition, and habitat size differed greatly between the fragment and contiguous forest, we hypothesized that the blue monkeys in the two forest types would differ markedly in their patterns of habitat use, daily path length, home range size, and dietary composition.
Methods
Study Area
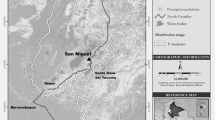
Jibat Forest (37°15′ – 37°30′E; 8°35′ – 8°50′N) is a montane forest in western Ethiopia covering an area of 320 km2 and ranging in elevation between 2000 and 3000 m asl (Bekele 1994). Jibat is of substantial conservation importance as both a National Forest Priority Area (SFCDD 1990) and an Important Bird Area for Ethiopia (BirdLife International 2009). Conversion to agricultural and grazing areas on the forest’s periphery began in the 1970s (B. Dandane, pers. comm.) and has resulted in ongoing fragmentation around the edges of the forest. Livestock currently graze legally both on grasses in recently cleared grazing land near the edge of the forest and on forest shrubs in some peripheral portions of the forest (D. Tesfaye, pers. obs.). Illegal tree cutting occurs throughout the forest, though reaches its highest intensity along the forest edge and in fragmenting areas of forest (D. Tesfaye, pers. obs.).
We obtained monthly rainfall and temperature data for the area between 1997 and 2006 from the Ethiopian Meteorology Agency’s Tikur Inchini Field Station, located ca. 25 km northeast of Jibat Forest. Mean annual rainfall was 1768 (SD ± 155) mm, and mean monthly low and high temperatures were 7.8 (SD ± 1.7) °C and 23.6 (SD ± 2.1) °C, respectively. The rainy season occurs from March to October with a peak in rainfall between June and September, and the dry season occurs from November to February. Because Tikur Inchini Field Station is in a less forested location than Jibat, we suspect that the total rainfall value used in our study is probably an underestimate, though we have no reason to suspect that the seasonal distribution of rainfall differs between these sites.
Focal Groups
We selected two groups of Boutourlini’s blue monkeys for detailed study. We identified these groups via the distinctive natural markings, facial features, coat colors, and sizes of members of each group. Forest Group occupied continuous tree-dominated forest experiencing low levels of human disturbance while Fragment Group occupied a heavily disturbed 2- to 3-km2 patch of forest consisting of tree- and bamboo-dominated habitat surrounded almost entirely by agricultural land (farms, settlements, and livestock grazing areas), with only a narrow corridor of riverine forest still linking the fragment to continuous forest (ca. 1 km away). During the study, Forest Group increased from 8 to 11 individuals while Fragment Group increased from 16 to 21 individuals, in both cases due to births. A second group inhabiting the continuous forest was counted at 24–26 individuals but was not selected for intensive study.
Data Collection and Analysis
Vegetation
Toward the end of our study, we established two 50 × 10 m quadrats in randomly selected locations within the home ranges of each of the two focal groups to provide a preliminary description of the vegetation composition in the study area (Tesfaye 2010). We counted (but did not measure the diameter at breast height [DBH] of) all individual plants including herbs, shrubs, lianas, and trees ≥2 m tall within the plots and identified them to species level at the National Herbarium, Addis Ababa University. Our vegetation enumeration methods placed greater emphasis on quantification of smaller plants than many previous studies of forest primate habitats, which have most often focused solely on stems ≥10 cm DBH irrespective of height.
The reason we chose our unconventional vegetation enumeration method is that it detects bamboo (Arundinaria alpina), a species characterized by culms of <10 cm DBH, but which was the top food species in the diet of Fragment Group at Jibat. The drawbacks of this method were that the density of plants in study plots was so great that we could not cover more than a small fraction (<1 %) of the home range area of each group in our vegetation surveys and we were able only to count (but not also measure) the number of stems in each plot. These quadrats were used to quantify the overall vegetation composition of the study area as well as to identify differences in forest composition between the home ranges of the focal groups. We calculated the relative density of each plant species as the total number of individuals of a species divided by the total number of all individuals sampled per hectare in the home range. In addition, we calculated plant species diversity via the Shannon–Wiener index, H′, and plant species evenness using the evenness index, J (Krebs 1989). Because of the small total area surveyed within the ranges of each blue monkey focal group, our vegetation enumeration must be regarded as preliminary, though the differences in plant composition between ranges are striking enough to warrant presenting them here.
Behavioral Sampling Protocol
To study the habitat use, ranging, and feeding ecology of Boutourlini’s blue monkeys at Jibat, D. Tesfaye conducted scan samples (Altmann 1974) at 15-min intervals between 07:30 h and 17:30 h. At the time of each scan, D. Tesfaye collected data on up to the first five adults or juveniles seen within the group he was following. Because of limited visibility in the forest at Jibat, however, the cutoff of five individuals per scan was not always reached. Scan samples were conducted for a total 5–6 d/mo in each focal group.
Habitat Use
We recorded habitat use as the habitat type in which the most members of the group were observed during each scan sample (Tesfaye 2010; Vié et al. 2001). We divided habitat types into four categories: tree-dominated forest, bamboo-dominated forest, bushland, and agricultural land (defined in Table I). We analyzed habitat use patterns by calculating the proportion of the number of group scans that the groups spent in each of the different habitats (Wallace 2006).
Ranging Ecology
At the time of each scan sample, we also recorded the location and elevation of the geographic center of the group (Cords 1987; Fashing 2001a) using a handheld Garmin GPS 12 unit (Tesfaye 2010). Our focal groups proved to be quite cohesive, never engaging in the subgrouping behavior typical of blue monkeys in some populations where group sizes are larger (Cords 1987). Ranging data points at Jibat were thus an accurate depiction of where most group members were at a given time. We obtained a GPS reading even if individuals could not be seen as long as group location could be confirmed via cues such as distinctive tree movements or vocalizations. We determined daily path length for each group based on the shortest linear distance between consecutive GPS locations for all full study days during which ranging data were collected at 15-min intervals from 07:30 h to 17:30 h (Forest Group: 39 d; Fragment Group: 31 d). We conducted daily path length analyses using the Hawths Tools extension for analyzing animal movements in ArcGIS 9.2 (Beyer 2004; Campbell-Smith et al. 2011).
We used the minimum convex polygon method (MCP) in Hawths Tools, ArcGIS 9.2 to determine the home range sizes (100 % MCPs) of the focal groups (Beyer 2004). To calculate home range size for each group, we used data from both full- and partial-day follows (Forest Group: 41 d, 1251 points; Fragment Group: 31 d, 1198 points).
Feeding Ecology
When an individual was feeding during a scan sample, we recorded both the food item and species it was consuming (Tesfaye 2010). We recorded feeding on plants when an individual was observed consuming, i.e., masticating, plant food items. We categorized food items as young leaves, mature leaves, shoots (newly growing aerial parts of a plant including leaf buds), stems (the supporting stalk of plants), flowers, fruits, seeds, bark, unknown plant parts, or animal prey. We recorded animal prey when we observed a monkey breaking off tree bark, exposing curled leaves, or masticating and ingesting invertebrates or vertebrates. We collected unidentified plant species for later taxonomic identification at the National Herbarium.
We determined diet composition by calculating the proportions of different 1) food items and 2) species consumed by the monkeys. We calculated dietary compositions for each group individually. We calculated the monthly proportion of each food item (and species) in the diet as the total number of monthly individual scans for each food item (and species) divided by the total number of monthly scan records for all food items (and species). We used the grand means of the monthly proportion of food items (and species) consumed to calculate the overall wet and dry season diets as well as the overall diet for the entire study period.
To assess dietary diversity of food plant species, we calculated the Shannon–Wiener diversity index (Krebs 1989) for each focal group for each month of study. We then calculated overall Shannon–Wiener diversity indices for each group over the entire study period by determining the means of the monthly indices. Similarly, we assessed dietary evenness using the evenness index, J (Krebs 1989), both monthly and over the entire study period.
We also measured the food selection ratio (a crude indicator of preference) for different food species in the diets of each focal group (Fashing 2001b). We calculated the food selection ratio by dividing the annual percentage of time spent feeding on species i (measured as the grand mean of monthly values) by the percentage species i contributed to the total stem density in the home range of a group (Chapman and Chapman 2002). We considered the food species with the largest selection ratios to be the most selected species (Fashing 2001b).
Because none of our data were normally distributed, we carried out all of our statistical analyses using the nonparametric Mann–Whitney U-test.
Results
Vegetation
We found 62 species in the vegetation plots in Forest Group’s home range and 53 species in the plots in Fragment Group’s range. Forest Group’s range contained 83 % (44 of 53) of the species found in Fragment Group’s range, while Fragment Group’s range contained 73 % (45 of 62) of the species found in Forest Group’s range. The most abundant plant species in the ranges of both groups was the herb Satureja simensis (Laminaceae), though this species accounted for a far greater percentage of the total stems in Fragment Group’s range (83 %) than in Forest Group’s range (30 %) (Table II). Further, the second most abundant species, Arundinaria alpina (Poaceae) or bamboo, in the range of Fragment Group (6.28 % of stems) was barely present in Forest Group’s range (0.01 % of stems) (Table II).
Shannon–Wiener diversity indices for plant species in the home ranges of the study groups were much higher for Forest Group (H′ = 2.02) than for Fragment Group (H′ = 0.80). Plant species evenness indices in the home ranges of the focal groups were 0.20 and 0.07 for Forest Group and Fragment Group, respectively.
Habitat Use
Tree-dominated forest was the most important habitat for Forest Group accounting for 89.7 % of scans (N = 4473). Bushland dominated by Rubus apetalus (Rosaceae) provided the remaining 10.3 % of scans. Fragment Group used a more diverse array of habitat types including tree-dominated forest (37.3 % of 6137 scans), bamboo forest (26.8 %), bushland (25.8 %), and farmland (10.1 %).
Ranging Ecology
Mean daily path lengths for Forest Group (mean = 799 m; range = 182–2341 m; SE ± 66 m; N = 39) and Fragment Group (mean = 783 m; range = 350–1612 m; SE ± 57 m; N = 31) did not differ significantly from one another (Z = –0.024, P = 0.981). There were also no seasonal differences in daily path length for either Forest Group (dry season mean = 807 m; range = 182 – 1711 m; SE ± 95; N = 23; wet season mean = 789 m; range = 303–2341 m; SE ± 93; N = 16) (Z = –0.542; P = 0.587) or Fragment Group (dry season mean = 734 m; range = 350–1093 m; SE ± 70; N = 13; wet season mean = 819 m; range = 353–1612 m; SE ± 86; N = 18; Z = –0.460; P = 0.645). Fragment Group ranged at a slightly higher mean elevation (mean = 2468 m; range = 2376–2593 m; SE ± 1; N = 500) than Forest Group (mean = 2419 m; range = 2338–2555 m; SE ± 2; N = 458; Z = –17.259; P < 0.001). The 100 % minimum convex polygon home range areas for Forest Group and Fragment Group over the study period were 72.0 ha and 61.2 ha, respectively.
Feeding Ecology
Dietary Composition
Fruit was the largest contributor to the overall diet of Forest Group, accounting for 52.5 % of all feeding records (N = 2232) (Fig. 1). Other common items in Forest Group’s diet included animal prey (14.7 %), young leaves (11.1 %), shoots (8.7 %), and flowers (7.3 %) (Fig. 1). The most frequently consumed food item by Fragment Group was shoots, which accounted for 29.8 % of feeding records (N = 2903) (Fig. 1). Young leaves (17.1 %), fruit (17.0 %), animal prey (13.1 %), and seeds (9.6 %) also made substantial contributions to Fragment Group’s diet. For both groups, animal prey included invertebrates, e.g., ants and spiders, and their larvae, as well as the meat and eggs of birds.
Overall percentage contribution of annual feeding time devoted to different food items by Boutourlini’s blue monkeys in two groups, Forest Group (N = 2232 feeding records) and Fragment Group (N = 2903 feeding records), at Jibat Forest, Ethiopia from July 2009 to April 2010. YL = young leaves; ML = mature leaves.
Fruits were the top food items, i.e., had the highest percent monthly contribution to the diet of any food item, during 8 of the 10 mo for Forest Group (range: 24.5–80.7 %), but were the top food items during only 4 mo for Fragment Group (range: 1.7–42.3 %) (Table III). Shoots were the top food items for Fragment Group (range: 15.6–48.1 %) during 4 additional months whereas they were the primary food items during only 1 month for Forest Group (range: 0.0–35.0 %). Seeds were never consumed heavily by Forest Group but accounted for at least one third of Fragment Group’s diet during December and January. During these 2 mo, Fragment Group entered agricultural areas and consumed the seeds of barley (Hordeum vulgare) and wheat (Triticum aestivum) extensively when these crops produced seeds that had not yet been harvested. Seeds from native trees such as Croton macrostachys and Syzygium guineense were only rarely consumed by either group.
There were no significant differences in monthly (N = 10) young leaf (Z = –1.512, P = 0.130), mature leaf (Z = –0.404, P = 0.686), or flower consumption (Z = – 0.154, P = 0.877) between the two focal groups. However, fruit consumption (Z = –3.099, P = 0.002) was significantly higher in Forest Group, whereas seed (Z = –2.221, P = 0.026) and shoot (Z = –2.878, P = 0.004) consumption were significantly higher in Fragment Group. The only food type whose consumption differed significantly on a seasonal basis (N = 6 rainy months vs. N = 4 dry months) in either group were seeds, which Fragment Group ate more often during the dry season (Z = –2.590, P = 0.010) when its members engaged in the aforementioned crop raiding of barley and wheat seeds.
Forest Group consumed a total of 24 plant species (Table IV) whereas Fragment Group exploited 33 plant species (Table V). For Forest Group, Ficus sur (39.9 %; range 14.9–70.0 %; SD ± 18.6 %), Ilex mitis (15.1 %; range 0.0–42.6 %; SD ± 16.7 %), Syzygium guineense (7.7 %; range 0.0–46.9 %; SD ± 16.8 %), Rubus apetalus (2.7 %; range 0.0–8.4 %; SD ± 2.9 %), and Landolphia buchananii (2.6 %; range 0.0–7.9 %; SD ± 2.4 %) were the five most consumed plant species, accounting for 68.0 % of the overall diet (Table IV). For Fragment Group, Arundinaria alpina (20.6 %; range 0.0–46.9 %; SD ± 15.3 %), Ilex mitis (9.3 %; range 0.0–26.0 %; SD ± 8.8 %), Rubus apetalus (7.5 %; range 0.0–24.7 %; SD ± 8.7 %), Triticum aestivium (6.1 %; range 0.0–35.5 %; SD ± 12.6 %), and Prunus africana (5.2 %; range 0.0–26.3 %; SD ± 7.8 %) were the top five plant species, accounting for 48.7 % of the overall diet (Table V).
Dietary Diversity and Food Choice
Mean monthly (N = 10) dietary species diversity (H′) was significantly higher for Fragment Group (mean: 2.06; range: 1.62–2.78; SE ± 0.11) than for Forest Group (mean: 1.36; range: 0.69–1.81; SE ± 0.11; Z = –3.477, P = 0.001; Table VI). Mean monthly dietary evenness index, J, was also significantly higher for Fragment Group (mean: 0.38; range: 0.29–0.51; SE ± 0.02) than for Forest Group (mean: 0.26; range: 0.13–0.34; SE ± 0.02; Z = –3.183, P = 0.001; Table VI).
The plant species with the 10 highest selection ratios for each group during the study period are listed in Table VII. In Forest Group, Ficus sur was the most eaten and by far the most selected for plant species with a selection ratio of 3987, and the four next most frequently selected for plant species were Apodytes dimidiata (220), Ilex mitis (216), Syzygium guineense (153), and Prunus africana (130). In Fragment Group, Prunus africana, the fourth most eaten species, had the highest selection ratio (524), followed by Ilex mitis (309), Rhynchosia resinosa (282), Olinia rochetiana (265), and Brucea antidysentrica (156).
Discussion
We found that Boutourlini’s blue monkeys inhabited both contiguous and fragmented forest at Jibat Forest, Ethiopia throughout our 10-mo study. Ranging patterns (daily path length and home range size) were similar in both habitats, whereas diet varied widely between them. Our focal group in contiguous forest (Forest Group) was mostly frugivorous, whereas our group in fragmented forest (Fragment Group) was primarily folivorous. The percent contributions of different plant species to the diets of each group also differed considerably and only Fragment Group engaged in crop raiding. Our results provide several important insights into the conservation requirements of Boutourlini’s blue monkeys, which we explore in the text that follows.
Forest Composition and Habitat Use
Consistent with patterns found in other tropical forests, contiguous intact forest at Jibat contained more plant species, higher species diversity, and higher species evenness than fragmented forest (Putz et al. 2011; Souza et al. 2012). The dense herb layer (dominated especially by Satureja simensis [Laminaceae]) in the fragmented forest at Jibat is also characteristic of disturbed forests elsewhere in Africa (Chapman and Chapman 1999; Fashing and Gathua 2004; Struhsaker 1997). Fragmented forest at Jibat also contained thick stands of bamboo, which were nearly absent in the intact forest, a vegetational difference that appeared to account for some of the large dietary differences between the two Boutourlini’s blue monkey focal groups.
The Boutourlini’s blue monkey group inhabiting relatively undisturbed contiguous forest (Forest Group) at Jibat was able to meet its needs almost exclusively in the tree-dominated forest, venturing out into nearby bushland only occasionally, mostly to feed on Rubus apetalus fruits. This pattern is similar to that for Stuhlmann’s blue monkeys (Cercopithecus mitis stuhlmanni) in Kakamega Forest, Kenya where an otherwise forest-dwelling group occasionally ventured out of the forest to access trees of Bischofia javanica planted in the surrounding human-dominated landscape to feed on fruit (Cords 1987; Pazol and Cords 2005). In contrast, the group inhabiting fragmented forest (Fragment Group) at Jibat divided their time more evenly among tree-dominated forest, bamboo forest, bushland, and agricultural areas. Not all blue monkey populations living in forest fragments venture into nearby matrix habitats like Boutourlini’s blue monkey at Jibat. For example, groups of samango monkeys (Cercopithecus mitis labiatus) inhabiting forest fragments of Podocarpus in South Africa never entered plantations of Acacia or other matrix habitats nearby (Lawes et al. 2000). The extent to which differences in habituation to humans or hunting threat might play a role in these differences in matrix use between blue monkey subspecies and populations is unclear.
Ranging Patterns
Primates in fragments often have compressed ranging patterns, traveling shorter distances per day or occupying smaller home ranges than conspecifics in contiguous forest (Dunbar 1987; Tutin 1999; Wong and Sicotte 2007). Among blue monkeys at Jibat, however, daily path lengths were relatively similar for Forest Group and Fragment Group and neither group exhibited much seasonal variation in this variable. Further, Forest Group at Jibat had only a moderately (18 %) larger home range than Fragment Group, despite the greater number of study days (41 vs. 31, or 32 % more) and larger number of total ranging points (1251 vs. 1198, or 4 % more) collected for Forest Group, variables known to influence MCP estimates of home range size (Jennrich and Turner 1969; Schoener 1981; Worton 1987). Fragment Group’s home range did not appear to be limited in size due to fragmentation given that they occupied only ca. 20–25 % of the 2–3 km2 area available in their fragment. They did, however, leave their fragment occasionally to exploit the surrounding matrix, including farmland, raising the possibility that they may not have been able to meet all of their nutritional needs within their fragment (see Dietary Ecology).
Overall, the two blue monkey groups at Jibat traveled considerably shorter distances per day than blue monkeys (Table VIII) and other guenons (Cercopithecus spp.) in most other African forests (Jaffe and Isbell 2011). This pattern suggests that blue monkeys at Jibat do not need to travel far to find sufficient food or will settle for nonpreferred food items to avoid increasing daily travel distances. Conversely, home range sizes at Jibat were within the typical size range for other blue monkey populations (Table VIII).
Dietary Ecology
As guenons, blue monkeys possess several anatomical features related to frugivory, including cheek pouches, simple stomachs, and low and rounded molars, yet the diets of blue monkeys have been found to be extremely variable (Jaffe and Isbell 2011). Indeed, as Jaffe and Isbell (2011, p. 282) recently noted, “dietary data for C. mitis account for the entire range of fruit composition in forest guenon diets (24.5 %–91.0 %)” across sites. Given this variability elsewhere in Africa, and the fact that we set out to study Boutourlini’s blue monkeys in two very different habitats (continuous forest and fragmented forest) at Jibat, we expected to see considerable variation in diet between the groups in our study. Our results show that Boutourlini’s blue monkeys do indeed consume very different diets in continuous and fragmented forest. Forest Group obtained 53 % of its diet from fruit, whereas fruit accounted for only 17 % of the diet of Fragment Group, the lowest level of frugivory recorded to date for a blue monkey population (Table VIII). This result is consistent with patterns from Gabon, where two guenon species, mustached guenons (Cercopithecus cephus) and greater spot-nosed monkeys (C. nictitans), were each found to consume far more fruit in a large continuous forest reserve (Lopé) than in a nearby forest fragment (Klainedoxa Bosquet) (Tutin 1999).
Another major dietary difference between the focal groups at Jibat was that Fragment Group (30 % of diet) relied much more heavily on shoots than Forest Group (9 % of diet). Most of the shoots in Fragment Group’s diet came from bamboo (Arundinaria alpina), a species that was so abundant in their range that it failed to appear among the top 10 most selected for species despite being their top food item, accounting for >20 % of their overall diet. In contrast, bamboo was very scarce (<0.01 % of stems) within the range of Forest Group, though they did consume it occasionally (0.8 % of their overall diet). The high level of bamboo consumption by Fragment Group at Jibat is not unprecedented among blue monkeys because Cercopithecus mitis kandti in Mgahinga, Uganda obtains 60 % of its diet from bamboo (Twinomugisha and Chapman 2008). Further, another more distant relation, sometimes classified as a guenon (Grubb et al. 2003), the Bale monkey (Cercopithecus djamdjamensis), was found to eat 77 % bamboo at Odobullu, Ethiopia (Mekonnen et al. 2010). Given that bamboo contains cyanide and the guenons possess simple stomachs, it is not entirely clear how they are able to subsist on such a toxic diet, though their focus on bamboo shoots and young leaves, which appear to be less heavily protected (Twinomugisha et al. 2006), probably plays a role in enabling them to feed so extensively on this species.
During 2 mo of the year (December and January), Fragment Group relied heavily on crop raiding, a strategy not adopted by Forest Group at Jibat. This crop raiding consisted of the consumption of barley and wheat seeds in agricultural areas adjacent to the forest fragment. Given the limitations of the data currently available, we cannot be certain whether crop raiding is essential to Fragment Group for surviving lean periods or whether it is simply an opportunistic supplement to an already complete diet. Primates that rely on crop raiding are generally in conflict with local human populations and are vulnerable to shooting, poisoning, and other eradication strategies (Campbell-Smith et al. 2010; Hill 1997; Lee and Priston 2005; Mekonnen et al. 2012; Swedell 2011). Although primates may be successful at crop raiding over the short term, crop raiding is rarely a sustainable long-term strategy, given that the people with whom primates are generally in conflict typically rely on the same crops for their own survival (Hill 1997; Lee and Priston 2005).
Despite inhabiting a more species-rich habitat, Forest Group consumed a less diverse diet than Fragment Group during 9 of 10 mo. Forest Group’s dietary diversity index was generally lower because it obtained 40 % of its diet from Ficus sur, a fig species that occurred at a low stem density but produced fruit throughout most of the year (D. Tesfaye, pers. obs.). Indeed, Forest Group’s selection ratio (3987) for Ficus sur was by far the highest for any food species for either group. The strong selection for figs by Forest Group is consistent with a pattern observed across many other tropical forest vertebrates, including many primates, worldwide (Fashing 2001b; Felton et al. 2008; Parr et al. 2011; Shanahan et al. 2001). Striking evidence of dietary flexibility in Boutourlini’s blue monkey comes from the fact that, despite their importance to Forest Group, figs scarcely factored into the diet (3 %) of Fragment Group (whose range contained only three fig trees; D. Tesfaye, pers. obs.). This result suggests that although they may be exploited heavily by Boutourlini’s blue monkeys in some habitats, figs are neither a keystone (Gautier-Hion and Michaloud 1989) nor a fallback species (Marshall et al. 2009) for these monkeys.
Conservation Implications
Several of our results suggest that the long-term conservation prospects of Boutourlini’s blue monkeys are promising. First, Boutourlini’s blue monkeys occupy both intact and fragmented forest at Jibat despite the markedly different vegetation compositions of these two forest types. Second, the groups inhabiting these different forest types are able to survive on very different diets, with the blue monkeys in Forest Group relying much more heavily on fruit and those in Fragment Group subsisting more on shoots, particularly from bamboo. Third, overall home range sizes and daily path lengths were comparable between the two focal groups, suggesting that forest fragmentation to date has had little impact on the ranging of Boutourlini’s blue monkeys at Jibat.
Despite the encouraging evidence of habitat, dietary, and ranging flexibility presented in our study, however, there are reasons to suggest that the long-term conservation of Boutourlini’s blue monkeys is far from assured. Their limited distribution in the forests of western Ethiopia, and the growing human population and related high rate of forest clearance in the region, highlight the need for protection of the remaining forests where Boutourlini’s blue monkeys occur. Once forest fragmentation begins in an area, the process tends to continue and the remaining fragments become smaller and less connected over time (Chapman et al. 2007; Chatelain et al. 1996). This pattern creates cause for concern because research on other blue monkey subspecies suggests that there is a size limit below which they are no longer able to survive in fragments (samango monkey: South Africa 45 ha; Lawes et al. 2000; Swart and Lawes 1996; Stuhlmann’s blue monkey: 130 ha; Onderdonk and Chapman 2000). The few hundred hectares of forest remaining for Fragment Group of Boutourlini’s blue monkey at Jibat places them not far above this threshold. Further, although bamboo played a major role in the diet of Fragment Group at Jibat, it is not known how widely bamboo is distributed across the forest fragments of western Ethiopia or whether the monkeys can survive in fragments where bamboo is absent. Lastly, the crop raiding of barley and wheat that contributed substantially to Fragment Group’s diet in December and January is unlikely to be sustainable over the long term because farmers tend to attempt to eradicate crop raiding primates (Hill 1997; Lee and Priston 2005).
Surveys are needed across western Ethiopia to assess the status of remaining Boutourlini’s blue monkey populations and to determine the minimum size and composition of fragments and surrounding matrix needed by the monkeys to ensure their long-term survival (cf. Lawes et al. 2000; Onderdonk and Chapman 2000; Swart and Lawes 1996). At locales where Boutourlini’s blue monkeys are raiding crops, mitigation strategies need to be developed to ensure that the needs of both the local people and the monkeys are being met (cf. Hill 2000; Strum 2010). Ultimately, given the high density and rapid growth of the human population in western Ethiopia, the ability to withstand fragmentation and other disturbance to their habitat will likely be the key factor in determining the long-term conservation prospects of Boutourlini’s blue monkeys.
References
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–267.
Anderson, J., Rowcliffe, J. M., & Cowlishaw, G. (2007). Does the matrix matter? A forest primate in a complex agricultural landscape. Biological Conservation, 135, 212–222.
Andren, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos, 71, 355–366.
Beeson, M. (1987). The origins of bark-stripping by blue monkeys (Cercopithecus mitis): implications for management. Zoological Journal of the Linnean Society, 91, 265–291.
Beeson, M., Tame, S., & Lea, S. E. G. (1996). Food habits of guenons (Cercopithecus spp.) in Afro-montane forest. African Journal of Ecology, 34, 202–210.
Bekele, T. (1994). Phytosociology and ecology of a humid Afromontane forest of the Central Plateau of Ethiopia. Journal of Vegetation Science, 5, 87–98.
Beyer, H. L. (2004). Hawth’s analysis tools for ArcGIS. Available at: http://www.spatialecology.com/htools. Accessed 01 May 2010.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In L. K. Marsh (Ed.), Primates in fragments: ecology and conservation (pp. 283–303). New York: Kluwer Academic/ Plenum Press.
BirdLife International (2009). Important bird area fact sheet: Jibat Forest, Ethiopia. Available at: http://www.birdlife.org/. Accessed 29 Oct 2012.
Boyle, S. A. (2008). The effects of forest fragmentation on primates in the Brazilian Amazon. Ph.D. dissertation, Department of Anthropology, Arizona State University.
Boyle, S. A. (2012). Implications of habitat fragmentation on the diet of bearded saki monkeys in central Amazonian forest. Journal of Mammalogy, 93, 959–976.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in high- and low-density subpopulations. Ecological Monographs, 60, 1–26.
Butynski, T. M., & Gippoliti, S. (2008). Cercopithecus mitis ssp. boutourlinii. In IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. Available at: www.iucnredlist.org. Accessed 26 July 2012.
Campbell-Smith, G., Simanjorang, H. V., Leader-Williams, N., & Linkie, M. (2010). Local attitudes and perceptions toward crop-raiding by orangutans (Pongo abelii) and other nonhuman primates in northern Sumatra, Indonesia. American Journal of Primatology, 72, 866–876.
Campbell-Smith, G., Campbell-Smith, M., Singleton, I., & Linkie, M. (2011). Apes in space: imperiled orangutan population in Sumatra. PloS One, 6, e17210.
Chapman, C. A., & Chapman, L. J. (1999). Forest restoration in abandoned agricultural land: a case study from East Africa. Conservation Biology, 13, 1301–1311.
Chapman, C. A., & Chapman, L. J. (2002). Foraging challenges of red colobus monkeys: influence of nutrients and secondary compounds. Comparative Biochemistry and Physiology, 133, 861–875.
Chapman, C. A., Lawes, M. J., & Eeley, H. A. C. (2006a). What hope for African primate diversity? African Journal of Ecology, 44, 116–133.
Chapman, C. A., Wasserman, M. D., Gillespie, T. R., Speirs, M. L., Lawes, M. J., & Ziegler, T. E. (2006b). Do nutrition, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? American Journal of Physical Anthropology, 131, 525–534.
Chapman, C. A., Naughton-Treves, L., Lawes, M. J., Wasserman, M. D., & Gillespie, T. R. (2007). Population declines of colobus in western Uganda and conservation value of forest fragments. International Journal of Primatology, 28, 513–528.
Chatelain, C., Gautier, L., & Spichiger, R. (1996). A recent history of forest fragmentation in southwestern Ivory Coast. Biodiversity and Conservation, 5, 37–53.
Cords, M. (1986). Interspecific and intraspecific variation in diet of two forest guenons. Cercopithecus ascanius and C. mitis. Journal of Animal Ecology, 55, 811–827.
Cords, M. (1987). Mixed-species association of Cercopithecus monkeys in the Kakamega Forest, Kenya. University of California, Publications in Zoology, 117, 1–109.
Cristóbal-Azkarate, J., & Arroyo-Rodríguez, V. (2007). Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: effects of habitat fragmentation and implications for conservation. American Journal of Primatology, 69, 1013–1029.
Dunbar, R. I. M. (1987). Habitat quality, population dynamics, and group composition in colobus monkeys (Colobus guereza). International Journal of Primatology, 8, 299–329.
Eniang, E. A. (2003). Effects of habitat fragmentation on the Cross River gorilla (Gorilla gorilla diehli): recommendations for conservation. In L. K. Marsh (Ed.), Primates in fragments: ecology and conservation (pp. 343–363). New York: Kluwer Academic/Plenum Press.
Estrada, A., & Coates-Estrada, R. (1996). Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. International Journal of Primatology, 17, 759–783.
Fairgrieve, C., & Muhumuza, G. (2003). Feeding ecology and dietary differences between blue monkey (Cercopithecus mitis stuhlmanni Matschie) groups in logged and unlogged forest, Budongo Forest Reserve, Uganda. African Journal of Ecology, 41, 141–149.
Fashing, P. J. (2001a). Activity and ranging patterns of guerezas in the Kakamega Forest: intergroup variation and implications for intragroup feeding competition. International Journal of Primatology, 22, 549–577.
Fashing, P. J. (2001b). Feeding ecology of guerezas in the Kakamega Forest, Kenya: the importance of Moraceae fruit in their diet. International Journal of Primatology, 22, 579–609.
Fashing, P. J., & Gathua, J. M. (2004). Spatial variability in the vegetation structure and composition of an East African rain forest. African Journal of Ecology, 42, 189–197.
Felton, A. M., Felton, A., Wood, J. T., & Lindenmayer, D. B. (2008). Diet and feeding ecology of Ateles chamek in a Bolivian semihumid forest: the importance of Ficus as a staple food resource. International Journal of Primatology, 29, 379–403.
Gautier-Hion, A., & Michaloud, G. (1989). Are figs always keystone resources for tropical frugivorous vertebrates? A test in Gabon. Ecology, 70, 1826–1833.
Grubb, P., Butynski, T. M., Oates, J. F., Bearder, S. K., Disotell, T. R., Groves, C. P., et al. (2003). Assessment of the diversity of African primates. International Journal of Primatology, 24, 1301–1357.
Harrison, S., & Bruna, E. (1999). Habitat fragmentation and large-scale conservation: what do we know for sure? Ecography, 22, 225–232.
Hill, C. M. (1997). Crop-raiding by wild vertebrates: the farmer’s perspective in an agricultural community in western Uganda. International Journal of Pest Management, 43, 77–84.
Hill, C. M. (2000). A conflict of interest between people and baboons: crop raiding in Uganda. International Journal of Primatology, 21, 299–315.
Isabirye-Basuta, G. M., & Lwanga, J. S. (2008). Primate populations and their interactions with changing habitats. International Journal of Primatology, 29, 35–48.
IUCN 2012. The IUCN Red List of Threatened Species. Version 2012.2. Available at: http://www.iucnredlist.org. Accessed 22 Feb 2013.
Jaffe, K. E., & Isbell, L. A. (2011). The guenons: polyspecific associations in socioecological perspective. In C. J. Campbell, A. Fuentes, K. C. Mackinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in perspective (pp. 277–300). New York: Oxford University Press.
Jennrich, R. I., & Turner, F. B. (1969). Measurements of non-circular home range. Journal of Theoretical Biology, 22, 227–237.
Kaplin, B. A. (2001). Ranging behavior of two species of guenons (Cercopithecus lhoesti and C. mitis doggetti) in the Nyungwe Forest Reserve, Rwanda. International Journal of Primatology, 22, 521–548.
Kaplin, B. A., & Moermond, T. C. (2000). Foraging ecology of the mountain monkey (Cercopithecus l’hoesti): implications for its evolutionary history and use of disturbed forest. American Journal of Primatology, 50, 227–246.
Kaplin, B. A., Munyaligoga, V., & Moermond, T. C. (1998). The influence of temporal changes in fruit availability on diet composition and seed handling in blue monkey (Cercopithecus mitis doggetti). Biotropica, 30, 56–71.
Kingdon, J., Gippoliti, S., Butynski, T. M., Lawes, M. J., Eeley, H., & Lehn, C. (2008). Cercopithecus mitis. In IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. Available at: www.iucnredlist.org. Accessed 26 July 2012.
Krebs, C. J. (1989). Ecological methodology. London: Harper Collins.
Laurance, W. F., Ferreira, L. V., Rankin-de Merona, J. M., & Laurance, S. G. (1998). Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology, 79, 2032–2040.
Lawes, M. J. (1990). The distribution of the Samango monkey (Cercopithecus mitis erythrarchus Peters, 1852 and Cercopithecus mitis labiatus I. Geoffroy, 1843) and forest history in southern Africa. Journal of Biogeography, 17, 669–680.
Lawes, M. J. (1991). Diet of samango monkeys (Cercopithecus mitis erythrarchus) in the Cape Vidal dune forest, South Africa. Journal of Zoology (London), 224, 149–173.
Lawes, M. J. (2002). Conservation of fragmented populations of Cercopithecus mitis in South Africa: The role of reintroduction, corridors and metapopulation ecology. In M. Glenn & M. Cords (Eds.), The guenons: Diversity and adaptation in African monkeys (pp. 375–392). New York: Kluwer Academic/Plenum Press.
Lawes, M. J., Mealin, P. E., & Piper, S. E. (2000). Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented Afromontane forest in South Africa. Conservation Biology, 14, 1088–1098.
Lee, P. C., & Priston, N. E. C. (2005). Perception of pests: human attitudes to primates, conflict and consequences for conservation. In J. D. Paterson & J. Wallis (Eds.), Commensalism and conflict: The human-primate interface (pp. 1–23). Norman: American Society of Primatologists.
Marsh, L. K. (2003). Primates in fragments: Ecology and conservation. New York: Kluwer Academic/Plenum Press.
Marshall, A. J., Boyko, C. M., Feilen, K. L., Boyko, R. H., & Leighton, M. (2009). Defining fallback foods and assessing their importance in primate ecology and evolution. American Journal of Physical Anthropology, 140, 603–614.
Martinez-Mota, R., Valdespino, C., Sánchez-Ramos, M. A., & Serio-Silva, J. C. (2007). Effects of forest fragmentation on the physiological stress response of black howler monkeys. Animal Conservation, 10, 374–279.
Mekonnen, A., Bekele, A., Fashing, P. J., Hemson, G., & Atickem, A. (2010). Diet, activity patterns, and ranging ecology of the Bale monkey (Chlorocebus djamdjamensis) in Odobullu Forest, Ethiopia. International Journal of Primatology, 31, 339–362.
Mekonnen, A., Bekele, A., Fashing, P. J., Lernould, J.-M., Atickem, A., & Stenseth, N. C. (2012). Newly discovered Bale monkey populations in forest fragments in southern Ethiopia: evidence of crop raiding, hybridization with grivets, and other conservation threats. American Journal of Primatology, 74, 423–432.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: the primates of Kibale National Park, Uganda. International Journal of Primatology, 21, 587–611.
Parr, N. A., Melin, A. D., & Fedigan, L. M. (2011). Figs are more than fallback foods: the relationship between Ficus and Cebus in a tropical dry forest. International Journal of Zoology. doi:10.1155/2011/967274.
Pazol, K., & Cords, M. (2005). Seasonal variation in feeding behavior, competition and female social relationships in a forest dwelling guenon, the blue monkeys (Cercopithecus mitis stuhlmanni), in the Kakamega Forest, Kenya. Behavioral Ecology and Sociobiology, 58, 566–577.
Plumptre, A. J. (2006). The diets, preferences, and overlap of the primate community in the Budongo Forest Reserve, Uganda: effects of logging on primate diets. In N. Newton-Fisher, H. Notman, J. D. Paterson, & V. Reynolds (Eds.), Primates of western Uganda (pp. 345–371). New York: Springer.
Putz, S., Groeneveld, J., Alves, L. F., Metzger, J. P., & Huth, A. (2011). Fragmentation drives tropical forest fragments to early successional states: a modeling study for Brazilian Atlantic forests. Ecological Modelling, 222, 1986–1997.
Riitters, K., Wickham, J., O’Neill, R., Jones, B., & Smith, E. (2000). Global-scale patterns of forest fragmentation. Conservation Ecology, 4, 3. Available at: http://www.consecol.org/vol4/iss2/art3/. Accessed 12 Nov 2012.
Rudran, R. (1978). Socioecology of the blue monkeys (Cercopithecus mitis stuhlmanni) of the Kibale Forest, Uganda. Smithsonian Contributions to Biology, 249, 1–88.
Schlichte, H.-J. (1978). The ecology of two groups of blue monkeys in an isolated habitat of poor vegetation. In G. G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 505–517). Washington, DC: Smithsonian Institution Press.
Schoener, T. W. (1981). An empirically based estimate of home range. Theoretical Population Biology, 20, 281–325.
Shanahan, M., Sampson, S. O., Compton, S. G., & Corlett, R. (2001). Fig-eating by vertebrate frugivores: a global review. Biological Reviews of the Cambridge Philosophical Society, 76, 529–272.
Souza, A. F., Cortez, L. S. R., & Longhi, S. J. (2012). Native forest management in subtropical South America: long-term effects of logging and multiple-use on forest structure and diversity. Biodiversity and Conservation, 21, 1953–1969.
State Forests Conservation and Development Department (SFCDD). (1990). Ethiopian forest resource base identification, conservation and rational use in Ethiopia. Addis Ababa: SFCDD.
Struhsaker, T. T. (1997). Ecology of an African rain forest: Logging in Kibale and the conflict between conservation and exploitation. Gainesville: University Press of Florida.
Strum, S. C. (2010). The development of primate raiding: implications for management and conservation. International Journal of Primatology, 31, 133–156.
Swart, J., & Lawes, M. J. (1996). The effect of habitat patch connectivity on samango monkey (Cercopithecus mitis) metapopulation persistence. Ecological Modelling, 93, 57–74.
Swedell, L. (2011). African papionins: diversity of social organization and ecological flexibility. In C. J. Campbell, A. Fuentes, K. C. Mackinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in perspective (pp. 241–277). New York: Oxford University Press.
Tesfaye, D. (2010). Ecology, behaviour and conservation of Boutourlini’s blue monkey (Cercopithecus mitis boutourlinii) in the Jibat Forest, Ethiopia. MSc thesis, Department of Biology, Addis Ababa University.
Turner, I. M. (1996). Species loss in fragments of tropical rain forest: a review of the evidence. Journal of Applied Ecology, 33, 200–209.
Tutin, C. E. G. (1999). Fragmented living: behavioural ecology of primates in a forest fragment in the Lopé Reserve, Gabon. Primates, 40, 249–265.
Twinomugisha, D., & Chapman, C. A. (2008). Golden monkey ranging in relation to spatial and temporal variation in food availability. African Journal of Ecology, 46, 585–593.
Twinomugisha, D., Chapman, C. A., Lawes, M. J., O’Driscoll Worman, C., & Danish, L. M. (2006). How does the golden monkey of the Virungas cope in a fruit-scarce environment? In N. E. Newton-Fisher, H. Notman, J. D. Paterson, & V. Reynolds (Eds.), Primates of Western Uganda (pp. 45–60). New York: Springer.
Vié, J., Richard-Hansen, C., & Fournier-Chambrillon, C. (2001). Abundance, use of space and activity pattern of white faced sakis (Pithecia pithecia) in French Guiana. American Journal of Primatology, 55, 203–221.
Wallace, R. B. (2006). Seasonal variations in black-faced black spider monkey (Ateles chamek) habitat use and ranging behavior in a southern Amazonian tropical forest. American Journal of Primatology, 68, 313–332.
Wong, S. N. P., & Sicotte, P. (2007). Activity budget and ranging patterns of Colobus vellerosus in forest fragments in Central Ghana. Folia Primatologica, 78, 245–254.
Worton, B. J. (1987). A review of models of home range for animal movement. Ecological Modeling, 38, 277–298.
Yalden, D. W., Largen, M. J., & Kock, D. (1977). Catalogue of the mammals of Ethiopia. 3. Primates. Monitore Zoologico Italiano, 1, 1–52.
Acknowledgments
This research was funded by Primate Conservation, Inc. (PCI) and by the Department of Zoological Sciences at Addis Ababa University. We thank the Department of Zoological Sciences at Addis Ababa University for logistical support and the Ethiopian Wildlife Conservation Authority, Finfine Forest Development Enterprise, and Jibat Forest Enterprise for permission to conduct this research. We thank Assefa Hailu of the National Herbarium of Addis Ababa University for identification of plant specimens. We also thank Nga Nguyen for assistance with the statistical analyses and for creating Fig. 1. This research would not have been possible without our field assistants, camp attendants, and local guides.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tesfaye, D., Fashing, P.J., Bekele, A. et al. Ecological Flexibility in Boutourlini’s Blue Monkeys (Cercopithecus mitis boutourlinii) in Jibat Forest, Ethiopia: A Comparison of Habitat Use, Ranging Behavior, and Diet in Intact and Fragmented Forest. Int J Primatol 34, 615–640 (2013). https://doi.org/10.1007/s10764-013-9684-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-013-9684-x