Abstract
We compared the feeding ecology of the Hali–Hali community of bonobos (Pan paniscus) at Kokolopori, a new field site in the Democratic Republic of the Congo, between two periods 5 months apart. During the first study period (SP1), bonobos relied heavily on the dry seeds of Guibourtia (Caesalpiniaceae), mostly eaten from the ground. The second period (SP2) was characterized by high consumption of ripe tree fruit. Terrestrial herbaceous vegetation (THV) contributed little to the diet in either study period. The low amount of ripe fruit and the high reliance on seeds in the diet during SP1 were associated with high cortisol production and low levels of urinary C-peptide in females, suggesting nutritional stress. However, female gregariousness was not constrained during the fruit-poor period, probably because high seed abundance on the ground ameliorated scramble feeding competition. This is the first description of extensive seed predation by bonobos. It suggests that bonobo feeding ecology may be more similar to that of chimpanzees than previously recognized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primates have two major types of relationship with tree seeds. First, they disperse them (Idani 1986; Tsuji et al. 2010; Lambert 2011). Second, they prey on them (Norconk et al. 1998). Seed predation by great apes is little studied. While its nutritional significance is not well known, some evidence suggests that among primates, tree seeds are principally eaten as part of a broadening of a mainly frugivorous diet during periods of fruit scarcity (e.g., Ateles: Wallace 2005). Despite similar patterns having been observed for some chimpanzee (Pan troglodytes) populations (Suzuki 1969), tree seeds need not necessarily be regarded as fallback foods (e.g., Pongo: Harrison and Marshall 2011), as their nutritional value may be high (Suzuki 1969; Rogers et al. 1990). For example, in Budongo, Uganda, during periods when chimpanzees fed on Cynometra alexandrii seeds, females tended to have high levels of ovarian hormones, suggestive of a positive energetic balance (Emery Thompson 2005). The significance of seed eating may therefore depend on the particular tree species and the ecological context of the period during which they are eaten. Here we report that bonobos at a new field site, at Kokolopori, Democratic Republic of the Congo, ate tree seeds extensively during a period when they ate few ripe fruits. Since bonobos have not been previously observed to rely on seeds for a major portion of their seasonal diet, we consider why Kokolopori bonobos ate seeds, and the socio-ecological consequences of seed eating.
We rely on direct observations to quantify dietary composition and gregariousness. To obtain preliminary data on whether bonobos at Kokolopori experienced energetic stress as a result of changes in diet composition, we use urinary C-peptide of insulin. The level of this molecule, produced during the activation of pro-insulin, has been shown to correlate significantly with dietary quality and energy intake in wild chimpanzees, orangutans (Pongo pygmaeus), black-and-white colobus monkeys (Colobus guereza), and free-ranging rhesus macaques (Macaca mulatta), and with changes in body weight in captive bonobos and two macaque species (M. mulatta and M. fascicularis) (Sherry and Ellison 2007; Deschner et al. 2008; Emery Thompson and Knott 2008; Emery Thompson et al. 2009; Harris et al. 2010; Higham et al. 2011; Girard-Buttoz et al. 2011). It is therefore a promising new tool for assessing energetic condition in wild primates that fits the aims of our study. Since cortisol has often been used in primate field studies to evaluate levels of physiological stress brought about by ecological factors, social factors, or a combination of the two (e.g., Emery Thompson et al. 2010), we also use glucocorticoid production to determine whether physiological stress increased or decreased during a period of seed predation compared to a period of ripe fruit eating.
Methods
Study site and subjects
A.V.G. conducted field work at Kokolopori, Democratic Republic of the Congo, during two study periods: SP1 was 8th November to 20th December 2006; SP2 was 18th June to 26th July 2007. Observations were based at Nsondo Camp (0°12′N, 22°51′E), which was set up by A.L.L. and lies within Kokolopori Bonobo Reserve approximately 30 km to the east of the bonobo study site at Wamba. The study subjects were members of the Hali–Hali bonobo community. We observed and identified a total of eleven adult and adolescent members of the Hali–Hali community (3 males, 5 mothers, and 2 nulliparous females in both periods, plus one further nulliparous female in 2007). Additionally ca. 5 juveniles and 5 infants were seen on a regular basis although they were not fully identified. Long-term observations by A.L.L. suggest that the ca. 21 individuals seen in this study represent the entire Hali–Hali community, but this awaits confirmation.
Data collection
Feeding behavior
We recorded the feeding of bonobos using instantaneous party scan sampling at 15-min intervals (Altmann 1974). If any food was eaten by any of the bonobos in sight on the sampling point, we noted the name of the food and the vegetative part eaten. For each feeding scan, we also noted whether party members fed on the ground, in the tree canopy, or both. We identified plants with the help of local field assistants and information from Wamba (Idani et al. 1994), and verified identities of the main food species against herbarium specimens at Royal Botanic Gardens, Kew and the Harvard University Herbaria. Daily diet composition was calculated as the percent of scans in which bonobos in the party fed on a particular food item from the total number of feeding scans recorded on that day. Dietary scores produced with this method were highly correlated with data on individual food intake obtained by the focal sampling of chimpanzee behavior at Kanyawara (Gilby et al. 2010). To maximize the sample size of observations lasting for at least half of the daylight hours, only observations ≥6 h in duration were used in this analysis (range 6–12.75 h). In SP1 we collected 16 observation days that satisfied this criterion (mean ± SEM hours per day: 10.2 ± 0.5 h/day). SP2 yielded 39 observation days (11.4 ± 0.2 h/day). The data-set on dietary intake thus comprises 608 observational hours spread over 55 days.
Party composition
We noted party composition continuously, including all independent individuals leaving or joining parties. Party size refers to the number of adults and adolescents travelling together within 50 m of each other, or arriving at the same feeding location within 15 min. See the Electronic supplementary material (ESM) for details on defining a feeding location. We calculated mean daily party size using party counts made following all new fission or fusion events on days when we were confident that the entire party had been observed. There were 58 parties recorded in this way on 34 days (24 in SP1, 10 in SP2). Observation times on the days for which we report mean party size varied between 50 min and 12 h (mean ± SEM hours per day: 5.6 ± 0.6 h/day). We refer to the proportion of individuals in a given party from the total community as the relative party size (Boesch 1996).
Urine sampling and analysis
We collected first-morning urine voids on disposable plastic bags or pipetted urine off vegetation when it was clear that only one individual contributed to that sample. Samples were stored on filter paper in the field and analyzed for C-peptide, cortisol and creatinine within 6 months of collection using standard laboratory protocols at the Primate Reproductive Ecology Laboratory at Harvard University. More details on sample collection, storage and urinalysis are provided in the ESM.
Data analysis
We analyzed data on dietary composition and party size with Mann–Whitney U tests using each observation day as a unit of analysis. We log-transformed all endocrine data and tested them for differences between study periods with a two-sample t test assuming unequal variance. Since we collected urine very early in the morning while visibility was poor, we could not normally record individual identity, despite being able to assign to age–sex class. We therefore present our results for adults by sex. We conducted all tests using two-tailed probabilities in PASW Statistics 18 with a significance level of 0.05.
Results
Feeding ecology
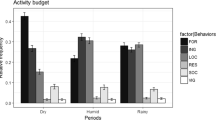
Ripe fruit accounted for over half of all feeding observations (53.6%, Table 1). Terrestrial herbaceous vegetation (THV) and young arboreal leaves comprised only minor portions of the diet. Tree seeds were an important component of the diet, particularly during SP1 (57.3%; Tables 1, 2). Bonobos at Hali–Hali were also seen to feed on meliponine honey, dead wood, truffles, colonial spiders, and the tissue of a holoparasitic plant (Chlamydophytum aphyllum). They once attempted to catch a scaly-tailed flying squirrel (Anomaluridae), but we did not see meat consumption. To our knowledge, holoparasitic plants (Georgiev et al. 2010) and colonial spiders are new dietary records for bonobos.
Bonobos spent less time eating ripe fruit pulp in SP1 (28.5%) than in SP2 (71.3%, Mann–Whitney, z = 4.9, N 1 = 16, N 2 = 39 days, p < 0.001, Table 2). They also consumed a smaller number of different fruit species in SP1 (6) than in SP2 (24; Tables 1, 2). By contrast, the contribution of dry seeds to the diet was higher in SP1 (57.3%) than SP2 (18.5%; z = −3.9, p < 0.001). There were no significant differences between study periods in the dietary contribution of either THV (z = −1.6, p = 0.12) or young arboreal leaves (z = −1.7, p = 0.09).
Across all observation days, the daily dietary proportions of fruit and dry seeds were inversely correlated (Spearman ρ = −0.8; N = 55 days; p < 0.001). A weaker but similar correlation occurred between the daily dietary proportions of ripe fruit and THV (Spearman ρ = −0.41; N = 55; p = 0.002), whereas there was no correlation between the consumption of THV and young arboreal leaves (Spearman ρ = 0.12; p = 0.4) or between ripe fruit and young arboreal leaves (Spearman ρ = −0.07; p = 0.6).
One species of seed dominated the diet during SP1. Guibourtia demeusei is a swamp-forest upper-canopy tree that produces seeds in dry dehiscent single-seed pods. In early November of SP1, Guibourtia seeds were available in trees, but few had fallen to the ground. At this time, bonobos ate Guibourtia seeds in the tree crowns. During November the seeds matured and fell in large numbers to the ground, where bonobos continued to eat them. As a result, ground foraging was much more common overall during SP1 than arboreal foraging for bonobos eating Guibourtia seeds (79.7% of 207 feeding observations with ground foraging vs. 20.3% for arboreal or mixed ground/arboreal). During SP1, Guibourtia alone comprised 57% of all feeding observations. By contrast, during SP2, no Guibourtia seeds were recorded in the diet, though dry seeds of four other tree species still comprised 18.5% of the diet (Table 1).
Gregariousness of Hali–Hali bonobos
The number of adults per party averaged 5.8–5.9 in both study periods (Table 2). There was no difference between study periods in mean party size (Mann–Whitney, z = −0.4, N 1 = 24, N 2 = 10 days, p = 0.7), relative party size (z = −1.1; p = 0.3), female party size (z = 0.153; p = 0.9), relative female party size (z = −0.9; p = 0.4), male party size (z = −1.3; p = 0.2) or relative male party size (z = −1.4; p = 0.2).
Physiological status
Data from females indicated substantial differences in both C-peptide and cortisol status between study periods. In SP1, during the period of heavy seed consumption, C-peptide levels (624.2 pg/mg Cr) were relatively low, averaging 47.8% of those in SP2 (1306.2 pg/mg Cr, t = −2.5, df = 36, N 1 = 17, N 2 = 26 samples, p = 0.02, Table 2), indicating decreased energy balance in SP1. By contrast, cortisol levels in SP1 (213.3 ng/mg Cr) were relatively high, averaging 305.6% of those in SP2 (69.8 ng/mg Cr; t = 3.9, df = 26, N 1 = 16, N 2 = 24, p = 0.0006). Similar absolute differences occurred in male urines, but these were not statistically significant, presumably due to the small sample size (Table 2).
Discussion
We observed bonobo behavior and physiology during two 2-month periods characterized by markedly distinct dietary composition. Yet, in both periods, tree seeds (mostly from Caesalpiniaceae) were an important part of the diet, occurring in between 15.9 and 67.7% of monthly scans. Tree seeds have not been reported to occupy such a large part of bonobo diets. Our study therefore extends the range of diet types known for bonobos. Chimpanzees have been previously recorded eating tree seeds (especially Caesalpiniaceae) at high concentrations in some months (Wrangham 1975; Goodall 1986; Reynolds 2005). At Budongo, the seeds of Cynometra alexandrii are described as being one of the “important foods” during a dry season lasting between December and March, a period characterized by a scarcity of fruiting figs (Sugiyama 1968). Sugiyama (1968) argued that the pattern of use of C. alexandrii seeds shown by the Budongo chimpanzees was similar to the reliance of savannah chimpanzees on the hard seeds of other Caesalpiniaceae trees during dry months (Suzuki 1969), and could thus be an important fallback food that buffers them against food shortages in some years. This interpretation fits well with our observations of seed eating by the bonobos during the months of November and December at Kokolopori, and should be explored further when long-term data on feeding ecology at Hali–Hali become available. Another unexpected finding was that the consumption of tree fruit by the bonobos varied twofold between the two study periods. This raised the question of whether seasonal fruit shortages occur regularly at Hali–Hali, a topic that requires future phenological observations. In summary, our observations on seed eating and variance in ripe fruit intake between different months indicate a closer similarity in the plant diets of bonobos and chimpanzees then previously recognized.
If either ripe fruit or seeds are preferred to the other, we expect that the preferred items should be more beneficial and associated with a more positive energy balance and less physiological stress. Our finding that ripe fruit diets were associated with high C-peptide and low cortisol therefore suggest that fruits were a more beneficial food item than tree seeds. The fact that our urine samples could not be identified to individual means the physiological data are preliminary, but our results clearly suggest that ripe fruits are preferred and that tree seeds are eaten when ripe fruits are scarce.
Bonobo females tend to be more gregarious than chimpanzee females (Furuichi 2009). One of the explanations proposed for this species difference is that bonobos have more seasonally stable and abundant fruit supplies than chimpanzees do (White and Wrangham 1988; Chapman et al. 1994; Hohmann et al. 2006). Another is that bonobos have greater access to THV than chimpanzees, allowing them to forage in a regime of low scramble competition (Wrangham 2000). The herbs available to bonobos have also been shown to be low in fiber and high in protein relative to similar plant foods in chimpanzee habitats (Sommer et al. 2010). Our data, however, show that even though ripe fruit was not eaten at uniformly high levels all year, and even though THV contributed little to the diet, gregariousness in the study community did not change. None of the prior explanations is therefore easily applicable to Kokolopori bonobos. Nevertheless, because bonobos often fed on tree seeds collected from the ground, scramble competition was apparently low, allowing female gregariousness to remain high. Our observations thus suggest that the mechanisms allowing bonobos to forage in relatively stable parties are more variable than previously considered.
Despite the short duration of this investigation, the contrast in dietary composition between our two study periods, together with the fact that extensive seed eating has not yet been recorded at other sites, suggests that much ecological variation in bonobos remains to be described and explained. Establishing multiple conservation areas across the DRC where bonobos are protected by involving the local community, as is the case at Kokolopori, can be expected to improve not just the survival prospects for bonobos but also our ability to understand the range of their ecological and behavioral adaptations.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Boesch C (1996) Social grouping in Tai chimpanzees. In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge University Press, Cambridge
Chapman CA, White FJ, Wrangham RW (1994) Party size in chimpanzees and bonobos: a reevaluation of theory based on two similarly forested sites. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (eds) Chimpanzee cultures. Harvard University Press, Cambridge, MA, pp 41–57
Deschner T, Kratzsch J, Hohmann G (2008) Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Horm Behav 54:620–626
Emery Thompson M (2005) Endocrinology and ecology of female chimpanzee reproduction. Ph.D. thesis, Harvard University, Cambridge, MA
Emery Thompson M, Knott CD (2008) Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Horm Behav 53:526–535
Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB (2009) Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm Behav 55:299–305
Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW (2010) Dynamics of social and energetic stress in wild female chimpanzees. Horm Behav 58:440–449
Furuichi T (2009) Factors underlying party size differences between chimpanzees and bonobos: a review and hypotheses for future study. Primates 50:197–209
Georgiev AV, Lokasola AL, Nkanga L, Lokondja A, Nsala J, Likenge J, Ilanga-Bomanga A, Likenge JP (2010) New observations of the terrestrial holoparasite Chlamydophytum aphyllum Mildbr. and its consumption by bonobos at Kokolopori, Democratic Republic of Congo. Afr J Ecol 48:849–852
Gilby IC, Pokempner AA, Wrangham RW (2010) A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol 81:254–264
Girard-Buttoz C, Higham JP, Heistermann M, Wedegärtner S, Maestripieri D, Engelhardt A (2011) Urinary C-peptide measurement as a maker of nutritional status in macaques. PLoS One 6(e18043):1–9
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. The Belknap Press of Harvard University Press, Cambridge, pp 674
Harris TR, Chapman CA, Monfort SL (2010) Small folivorous primate groups exhibit behavioral and physiological effects of food scarcity. Behav Ecol 21:46–56
Harrison M, Marshall A (2011) Strategies for the use of fallback foods in apes. Int J Primatol. doi:10.1007/s10764-010-9487-2
Higham JP, Heistermann M, Maestripieri D (2011) The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim Behav 81:1001–1007
Hohmann G, Fowler A, Sommer V, Ortmann S (2006) Frugivory and gregariousness of Salonga bonobos and Gashaka chimpanzees: the influence of abundance and nutritional quality of fruit. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology in apes and other primates: ecological, physical and behavioural aspects. Cambridge University Press, Cambridge, pp 123–159
Idani G (1986) Seed dispersal by pygmy chimpanzees (Pan paniscus)—a preliminary report. Primates 27:441–447
Idani G, Kuroda S, Kano T, Asato R (1994) Flora and vegetation of Wamba forest, central Zaire with reference to bonobo (Pan paniscus) foods at Wamba. Tropics 3:309–332
Lambert JE (2011) Primate seed dispersers as umbrella species: a case study from Kibale National Park, Uganda, with implications for Afrotropical forest conservation. Am J Primatol 73:9–24
Norconk MA, Grafton BW, Conklin-Brittain NL (1998) Seed dispersal by neotropical seed predators. Am J Primatol 45:103–126
Reynolds V (2005) The chimpanzees of Budongo Forest: ecology. behaviour and conservation. Oxford University Press, Oxford
Rogers ME, Maisels F, Williamson EA, Fernandez M, Tutin CEG (1990) Gorilla diet in the Lope Reserve, Gabon: a nutritional analysis. Oecologia 84:326–339
Sherry DS, Ellison PE (2007) Potential applications of urinary C-peptide of insulin for comparative energetics research. Am J Phys Anthropol 133:771–778
Sommer V, Bauer J, Fowler A, Ortmann S (2010) Patriarchal chimpanzees, matriarchal bonobos: potential ecological causes of a Pan dichotomy. In: Sommer V, Ross C (eds) Primates of Gashaka: socioecology and conservation in Nigeria’s biodiversity hotspot. Springer, New York
Sugiyama Y (1968) Social organization of chimpanzees in the Budongo Forest, Uganda. Primates 9:225–258
Suzuki A (1969) An ecological study of chimpanzees in a savanna woodland. Primates 10:103–148
Tsuji Y, Yangozene K, Sakamaki T (2010) Estimation of seed dispersal distance by the bonobo, Pan paniscus, in a tropical forest in Democratic Republic of Congo. J Trop Ecol 26:115–118
Wallace R (2005) Seasonal variations in diet and foraging behavior of Ateles chamek in a Southern Amazonian Tropical Forest. Int J Primatol 26:1053–1075
White F, Wrangham RW (1988) Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105:148–164
Wrangham RW (1975) The behavioural ecology of chimpanzees in Gombe National Park, Tanzania (Ph.D. thesis). Cambridge University, Cambridge
Wrangham RW (2000) Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In: Kappeler P (ed) Primate males: causes and consequences of variation in group composition. Cambridge University Press, Cambridge, pp 248–258
Acknowledgments
We thank Sally Coxe and Michael Hurley from the Bonobo Conservation Initiative (BCI) for the invitation to work at Kokolopori and for their support in the field. Our study was made possible by research permission obtained by the BCI and with the cooperation of Dr. Mwanza Ndunda at the Centre Recherche de Ecologie et Forestrie at Mbandaka. BCI staff in Kinshasa and Mbandka, the late Veronique Lokasola, and her colleagues at Vie Sauvage provided guidance and assistance in the DRC. Barbara McKinder arranged access to and helped track down specimens at the Royal Botanical Gardens Kew. Leonard Nkanga, Antoine Lokondja, Jean Nsala, Jacques Likenge, Antoine Ilanga-Bomanga and Jean-Pierre Likenge assisted in the field. For their help before, during and in-between trips, AG would further like to thank the late B. Carey, D. Carey, M. Reiches, E. Aggianiotakis and M. Lynch. Funding was provided by Harvard University, the Arthur L. Greene Fund, and a Bristol Myers Freedom to Discover Award to B. Hahn at University of Alabama at Birmingham. We also thank Takeshi Furuichi and an anonymous reviewer for their valuable comments on the manuscript. This study complied with the requirements of the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Arts and Sciences at Harvard University, as well as with relevant legislation of the host country, the Democratic Republic of the Congo.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Georgiev, A.V., Thompson, M.E., Lokasola, A.L. et al. Seed predation by bonobos (Pan paniscus) at Kokolopori, Democratic Republic of the Congo. Primates 52, 309–314 (2011). https://doi.org/10.1007/s10329-011-0256-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-011-0256-4