Abstract
Understanding how primates adjust their behavior in response to seasonality in both continuous and fragmented forests is a fundamental challenge for primatologists and conservation biologists. During a 15-mo period, we studied the activity patterns of 6 communities of spider monkeys (Ateles geoffroyi) living in continuous and fragmented forests in the Lacandona rain forest, Mexico. We tested the effects of forest type (continuous and fragmented), season (dry and rainy), and their interaction on spider monkey activity patterns. Overall, monkeys spent more time feeding and less time traveling in fragments than in continuous forest. A more leafy diet and the spatial limitations in fragments likely explain these results. Time spent feeding was greater in the rainy than in the dry season, whereas time spent resting followed the opposite pattern. The increase in percent leaves consumed, and higher temperatures during the dry season, may contribute to the observed increase in resting time because monkeys probably need to reduce energy expenditure. Forest type and seasonality did not interact with activity patterns, indicating that the effect of seasonality on activities was similar across all sites. Our findings confirm that spider monkeys are able to adjust their activity patterns to deal with food scarcity in forest fragments and during the dry season. However, further studies are necessary to assess if these shifts are adequate to ensure their health, fitness, and long-term persistence in fragmented habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival and reproduction of tropical primates are noticeably altered in forest fragments (Arroyo-Rodríguez and Dias 2010; Chapman et al. 2007), as most primates rely on the presence and abundance of large canopy food trees (Arroyo-Rodríguez et al. 2007a; Dunn et al. 2010; Stevenson 2001), and changes in vegetation derived from fragmentation can result in reduced food availability, e.g., reduced tree basal area (Arroyo-Rodríguez and Mandujano 2006; Laurance et al. 1997) and reduced plant species richness (López et al. 2005). Further, in seasonal forests fruit availability is often lower during the dry season than during the rainy season (Asensio et al. 2009; Hemingway and Bynum 2005; Zimmerman et al. 2007), and hence could have negative effects on food availability for primates. Although some tropical primates can make behavioral adjustments to cope with resource limitations in fragmented or seasonal habitats (Boyle and Smith 2010; Hemingway and Bynum 2005; Irwin 2008a,b; Tutin 1999), our current knowledge about these adjustments is scarce, even when considering the most studied primates, e.g., Colobus guereza and Cercopithecus spp. (Onderdonk and Chapman 2000) and Alouatta spp. (Arroyo-Rodríguez and Dias 2010; Bicca-Marques 2003).
A few studies have demonstrated that primates can adjust their behavior in fragmented forests. For example, in response to fruit scarcity in fragmented forests, frugivorous primates often switch to a more leafy diet (Chaves et al. 2011; Juan et al. 2000; Onderdonk and Chapman 2000), which in some cases may increase time spent feeding (Boyle and Smith 2010; Silva and Ferrari 2009) or limit time devoted to other core activities such as traveling and resting (Dunbar 1992; Irwin 2008a,b; Juan et al. 2000; Korstjens et al. 2010). Other researchers also have found that primates spend more time feeding after partial deforestation of their habitats (Clarke et al. 2002). Moreover, in comparison to those in continuous forest, monkeys in fragments use smaller home ranges (Propithecus diadema: Irwin 2008a; Alouatta palliata; Cristóbal-Azkarate and Arroyo-Rodríguez 2007), travel greater daily distances, and spend less time traveling and more time resting (Chiropotes satanas: Boyle et al. 2009; Boyle and Smith 2010).
Seasonal variations in both food availability and climatic variables may contribute to shifting activity patterns in primates. During the dry season, animals in some Neotropical forests are exposed to more stressful conditions than during the rainy season, e.g., temporal food scarcity, drought, and high ambient temperature (Hemingway and Bynum 2005; Murphy and Lugo 1995; Stoner and Timm 2004). To deal with these conditions, tropical primates often reduce energy expenditure during the dry season by spending more time resting, an energy-saving activity, and less time traveling, an energetically costly activity (Asensio et al. 2009; Dunbar et al. 2009; Korstjens et al. 2006; 2010; Masi et al. 2009; Stelzner 1988). Because fleshly ripe fruits are particularly abundant during the rainy season (Asensio et al. 2009), this season is frequently a period of high frugivory (Felton et al. 2009; Masi et al. 2009). Moreover, some primates spend less time feeding during the rainy season (Masi et al. 2009), as fleshly ripe fruits are more digestible and higher in energy than leaves (Felton et al. 2009; Lambert 1998; Milton 1981a,b). Other studies suggest that some primates spend more time feeding during the rainy season (Felton et al. 2009), and they attribute this to the fact that feeding is an energetically costly activity that needs to be minimized during the dry season (Campos and Fedigan 2009). In addition, by increasing time feeding during the rainy season, monkeys may ingest surplus energy and store it as fat in preparation for the impending period of food scarcity (Felton et al. 2009).
Studies evaluating simultaneously the influence of forest type, i.e., continuous and fragmented, and seasonality on primate activity patterns are rare, particularly in the Neotropics (cf. studies with Chiropotes satanas: Boyle et al. 2009; Boyle and Smith 2010). Ateline primates may be particularly amenable subjects for this type of research because throughout their wide geographical distribution they typically live in a large variety of nonseasonal and seasonal habitats where food abundance and distribution can vary greatly over the course of a year (Asensio et al. 2009; Di Fiore et al. 2010). Although a number of researchers have briefly described the activity patterns of frugivorous Ateline primates such as Lagothrix spp. (Defler 1995; Stevenson et al. 1994) and Ateles spp. in continuous forests (Di Fiore et al. 2010; Wallace 2001), no studies to date have attempted to determine how resource limitation in fragmented and seasonal forests modify their activity patterns.
Here we assess variations in activity patterns of spider monkeys (Ateles geoffroyi vellerosus) in 2 contrasting forest types (continuous and fragmented) and during 2 seasons (dry and rainy) in the Lacandona rain forest, southern Mexico. We hypothesize that spider monkeys can adjust their activity patterns to deal with food scarcity in forest fragments and during the dry season. We expect that feeding time will be greater in fragments than in continuous forest to compensate for the lower abundance and quality of foods for primates frequently found in fragments (Arroyo-Rodríguez and Mandujano 2006; Dunn et al. 2010; Irwin 2008b; Onderdonk and Chapman 2000). Further, because fruit scarcity in fragments and during the dry season contributes to an increase in consumption of low-energy content items, such as leaves (González-Zamora et al. 2009; Hemingway and Bynum 2005), we predict that monkeys will increase time resting and reduce time traveling in fragments and during the dry season to reduce energy expenditure (Korstjens et al. 2010; Milton 1981a).
Methods
Study Area
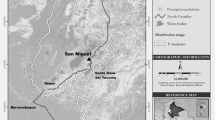
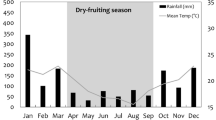
We conducted the study in the Lacandona rain forest, southern Chiapas, Mexico (16°05′58″N, 90º52′36″W; elevation 10–50 m a.s.l.). This region encompasses the largest portion of tropical rain forest in Mesoamerica, covering parts of Mexico, Guatemala, and Belize (ca. 800,000 ha, De la Maza and De la Maza 1991). The original vegetation in the area is lowland tropical rain forest and semideciduous rainforest. The region is highly seasonal, presenting 2 clearly defined seasons: a rainy season (June–December) and a dry season (January–May). Average annual rainfall is 2881 mm; the greatest rainfall concentration occurs in June–September (ranging from 423 to 511 mm/mo), and the lowest occurs in February–April (ranging from 46 to 60.6 mm/mo). Average annual temperature is 24°C, and is greater in the dry season (average per month: 26.3°C; range 22–28°C) than in the rainy season (average per month: 23.5°C; range: 20–25°C). Average maximum daily temperature occurs in the dry season from March to May (ca. 38°C in each month; Comisión Federal de Electricidad, Mexico unpubl. data). Although there are no long-term published records of phenological data for the region, an 8-yr study of tree community dynamics indicates that fleshy fruit (the main food item for Ateles geoffroyi: Chaves et al. 2011; González-Zamora et al. 2009) production at Lacandona is concentrated within the rainy season, while fruit is scarce during the dry season (M. Martínez-Ramos unpubl. data). This pattern is consistent with that observed in other Neotropical forests (Asensio et al. 2009; Zimmerman et al. 2007).
We worked in 2 adjacent areas separated by the Lacantún River (>100 m wide): the Marqués de Comillas region (MCR, eastern side of the river) encompassing ca. 176,200 ha of disturbed forests and human settlements (Marquez-Rosano 2006) and the Montes Azules Biosphere Reserve (MABR, western side) comprising ca. 331,000 ha of old-growth undisturbed forest (Gómez-Pompa and Dirzo 1995). Human colonization and deforestation of MCR began in the 1960s and cattle ranching resulted in the rapid disappearance and fragmentation of the forest (Marquez-Rosano 2006). Approximately 50% of the land surface of MCR is now used for agricultural purposes, but several forest fragments (0.5–1500 ha) remain in the area.
Study Sites and Spider Monkey Communities
We studied activity patterns of 6 independent spider monkey communities: 3 communities located in 3 different areas within the MABR continuous forest separated by ≥4 km, and 3 communities located in 3 different forest fragments within the MCR. We chose these sites because well habituated monkey communities occupied them. In addition, the fragments were easily accessible and protected by the landowners since 1985, avoiding confounding effects of hunting and logging. These communities ranged from 35 to 44 individuals, and their home ranges varied from 32 to 90 ha (Table I). All fragments in MCR were isolated ≥24 yr ago, and their sizes were 14, 31, and 1125 ha. The isolation distance among fragments ranged from 1 km (F1–F2) to 9.5 km (F1–F3). Despite the variation in size, we consider all 3 sites as fragments because all 1) are surrounded by an anthropogenic matrix, i.e., crops, pastures, and human settlements, and are isolated from the continuous forest by 200–1200 m; and 2) the vegetation has noticeable signs of disturbance, e.g., plantations such as Theobroma cacao and Aechmea magdalenae, and lower density of emergent trees in the canopy. Further, because home range size of monkeys in the 1125-ha fragment was 63 ha, and this figure can reflect a more realistic measure of the area of forest used by the monkeys (Boyle and Smith 2010), differences in area used by the monkeys among fragments are surely much lower than the differences in fragment size. Fragments F1 and F2 each contained 1 monkey community. Although fragment F3 contained 3 monkey communities, we studied only 1 of the 3 communities. Distinct home ranges, group composition, and individuals with unique marks distinguished the communities.
In addition to the forest fragment, the monkey community inhabiting fragment F1 frequently (ca. 34% of the time) exploited adjacent areas in the matrix. These areas consisted of Theobroma cacao and Manguifera indica plantations (2.5 ha), a 9-yr-old secondary forest (5 ha), and live fences and pastures (ca. 10 ha) with isolated adult trees of several top food species for Ateles geoffroyi including Ficus tecolutensis, F. insipida, Brosimum alicastrum, Dialium guianense, Inga spp., and Spondias spp. (González-Zamora et al. 2009). Similarly, the monkey community inhabiting fragment F2 exploited an additional area, different from the area exploited by the monkeys inhabiting fragment F1, composed of cocoa plantations (2 ha) and a 12-yr-old secondary forest (3.7 ha). In spite of the differences in size among the 3 fragments (F1, F2, and F3), neither the density of large trees (1, 1, and 2 stems/1000 m2, respectively), nor the sum of the importance value index (IVI) of top food species differ noticeably among fragments (IVI = 115, 159, and 171, respectively; Table SI). Overall, the diet of the communities of Ateles geoffroyi within the study sites comprises 121 plant species belonging to 39 families, with fruits and leaves the most eaten items (56% and 18.5% of the total feeding time, respectively, Chaves et al. 2011).
We followed the monkeys through the entire fragment area and the matrix. Monkey communities did not move between fragments. The landowners that are permanent residents also confirmed no intersite movement between fragments. Similarly, we did not observe movements between our studied fragments and the continuous forest, i.e., MABR.
Activity Pattern and Diet Data Collection
We studied activity patterns of spider monkey communities during a 15-mo period: 6 mo in the dry season (February–April 2007 and 2008), and 9 mo in the rainy season (May–October 2007 and August–October 2008). We recorded the activity patterns of each of the 6 communities during 3 consecutive days from 07:00 h to 17:30 h once every 3 wk, using focal animal sampling (Altmann 1974). We alternated observations among communities every 3 d. During each observation day, we first selected the closest subgroup, or subgroups, and then identified as many individuals as we could (commonly 2–4 individuals/subgroup). We identified individuals using unique marks found in skin pigmentation, hair, genitals (shape, size, and pigmentation of clitoris and penis), face (distinctive facial shapes), body size, and other distinguishing marks such as scars. In each subgroup, we randomly changed focal individuals in most cases after each 5-min interval or when individuals moved out of sight. Whenever possible, we collected data from 1–4 subgroups throughout the day. During the focal observations we recorded 4 mutually exclusive activities: 1) feeding (masticating or consuming food items), 2) resting (period of inactivity), 3) traveling (movement between tree crowns or within the crown of a tree that was not directly food related), and 4) other activities, e.g., social activities.
We used the same data and methods described by Chaves et al. (2011) to calculate seasonal variations in diet.
Data Analyses
We base our analyses on 1010 h of focal observations (496 h in continuous forest and 514 h in fragments, and 517 h in the rainy season and 493 h in the dry season); we discarded 900 unsuitable focal observations because of poor visibility (Fig. S1). To minimize the potential effect of age class, i.e., adults, subadults, juveniles, and infants, on monkey behavior (Arroyo-Rodríguez et al. 2007b), all focal individuals included in our analyses were adults or subadults.
We used an analysis of deviance (ANODE) with a generalized linear model (Crawley 2002) to compare activity patterns among forest types and seasons. Because we observed an independent monkey community in each study site, we considered each of the sites per forest type as replicas. We first made arcsine transformations for proportion time data, and we used a normal distribution with an identity link function to the response variable (Crawley 2002). The whole model was: TIME = ACTIVITY + FOREST + ACTIVITY*FOREST + SEASON + SEASON*ACTIVITY + SEASON*FOREST + ACTIVITY*FOREST*SEASON (asterisks indicate interactions among factors). To identify the levels of each factor that were statistically different between each other we used post hoc analyses with contrasts (Crawley 2002). We used a similar statistical procedure to compare the proportion of time devoted to each plant item, i.e., fruits, leaves, branch piths, flowers, and others, between seasons. We used JMP version 7.0 (SAS Institute, Cary, N.C.) to perform all statistical analyses.
We conducted the aforementioned analyses both including and excluding the largest fragment size as a replica, because of the large differences in fragment size, and because some studies have shown that activity patterns are affected by fragment size (Boyle and Smith 2010; cf. Bicca-Marques 2003; Cristóbal-Azkarate and Arroyo-Rodríguez 2007). This allowed us to assess the potential effect of the largest fragment on the observed differences in activity patterns between continuous and fragmented forests. As we found that the results were similar (Table SII), we decided to include the largest fragment in the analyses to have a larger sample size.
Results
Overall, spider monkeys spent different amounts of time per activity (ACTIVITY; χ 2 = 94.2, df = 3, p < 0.01), with time feeding (average percentage ± SD, 44 ± 9% of total time) and resting (34 ± 7%) higher than time spent traveling (12 ± 4%) and other activities (10 ± 3%) (contrast tests, p < 0.01 in all cases). However, we observed notable variations in percentages of time spent in each activity across sites and seasons (Table II).
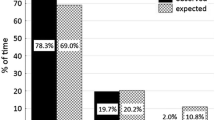
Activity patterns differed between forest types (ACTIVITY*FOREST; χ 2 = 10.3, df = 3, p = 0.01, Fig. 1a) with the percentage of time spent feeding higher in forest fragments than in continuous forest, whereas traveling was higher in continuous forest than in fragments (contrast test, p < 0.03; Fig. 1a). Time spent resting and in other activities did not differ between forest types (contrast tests, p = 0.13 and p = 0.11, respectively; Fig. 1a).
Activity patterns also differed between seasons (ACTIVITY*SEASON; χ 2 = 16.6, df = 3, p < 0.01; Fig. 1b). The percentage of time spent feeding was higher in the rainy than in the dry season (contrast test, p < 0.01; Fig. 1b), whereas resting was higher in the dry than in the rainy season (contrast test, p = 0.02; Fig. 1b). We observed both of these patterns across most study sites (with the exception of C2; Table II). Time dedicated to traveling and to other activities did not differ between seasons (contrast test, p = 0.07 and p = 0.12, respectively; Fig. 1b). Finally, we found that forest type and season did not interact with activity patterns (ACTIVITY*FOREST*SEASON; χ 2 = 1.5, df = 3, p = 0.70), indicating that the effect of season on activity patterns was similar across all study sites.
We found differences in consumption of leaves (young and mature) between seasons (χ 2 = 11.5, df = 1, p < 0.01), being higher in the dry than in the rainy season (contrast tests, p < 0.02 in both cases; Fig. 2). Nevertheless, time spent on fruits (immature and mature) was similar (χ 2 = 0.60, df = 1, p = 0.60; Fig. 2), as was time spent on branch pith, flowers, and other plant items (contrast tests, p > 0.05 in all cases; Fig. 2).
Seasonal diet composition of spider monkeys. The percentages of total feeding time (mean ± SD) consuming different plant items are indicated: mature fruits (MF), immature fruits (IF), mature leaves (ML), immature leaves (IL), young branch piths (BR), flowers (FW), and other plant items (OT, decayed wood, plant secretions, moss, and epiphyte roots). Different letters above bars indicate significant differences (p < 0.05).
Discussion
In general, our results demonstrate that spider monkeys in Lacandona adjusted their activity patterns to cope with forest type and season. Nevertheless, our results did not support all of our predictions. We demonstrated that 1) feeding time was higher in fragments than in continuous forest, 2) traveling was higher in continuous forest than in fragments, and 3) monkeys spent more time resting in the dry than in the rainy season. However, contrary to our expectations, we did not find differences in time spent resting between forest types, and time traveling did not differ between seasons. Overall, these habitat- and seasonal-related shifts in activity patterns are consistent with previous reports in different primates (Boyle and Smith 2010; Bronikowski and Altmann 1996; Dunbar 1992; Korstjens et al. 2010), and can be better understood by taking into account variations in diet and food availability documented for the same monkeys across the study sites (Chaves et al. 2011).
Habitat-Related Shifts in Activity Patterns
The spider monkeys we studied ate more immature than mature fruits and devoted more time to leaves in fragments than in continuous forest (Chaves et al. 2011). This was probably a consequence of the lower availability of fleshy fruit species in this habitat, i.e., fragments present a lower density of large trees of fleshy fruit species and importance value index of top food species than continuous forest (Chaves et al. 2011). Because the digestive system of Ateles geoffroyi is designed essentially for a diet composed mainly of easily digestible food items such as fleshly ripe fruits (Lambert 1998; Milton 1981a,b), monkeys probably invest more time feeding when eating leaves and immature fruits to obtain sufficient amounts of energy (Chapman and Chapman 1991). This idea is supported by several studies, e.g., Papio spp. (Dunbar 1992), Eulemur spp. (Overdorff 1996), and Gorilla gorilla (Masi et al. 2009), which indicate that time spent feeding is directly related to the proportion of leaves in the diet. Similarly, time spent feeding by populations of Theropithecus gelada in 3 different habitats is inversely related to variation in food quality among habitats (Iwamoto and Dunbar 1983), a pattern similar to that reported by Watts (1988) for Gorilla gorilla.
Nevertheless, our findings contrast with those of some studies conducted on primates in fragments in South America. For example, in Brazil, Silva and Ferrari (2009) reported that Chiropotes satanas spends a similar time feeding in continuous forest and in fragments, while the time spent resting is greater in fragments. Similarly, Boyle and Smith (2010) found no relationship between fragment size and time devoted to feeding in 7 saki groups, but groups in smaller fragments rested more. These discrepancies are probably explained, at least in part, by the fact that plant species diversity (and hence food availability for frugivores) is higher in South America vs. Mesoamerica (Gentry 1982). Proximity to the equator and mean annual rainfall affect diet diversity in Ateles spp. (Di Fiore et al. 2008). Further, it is reasonable to expect that Mesoamerican frugivorous primates, such as Ateles geoffroyi, inhabiting fragments experience stronger limitations in the abundance or quality of fruits and other plant foods, “forcing” them to invest more time in feeding on low-quality foods.
The limited foraging area available in fragments partially explains the lower traveling time in fragments vs. continuous forest. In fragments as small as 14 ha, food is scarce and space is limited, and hence monkeys cannot travel very much, with the exception of some elements in the matrix, such as secondary forest and cocoa and mango plantations. Boyle and Smith (2010) and Silva and Ferrari (2009) have reported similar results for Chiropotes satanas, concluding that this species spends less time traveling in fragments than in continuous forest because of the larger home ranges, and hence the larger space available for moving in continuous forest. Further, because traveling is an energetically costly activity (Chapman and Chapman 2000), the reduction of time traveling in fragments could also be an energy-saving strategy to cope with resource limitations in fragments rather than an effect of the area. For instance, in Punta Laguna, Mexico, Rebecchini et al. (2008) found that after Hurricane Emily, which produced severe fruit scarcity, spider monkeys spent less time traveling than during the pre-hurricane period.
The finding that time spent resting did not differ between forest types could indicate that resting is more constrained by changes in temperature between seasons (Korstjens et al. 2010) than changes in food availability between forest types because in Lacandona differences in mean daily temperature are higher between seasons than between forest types. More information concerning the potential effect of temperature on resting time of spider monkeys is necessary to evaluate this hypothesis.
Seasonal-Related Differences in Activity Patterns
Primate socioecological models indicate that time spider monkeys and other tropical primates spend resting is a function of seasonality, the percentage of leaves in the diet, and the annual mean temperature (Korstjens et al. 2006; 2010). Evidence indicates that as the percentage of leaves in the diet and the mean temperature increase, the resting time also increases (Dunbar et al. 2009; González-Zamora et al. submitted; Korstjens et al. 2010). In concurrence with this hypothesis, we found that time resting was higher during the dry season, when consumption of leaves was greater, and ambient temperature reached maximum values (Fig. 2 and González-Di Pierro et al. 2011).
Overall, independent of forest type, time spent feeding was higher during the rainy than during the dry season. Evidence suggests that seasonal increases in ambient temperature, such as that occurring during the dry season (ca. 2°C; González-Di Pierro et al. 2011), may stimulate primates to reduce heat-generating activities such as feeding to avoid thermal overload and its associated energetic costs (Dunbar et al. 2009; Korstjens et al. 2010). This explanation is consistent with several studies on spider monkeys (Chapman 1988; Korstjens et al. 2006). Felton et al. (2009) also found that time spent feeding by spider monkeys is higher during the rainy season. They interpret a greater feeding in the rainy season as a strategy for spider monkeys to take advantage of peak seasonal foods, allowing them to ingest surplus energy and store it as fat in preparation for the impending period of food scarcity. Our data partially support this possibility because we observed that time spent feeding on fruits and other plant items was slightly higher in the rainy season than in the dry season (Fig. 2). However, to determine the relative influence of each of these factors, more studies need to evaluate energetic strategies of spider monkeys.
In spite of some limitations of our study, e.g., we did not measure directly spatial and temporal food availability and ambient temperature within each site, we hope that the patterns we describe will serve as a stimulus for future studies. Although our findings indicate that spider monkeys in Lacandona adjust their activity patterns and diet to cope with food scarcity in forest fragments and during the dry season, it is not clear if this behavioral flexibility is large enough to ensure their health and persistence over time. More long-term behavioral studies of spider monkeys will help us improve our understanding of the behavioral responses of primates to environmental stresses imposed by fragmentation and seasonality.
References
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behavior, 49, 227–267.
Arroyo-Rodríguez, V., & Dias, P. A. (2010). Effects of habitat fragmentation and disturbance on howler monkeys: A review. American Journal of Primatology, 72, 1–16.
Arroyo-Rodríguez, V., & Mandujano, S. (2006). Forest fragmentation modifies habitat quality for Alouatta palliata. International Journal of Primatology, 27, 1079–1096.
Arroyo-Rodríguez, V., Mandujano, S., Benítez-Malvido, J., & Cuende-Fanton, C. (2007a). The influence of large tree density on howler monkey (Alouatta palliata mexicana) presence in very small rain forest fragments. Biotropica, 39, 760–766.
Arroyo-Rodriguez, V., Serio-Silva, J. C., Alamo-Garcia, J., & Ordano, M. (2007b). Exploring immature-to-mother social distances in Mexican mantled howler monkeys at Los Tuxtlas, Mexico. American Journal of Primatology, 69, 163–181.
Asensio, N., Korstjens, A. H., & Aureli, F. (2009). Fissioning minimizes ranging costs in spider monkeys: A multiple-level approach. Behavioral Ecology and Sociobiology, 63, 649–659.
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In L. K. Marsh (Ed.), Primates in fragments: Ecology and conservation (pp. 283–303). New York: Kluwer Academic/Plenum Press.
Boyle, S. A., & Smith, A. T. (2010). Behavioral modifications in northern bearded saki monkeys (Chiropotes satanas chiropotes) in forest fragments of central Amazonia. Primates, 51, 43–51.
Boyle, S. A., Lourenco, W. C., da Silva, L. R., & Smith, A. T. (2009). Travel and spatial patterns change when Chiropotes satanas chiropotes inhabit forest fragments. International Journal of Primatology, 30, 515–531.
Bronikowski, A. M., & Altmann, J. (1996). Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology, 39, 11–25.
Campos, F. A., & Fedigan, L. M. (2009). Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. American Journal of Physical Anthropology, 138, 101–111.
Chapman, C. A. (1988). Patterns of foraging and range use by three species of Neotropical primates. Primates, 29, 177–194.
Chapman, C. A., & Chapman, L. J. (1991). The foraging itinerary of spider monkeys: When to eat leaves? Folia Primatologica, 56, 162–166.
Chapman, C. A., & Chapman, L. J. (2000). Determinants of group size in primates: The importance of travel costs. In S. Boinski & P. A. Garber (Eds.), On the move: How and why animals travel in groups (pp. 24–42). Chicago: University of Chicago Press.
Chapman, C. A., Naughton-Treves, L., Lawes, M. J., Wasserman, M. D., & Gillespie, T. R. (2007). Population declines of colobus in Western Uganda and conservation value of forest fragments. International Journal of Primatology, 28, 513–528.
Chaves, O. M., Stoner, K. E., & Arroyo-Rodríguez, V. (2011). Differences in diet between spider monkey groups living in forest fragments and continuous forest in Lacandona, Mexico. Biotropica, doi:10.1111/j.1744-7429.2011.00766.x.
Clarke, M. R., Collins, A. D., & Zucker, E. L. (2002). Responses to deforestation in a group of mantled howlers (Alouatta palliata) in Costa Rica. International Journal of Primatology, 23, 365–381.
Crawley, M. (2002). Statistical computing: An introduction to data analysis using S-Plus. Chichester: John Wiley & Sons.
Cristóbal-Azkarate, J., & Arroyo-Rodríguez, V. (2007). Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: Effects of habitat fragmentation and implications for conservation. American Journal of Primatology, 69, 1013–1029.
De la Maza, J., & De la Maza. R. (1991). El ‘Monte Alto’: Esbozo de una región. In Agrupación Sierra Madre, Lacandonia, el último refugio (pp. 21–35). Distrito Federal, México: Universidad Nacional Autónoma de México.
Defler, T. R. (1995). The time budget of a group of wild woolly monkeys (Lagothrix Lagotricha). International Journal of Primatology, 16, 107–120.
Di Fiore, A., Link, A., & Dew, J. L. (2008). Diets of wild spider monkeys. In C. J. Campbell (Ed.), Spider monkeys: Behavior, ecology and evolution of the genus Ateles (pp. 81–137). New York: Cambridge University Press.
Di Fiore, A., Link, A., & Campbell, C. J. (2010). The atelines: Behavioral and socioecological diversity in a New World radiation. In C. J. Campbell, A. Fuentes, K. C. Mackinnon, M. Panger, & S. K. Bearder (Eds.), Primates in perspective (pp. 784–798). Oxford: Oxford University Press.
Dunbar, R. I. M. (1992). Time: A hidden constraint on the behavioural ecology of baboons. Behavioral Ecology and Sociobiology, 31, 35–49.
Dunbar, R. I. M., Korstjens, A. H., & Lehmann, J. (2009). Time as an ecological constraint. Biological Reviews, 84, 413–429.
Dunn, J. C., Cristóbal-Azkarate, J. C., & Vea, J. (2010). Seasonal variations in the diet and feeding effort of two groups of howler monkeys in different sized forest fragments. International Journal of Primatology, 31, 887–903.
Felton, A. M., Felton, A., Wood, J. F., Foley, W. J., Raubenheimer, D., Wallis, I. R., et al. (2009). Nutritional ecology of Ateles chamek in lowland Bolivia: How macronutrient balancing influences food choices. International Journal of Primatology, 30, 675–696.
Gentry, A. H. (1982). Patterns of neotropical plant species diversity. Evolutionary Biology, 15, 1–84.
Gómez-Pompa, A., & Dirzo, R. (1995). Atlas de las áreas naturales protegidas de México. Distrito Federal, México: CONABIO-INE.
González-Di Pierro, A. M., Benítez-Malvido, J., Méndez-Toribio, M., Zermeño, I., Arroyo-Rodríguez, V., & Stoner, K. E. (2011). Effects of the physical environment and primate gut passage on the early establishment of Ampelocera hottlei Standley in rainforest fragments. Biotropica, doi:10.1111/j.1744-7429.2010.00734.x.
González-Zamora, A., Arroyo-Rodríguez, V., Chaves, O. M., Sánchez-López, S., & Stoner, K. E. (in review). Influence of climatic variables, forest type and condition on activity patterns of Geoffroyi´s spider monkeys throughout Mesoamerica. American Journal of Primatology.
González-Zamora, A., Arroyo-Rodríguez, V., Chaves, O. M., Sánchez-López, S., Stoner, K. E., & Riba-Hernández, P. (2009). Diet of spider monkeys (Ateles geoffroyi) in Mesoamerica: Current knowledge and future directions. American Journal of Primatology, 71, 8–20.
Hemingway, C. A., & Bynum, N. (2005). The influence of seasonality on primate diet and ranging. In D. K. Brockman & C. P. van Schaick (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 57–104). Cambridge: Cambridge University Press.
Irwin, M. T. (2008a). Diademed sifaka (Propithecus diadema) ranging and habitat use in continuous and fragmented forest: Higher density but lower viability in fragments? Biotropica, 40, 231–240.
Irwin, M. T. (2008b). Feeding ecology of Propithecus diadema in forest fragments and continuous forest. International Journal of Primatology, 29, 95–115.
Iwamoto, T., & Dunbar, R. I. M. (1983). Thermoregulation, habitat quality and the behavioural ecology in gelada baboons. The Journal of Animal Ecology, 53, 357–366.
Juan, S., Estrada, A., & Coates-Estrada, R. (2000). Contrastes y similitudes en el uso de recursos y patrón general de actividades en tropas de monos aulladores (Alouatta palliata) en fragmentos de selva de Los Tuxtlas, México. Neotropical Primates, 8, 131–135.
Korstjens, A. H., Verhoeckx, I. L., & Dunbar, R. I. M. (2006). Time as a constraint on group size in spider monkeys. Behavioral Ecology and Sociobiology, 60, 683–694.
Korstjens, A. H., Lehman, J., & Dunbar, R. I. M. (2010). Resting time as an ecological constraint on primate biogeography. Animal Behaviour, 79, 361–374.
Lambert, J. E. (1998). Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evolutionary Anthropology, 7, 8–20.
Laurance, W. F., Laurance, S. G., Ferreira, L. V., Rankin-de Merona, J. M., Gascon, C., & Lovejoy, T. E. (1997). Biomass collapse in Amazonian forest fragments. Science, 278, 1117–1118.
López, G. O., Terborgh, J., & Ceballos, N. (2005). Food selection by a hyperdense population of red howler monkeys (Alouatta seniculus). Journal of Tropical Ecology, 21, 445–450.
Marquez-Rosano, C. (2006). Environmental policy and dynamics of territorial appropriation: The tensions between the conservation of tropical forests and the expansion of cattle ranching in the Mexican tropics. Retrieved from http://iasc2008.glos.ac.uk/conference%20papers/papers/M/Marquez-Rosano_110701.pdf.
Masi, S., Cipolletta, C., & Robbins, M. M. (2009). Western lowland gorillas (Gorilla gorilla gorilla) change their activity patterns in response to frugivory. American Journal of Primatology, 71, 91–100.
Milton, K. (1981a). Food choice and digestive strategies of two sympatric primate species. The American Naturalist, 117, 476–495.
Milton, K. (1981b). Distribution patterns of tropical plant foods as an evolutionary stimulus to primate mental development. American Anthropologist, 83, 534–548.
Murphy, P., & Lugo, A. E. (1995). Dry forests of Central America and the Caribbean. In S. H. Bullock, H. M. Mooney, & E. Medina (Eds.), Seasonally dry tropical forests (pp. 146–194). Cambridge: Cambridge University Press.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology, 21, 587–611.
Overdorff, D. J. (1996). Ecological correlates to activity and habitat use of two prosimian primates: Eulemur rubriventer and Eulemur fulvus rufus in Madagascar. American Journal of Primatology, 40, 327–342.
Rebecchini, L., Schaffner, C. M., Auleri, F., Vick, L., & Ramos-Fernández, G. (2008). The impact of hurricane Emily on the activity budget, diet and subgroup composition of wild spider monkeys (Ateles geoffroyi yucatanensis). In 22nd Congress of the International Primatological Society, Abstract Book, p. 147.
Silva, S. S. B., & Ferrari, S. F. (2009). Behavior patterns of southern bearded sakis (Chiropotes satanas) in the fragmented landscape of eastern Brazilian Amazonia. American Journal of Primatology, 71, 1–7.
Stelzner, J. K. (1988). Thermal effects on movement patterns of yellow baboons. Primates, 29, 91–105.
Stevenson, P. R. (2001). The relationship between fruit production and primate abundance in Neotropical communities. Biological Journal of the Linnean Society, 72, 161–178.
Stevenson, P. R., Quiñones, M. J., & Ahumada, J. A. (1994). Ecological strategies of woolly monkeys (Lagothrix lagotricha) at Tinigua National Park, Colombia. American Journal of Primatology, 32, 123–140.
Stoner, K. E., & Timm, R. M. (2004). Tropical dry-forest mammals of Palo Verde: Ecology and conservation in a changing landscape. In G. W. Frankie, A. Mata, & S. B. Vinson (Eds.), Biodiversity conservation in Costa Rica: Learning the lessons in a seasonal dry forest (pp. 48–66). Berkeley, CA: University of California Press.
Tutin, C. E. (1999). Fragmented living: Behavioural ecology of primates in a forest fragment in Lopé Reserve, Gabon. Primates, 40, 249–265.
Wallace, R. B. (2001). Diurnal activity budgets of black monkeys, Ateles chamek, in a southern Amazonian tropical forest. Neotropical Primates, 9, 101–107.
Watts, C. H. (1988). Environmental influences on mountain gorilla time budgets. American Journal of Primatology, 15, 195–211.
Zimmerman, J. K., Wright, S. J., Calderon, O., Pagan, M. A., & Paton, S. (2007). Flowering and fruiting phenologies of seasonal and aseasonal Neotropical forests: The role of annual changes in irradiance. Journal of Tropical Ecology, 23, 231–251.
Acknowledgments
This research was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONACyT grants CB2005-C01-51043 and CB2006-56799). Ó. M. Chaves obtained a scholarship from the Dirección General de Estudios de Posgrado, UNAM, as part of the Programa de Posgrado en Ciencias Biológicas and from Secretaría de Relaciones Exteriores (SRE) of Mexico. A postdoctoral fellowship awarded to V. Arroyo-Rodríguez by the Consejo Técnico de la Investigación Científica (UNAM) is gratefully acknowledged. The Instituto para la Conservación y el Desarrollo Sostenible, Costa Rica (INCODESO) provided logistical support. This study would not have been possible without the collaboration of the local people in Loma Bonita, Chajul, Reforma Agraria, and Zamora Pico de Oro Ejidos. We thank C. Hauglustaine, C. Balderas, K. Amato, S. Martínez, J. Herrera, and R. Lombera for field assistance. A. Estrada and J. Benítez-Malvido made useful suggestions during the design of this research. J. M. Lobato, G. Sánchez, H. Ferreira, and A. Palencia provided technical support. We also thank J. Rothman, J. Setchell, and 2 anonymous reviewers for valuable criticisms and suggestions that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Table SI
Importance value index (IVI) of the top food species for spider monkeys in the three study forest fragments within the Marqués de Comillas region, Lacandona, Chiapas, Mexico (DOC 55.0 kb)
Table SII
Values of the general linear model either excluding or including the larger fragment inhabited by the spider monkeys in Lacandona, Southern Mexico (DOC 36.5 kb)
Fig. S1
Percent of focal observations discarded in each forest type according to the activity pattern. The number of discarded observations is indicated above the bars (DOC 27.5 kb)
Rights and permissions
About this article
Cite this article
Chaves, Ó.M., Stoner, K.E. & Arroyo-Rodríguez, V. Seasonal Differences in Activity Patterns of Geoffroyi´s Spider Monkeys (Ateles geoffroyi) Living in Continuous and Fragmented Forests in Southern Mexico. Int J Primatol 32, 960–973 (2011). https://doi.org/10.1007/s10764-011-9515-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-011-9515-x