Abstract
The presence of other animals, both conspecifics and heterospecifics, is a major driving force for how animals organize themselves in space and time. Although theoretical models are available to explain the role of each in animal movement, fine-scale assessments of daily movement are scarce, particularly for primates. Hence, our goal was to assess whether and how the presence of conspecifics and heterospecifics influence spatiotemporal landscape use in two, wild, howler monkey (Alouatta guariba) groups. We followed the groups for 14 months in a large, continuous forest, during which we recorded their daily path length (DPL), home range, activity budget, feeding, and the presence of other groups (conspecifics) and other species (heterospecifics). The two groups differed in DPL, home range, proportion of fruits ingested, and time devoted to moving and resting. Partial least squares path modelling showed that variation in DPL was explained by the percentage of leaves or fruits ingested and by the presence of conspecifics, but not of heterospecifics. Group differences in several ecological variables emphasise the need to conduct further studies of space use with more groups in the same area to understand the underlying mechanisms of these differences. Moreover, our analysis shows that within-species interactions may be a stronger force in spatiotemporal organisation than interspecies interactions, at least in this folivorous primate. This is relevant from both a theoretical standpoint, and also when considering the consequences of habitat fragmentation and reduction. Deforestation leads to decreased resource availability and increased likelihood of encounters with conspecifics, which ultimately alters the proportion of food items ingested and increases the DPL, disrupting energy balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent advances in technology have given us a broad understanding of large and global scales of animal movement (Cooke et al., 2004; Nathan et al., 2022). Simultaneous tracking of animals and environmental data allowed the prediction of how animals respond to environmental changes (Courbin et al., 2014; Bestley et al., 2015; Nathan et al., 2022), how animals perceive the landscape (Janmaat et al., 2021), where do animals go while migrating (Hobson et al., 2019), and the requirements, avoidances, and motivations of animal movement (Nathan et al., 2022). However, primates, despite being well-known for many aspects of their behaviour, many species still lack fine-scale assessment of their daily movements, particularly because many species are elusive or rare or because of the inherent challenges of data collection (Kamilar & Beaudrot, 2013; Pinto et al., 2013; Sanz et al., 2022).
Resource distribution is one of the main external factors that influence animal movement. While leaves are assumed to be abundant and evenly distributed, young leaves may be spatially and temporally aggregated (Agetsuma, 1995; Harris & Chapman, 2007) and ripe fruits are even more so (Milton, 1979). Energetic and chemical composition differences influence the digestion of leaves, meaning that some species prefer young leaves and soft parts over mature leaves as the former are easier to digest or rich in protein (Matsuda et al., 2017). Moreover, because leaves are generally abundant and nonmonopoliseable, food competition is considered to be relaxed in folivores (Harris & Chapman, 2007; Isbell, 1991), with scramble competition for food predominating (Isbell, 1991). However, many dietary specialists (e.g., folivores) also complement their diet with other food sources (e.g., fruits) (Bonvicino, 1989; dos Santos-Barnett et al., 2022; Snaith & Chapman, 2007). Hence, animals travel to find good food patches or items (Cunningham & Janson, 2007) aiming to maximize energy intake while minimizing the costs of travelling (Pyke, 2019).
Interactions with conspecifics or heterospecifics also influence animal movement. Animals may move in response to physical (Sanz et al., 2022), visual (Markham et al., 2012; Bonadonna et al., 2020) and acoustic cues and signals (Kurihara & Muto, 2021; Van Belle & Estrada, 2020) from other individuals, other groups, or other species. Although some species benefit from mixed-species aggregations to avoid predators or to increase foraging efficiency (e.g., mixed-species troops of callitrichine primates: Heymann & Buchanan-Smith, 2000), other interactions between individuals or between species may be adverse. Adverse interactions comprise the well-known predator-prey relationship (Di Bitetti et al., 2009; Schoener, 1974; Sih, 2005; Singh et al., 2000) or foraging competition. Food competition is a major cost of group living among social animals (Janson, 1985) and can also occur between sympatric species (Sobroza et al., 2021). Individuals or species that compete frequently may have to forage for longer or travel further (Harris & Chapman, 2007; Isbell, 1991; Janson & Van Schaik, 1988).
Howler monkeys (genus Alouatta) are an excellent model for addressing the influence of intra- and interspecific interactions on movement. Many howler populations live at high densities (Chiarello, 1992; Bravo & Sallenave, 2003; Kowalewski, 2007; Goméz-Posada & Londoño, 2012), and they may increase their daily range in response to intergroup encounters (Agostini et al., 2010a; Ceccarelli et al., 2019; Raño et al., 2016). The genus is characterised by intragroup scramble competition for food (Behie et al., 2010; Isbell, 1991). Such scramble competition is difficult to measure so measures of spatiotemporal use of the landscape, such as daily path length (DPL) and moving/feeding time, often are used as proxies (Isbell, 1991; Raño et al., 2016). To reduce competition, animals may increase either feeding time or their daily travel movements (Harris & Chapman, 2007; Isbell, 1991; Janson & Van Schaik, 1988). For instance, DPL increases in response to a greater proportion of mature fruits ingested or decreasing with a greater proportion of mature leaves ingested (Raño et al., 2016). In contrast, similar values in DPL and activity budget among groups may indicate similar levels of competition or similar habitat quality (Jung et al., 2015; Schreier et al., 2021), whereas the presence of heterospecifics does not necessarily influence DPL due to other mechanisms, such as niche partitioning as observed among sympatric strepsirrhines (Bersacola et al., 2015).
Howler monkeys also have a wide geographic range (Cortés-Ortiz et al., 2003), frequently overlapping in distribution with other primate species (Cristóbal-Azkarate et al., 2015). A series of studies of two sympatric howler species found no evidence that interspecific interaction influenced daily range (Agostini et al., 2010a, 2010b; Agostini et al., 2012). Other studies addressing heterospecific interactions show that these interactions can include physical aggression (e.g., capuchins, Falótico et al., 2021) or in howlers being chased or result in displacing howlers from their feeding trees (Mendes, 1989; Dias & Strier, 2000; Rose et al., 2003; Martins, 2008), potentially influencing their DPL.
Brown howler monkeys (Alouatta guariba) are one of the 25 most threatened primate species in the world (Buss et al., 2019; Oklander et al., 2022) following a yellow-fever outbreak, which increased their threat level from Least Concern to Vulnerable (Jerusalinsky et al., 2020). Our focal population in Carlos Botelho State Park (PECB), São Paulo, Brazil, is a low-density population (González-Solís et al., 2001), which inhabits one of the few, large, continuous, protected remnants of the Atlantic Forest (approx. 38,000 ha) (Rosa et al., 2021). These two characteristics are unlike the majority of studies conducted with Alouatta, because most studies have been conducted with high-density populations (Chiarello, 1992; Bravo & Sallenave, 2003; Kowalewski, 2007; Goméz-Posada & Londoño, 2012) inhabiting small, fragmented, and altered landscapes (Chaves et al., 2019). Studies of activity budget and space use conducted in these small fragments may not show the full extent of variation in this species. Moreover, PECB howlers live in sympatry with two primate species: the southern muriqui (Brachyteles arachnoides) and the capuchin monkey (Sapajus nigritus). All three species consume local fruits (B. arachnoides: Carvalho Jr. et al., 2004; S. nigritus: Izar et al., 2012), a food resource of high-energetic value and important in food competition (Caillaud et al., 2010; Chapman, 1990; Chapman et al., 1995; Strier, 1989). In terms of habitat quality, reduction in forest size has several direct and indirect implications for species’ movement. The cascade of consequences include increased travel distances, blocked migration, increased population density, reduced resource availability, and increased competition (Knowlton & Graham, 2010).
We tested the hypothesis that changes in activity budget and DPL in these howlers will be due to the presence of conspecifics or heterospecifics. If this is the case, then we predict that groups will move further and for a longer time when encounters are more frequent.
Methods
Study Area
We conducted our study in Carlos Botelho State Park (PECB), São Paulo, Brazil. The park is approximately 38,000 ha. Together with other public and private-owned parks, it forms part of the Serra de Paranapiacaba Ecological Continuum, an area of some 250,000 ha of Atlantic Forest, and one of the largest surviving remnants of this highly-threatened domain (Pisciotta, 2002; Ribeiro et al., 2009).
The park is considered a nonseasonal ecosystem in terms of rainfall and temperature but has marked fruiting seasons (Morellato et al., 2000). Rainfall at the study site is more abundant in the summer, with lower amounts during winter, without a real dry period (Plano de Manejo, 2015). The mean annual temperature varies between 15 °C in winter and 24 °C in summer, with the lowest temperature (1.8 °C) recorded in July and the highest (35 °C) in December (Instituto Nacional de Meteorologia, INMET, data). Elevation varies between 700 and 850 m a.s.l., and the entire study area is in mountainous terrain where the hilltops are composed of montane and altimontane dense vegetation and lowlands are composed of ombrophilous forest (Plano de Manejo, 2015).
Study Groups
In the study region, we identified six groups individually based on their unique characteristics, such as face shape, natural markings, scars, and depigmentation. We monitored two of these groups (G3 and G4) between November 2017 and December 2018. We monitored G1 for 17 days during March, November, and December 2017. We followed a fourth group (G6) for one full day and counted individuals and recorded GPS location whenever we saw other groups.
We followed G3 and G4 between dawn and sunset for 4 to 6 days per month (1,361 h of total observation, 640 h for G3, 570 h for G4, 141 h for G1, and 10 h for G6). We provide data from 52 full-day follows for G3 and 48 full-day follows for G4, disregarding partial day-follows.
G3 and G4 were of similar size and composition. G3 was composed of one adult male, two adult females (one with a dependent infant), and two subadult males of unknown kinship. G4 was composed of one adult male, two adult females, and their offspring and one subadult male of unknown kinship. Moreover, G3 inhabited an area at the park boundary and its movement was limited by open grasslands, roads, and houses. Canopy was either naturally connected or artificially connected with canopy bridges. G3 lived close to the park headquarters and had daily contact with humans. Conversely, G4 occupied an area approximately 3 km from the headquarters, accessible only to researchers.
Home Range and Daily Path Length
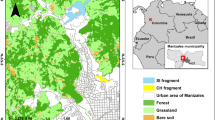
While we followed the groups, a GPS collected 260 location records per day. From these points, we selected a subset of locations every 20 minutes if the groups were moving or once an hour if the groups were resting, because they could spend several hours without moving. Whenever we detected nontarget groups, we took the GPS location and identified individuals. We used these points to create a home-range map for each group, connecting the outermost GPS points with QGIS. Hence, we created polygons for graphic representation (Fig. 3), but this does not represent their true home range.
Using the GPS points, we calculated DPL by summing the linear distances between the GPS interval points (Raño et al., 2016). We also stimated home range (95%) and core area (50%) for G3 and G4 based on Minimum Convex Polygon method (MCP, Worton, 1995) and the Kernel Density Estimate (KDE, Powell, 2000; Kernohan et al., 2001). For KDE, we used an ad hoc method for choosing the smoothing parameter (“href”), with metres as the unit in and hectares (ha) as the unit out. We provide both estimates to allow for comparison with previous works, which used MCP only while also providing the most widely used home-range estimation methods, KDE. We calculated areas with the mcp.area and kernelUD functions in the adeHabitatHR package (R Core Team, 2019). Although we have enough data to calculate G1’s home range, two individuals were not habituated, potentially altering group behaviour and their DPL so opted to not include this group in our study. However, because we followed G1, G3, and G4 consistently, we are confident enough to state whether two or more groups shared the same areas. We considered home ranges to overlap if two or more groups shared some GPS locations, that is, if we observed two or more groups in the same areas.
Activity Budget
We calculated the activity budget for G3 and G4 using scan sampling (Altmann, 1974). Every 20 minutes, we recorded the following categories for each individual during a 2-min window: foraging (feeding, manipulating, and ingesting food or drinking water), moving (movement in any direction and speed), resting (immobile or sleeping), socialising (play, grooming, and any other social interactions between individuals), and others (any behaviour that did not fit within these categories). We summed all cases in which visible individuals were involved in a given behaviour and divided this by the number of individuals visible to obtain the proportion of each behaviour during the scan sample. Then, we took the mean of the proportions for each scan sample for the whole day to obtain daily proportions of each behaviour and monthly proportions of each behaviour. The groups were small and cohesive, so it was unlikely that we missed an individual in a scan. In total, we recorded 3,656 individual activities for G3 and 4,656 for G4.
Feeding Bouts
To understand whether the presence of conspecifics or heterospecifics influenced the amount of leaves and fruits ingested, we recorded a feeding bout whenever more than 50% of scanned individuals were foraging during a scan sample, identified the plant part consumed (if possible), and recorded the GPS location, similar to Bryson-Morrison et al. (2017) and Back & Bicca-Marques (2019). We then calculated daily percentages of fruits or leaves consumed, including records when we could not identify the food item in the total.
Presence of Conspecifics or Heterospecifics
Because the muriquis and capuchins have been studied over the past 20 years, many howler monkey groups are habituated to human presence, allowing us to witness interspecific encounters in detail. We recorded audible loud calls emitted both by neighbouring groups and by the group we were following. We considered howling bouts independent when calling had ceased for ten consecutive minutes between two calling bouts (Van Belle et al., 2013). Loud calls could occur during intergroup encounters, (i.e., when two groups saw each other). We recorded the presence of conspecifics daily, including both audible vocalisation bouts and visual intergroup encounters.
We used the same rationale for the presence of heterospecifics, including both audible vocalizations and visual encounters of other species. There are no standard methods for recording interspecific encounters (Falótico et al., 2021), so we recorded heterospecific encounters as events, regardless of the number of individuals involved, and the duration of the interaction (Scarry, 2013). Encounters with the same species on the same day were rare, usually occurring between 30 minutes and 2 hours apart. We considered each encounter as an independent event and identified the outcome for howler monkeys.
Rainfall, Temperature, and Season
We obtained daily rainfall data from the park pluviometric database made available to us by the manager. The National Institute of Meteorology (INMET) provided hourly temperature data. Because the region does not have a dry period, we divided the seasons by combining water surplus (Walter, 1973) and peak fruit availability (Talebi et al., 2005). A water surplus occurs when the rainfall exceeds twice the mean maximum temperature in degrees Celsius (20.4 °C). Thus, we considered the period between October and March as the wet-nonfruiting season and April to September as the dry-fruiting season (Fig. 1).
Graph showing the accumulated rainfall (black bars, mm) and the mean monthly temperature (grey line, °C) for each month of 2018, in Carlos Botelho State Park (PECB), São Paulo, Brazil, combined with fruiting and non-fruiting season according to Talebi et al. (2005).
Statistical Analysis
We tested data for normality and, because it was nonnormally distributed, we used Kruskal Wallis tests with Dunn a posteriori tests to test for differences between groups in home range, DPL, and percentages in each activity budget behaviour.
To evaluate the direct and indirect effects of season, group identity (group ID), activity budget, feeding bouts, and presence of heterospecifics and conspecifics on DPL, we used partial least squares path modelling (PLS) based on the plspm package (Sanchez, 2013) in the R software (R Core Team, 2019). Our dataset for these analyses consisted of 90 daily values. We converted season into factors, with 1 being wet-nonfruiting and 2 being dry-fruiting. We used the DHARMa package (Hartig, 2017) to test the model’s residual distribution and assessed the variance inflation factor (VIF) to test for model inflation. All VIF values were <5. Because the activity budget behaviours were highly correlated among each other as well as with the consumption of leaves and fruits (assessed via ggpairs package in R), we included only resting and the percentage of fruits or leaves ingested during the feeding bouts in our model.
We built two models: one with the percentage of feeding bouts where fruits were ingested (fruit model), and one for leaves (leaf model) (Fig. 2a). Specifically, 0 means no influence and 1 means that we expect an effect from the row above to the row below (Fig. 2b). Given expected direction of effects, season cannot influence group ID, so the effect is 0. However, season can influence the presence of conspecifics and heterospecifics, activity (moving, feeding, or resting), and resource availability (leaves or fruits), so we assigned 1 to each of these. Because we detected differences across groups in the presence of heterospecifics and conspecifics, we expected that group ID would influence these variables and assigned 1 to these relationships. Finally, because our initial hypothesis was that season, group ID, activity budget, feeding bouts, and presence of heterospecifics and conspecifics influence DPL, the bottom row is composed of ones (Fig. 2b). We counted encounters with each species separately, but they were analysed together into a single block called “presence of heterospecifics.” The combination of variables into blocks is a requirement for package use (Sanchez, 2013) without inflating the model. We based effect estimation on 200 bootstraps resampling and the explanatory power of the models is provided by the Goodness of Fit (GoF). All statistical analyses used software R v.4.0.3. We considered p < 0.05 as significant.
a Path diagram depicting our model tested with partial least square modelling (path analysis) to assess the effects of these variables on daily path length (DPL) for brown howler monkeys (Alouatta guariba) in Carlos Botelho State Park (PECB), São Paulo, Brazil, between 2017 and 2018. Dark grey boxes are the variables, light grey boxes are the blocks, and white dashed box is the assessed variable. b Expected direction of effects. We included the percentage of leaves or fruits recorded in feeding scans separately in each model. Zero predicts no influence of a row on the row below and 1 predicts an influence of a row on the row below. Group ID: group identity. % Leaves or Fruits: percentage of feeding bouts in which leaves or fruits were the food item being consumed. DPL: daily path length.
Ethical Note
This research was approved by the Ethics Committee on Animal Use of the School of Veterinary Medicine and Animal Science (University of São Paulo), under protocol number 4864040618. We have no conflicts of interest to declare.
Data Availability
Made available under reasonable request.
Results
Group Differences in Space Use and Activity Budget
Howlers were exclusively arboreal, except for a single day on which we recorded one group using the forest floor to escape from an intergroup encounter that happened at their home range border. We never saw two groups using the same area. Mean DPL was significantly shorter for G3 than for G4 (Table I). G3’s home range and core home range were much smaller than those of G4 (Fig. 3; Table I). The 95% Kernel estimate was larger than that using MCP.
Park location and home range maps of the six brown howler monkey (Alouatta guariba) groups known to live in Carlos Botelho State Park, Brazil, between 2017 and 2019. Colors show each group’s home range. Polygons for G3 (green) and G4 (orange) were estimated based on 14 months of survey (15.0 ha and 41.3 ha, respectively). Home ranges for other groups do not represent exact values. White bold line shows the park edge. Explosion symbols show intergroup encounters. Monkey symbols show individuals that we saw but could not identify or assign to any of the known groups.
In terms of activity budget, howler monkeys spent most of their time resting (53.3%), followed by foraging (22.5%), moving (13.7%), social (4.6%), and other behaviours (4.5%). However, only moving and resting were statistically different between groups (Table I).
We recorded 1,234 feeding bouts (548 for G3 and 686 for G4), and recorded the item eaten for 70% (864) of bouts (73%, n = 401 for G3 and 67%, n = 463 for G4) (Table I). Leaves were more commonly consumed than fruits in G3, but not G4. We also recorded occasional feeding on flowers (n = 2 feeding bouts) and gum (n = 7), and we observed howlers drinking water (n = 17) from bromeliads and tree holes.
Presence of Conspecifics and Heterospecifics
We recorded only eight intergroup encounters but 116 neighbouring group vocalisations (total 124), with a rate of 1.17 calls per day. Vocalizations heard from neighbouring groups were similar for the two groups (20 for G3 and 22 for G4), although G3 howled more than G4 (n = 57 and 17, respectively). Considering the number of full-day follows, the rate of intergroup interactions per day were 0.38 for G3 and 0.47 for G4.
We recorded 63 encounters with other species, with muriquis (n = 45) being encountered more frequently than capuchins (n = 18). Combined, these two species were present 0.63 times per day. During visual encounters, howlers were chased 22 times, remained alert 9 times, escaped with no signs of chase 15 times, did not change their behaviour in 16, and escalated to physical interactions in three. They encountered the two other species simultaneously twice. The maximum presence recorded per day was three for each species. Of these, we could relate 50 heterospecific and 59 conspecifics to a GPS track and included these encounters in the path analysis.
Path Analysis
Our leaf model explained 43% of the variation in DPL (Fig. 4b). Group ID influenced the number of records for heterospecifics, resting, proportion of feeding bouts in which leaves were ingested and DPL. Season had a negative influence on the percentage of leaves consumed in feeding bouts (fewer leaves ingested in the dry-fruiting season). Season also had a negative effect on DPL (shorter distance travelled during the dry-fruiting season), the presence of heterospecifics recorded (fewer records during the dry-fruiting season), and the presence of conspecifics (fewer records during the dry-fruiting season). The percentage of leaves ingested also positively impacted DPL. Finally, the presence of conspecifics had a positive influence on the percentage of feeding bouts, including leaves and on the DPL. The presence of heterospecifics was not a significant explanator of DPL.
Representative scheme of a leaf model (a) and fruit model (b) of two groups of brown howler monkeys (Alouatta guariba) in Carlos Botelho State Park, São Paulo, Brazil, between 2017 and 2018. Figures show the direct and significant effects obtained via partial least square analysis of daily path length (DPL). We based effect estimation on 200 bootstraps resampling. The model explained 43% of the variation in DPL. Black boxes show the variable assessed (DPL), grey boxes are the blocks included in the model and white boxes are variables that did not have significant relationships with any other variables.
In the fruit model, explanatory power was smaller (37%). The significant effects and directions were similar to those of the leaf model (Fig. 4a and b). However, season had an opposite influence on fruits than on leaves (more fruits in the dry-fruiting season). The most striking difference between the two modes was the negative influence of the percentage of fruits on DPL (the more fruits ingested, the shorter the DPL). The presence of heterospecifics was not significant in explaining DPL.
Discussion
Our results partially support our hypothesis as the presence of conspecifics did alter daily movement, but the presence of other species did not. Space use varied widely between the two main study groups, and although we cannot draw strong conclusions based on this comparison, we discuss some group differences below.
Home Range
G3’s home range falls within the size typically reported for the genus (average 22 ha for several howler monkey species; Fortes et al., 2015), whereas G4 showed one of the highest values ever reported for the genus (exceptions include two A. guariba groups from Argentina, with home ranges varying between 47.4 ha and 70.3 ha; Agostini, 2009). One-third of studies of Alouatta guariba have been conducted in fragments smaller than the home range found in the present work (Bicca-Marques, 2003; Fortes et al., 2015).
Varying home range sizes, as observed in our study, are common in primates (e.g., Cercopithecinae, Gibson & Koenig, 2012; Sha & Hanya, 2013; other Platyrrhini, Alba-Mejia et al., 2013). Explanations for such differences include group size, predation risk, food resources, and neighbouring groups (Fortes et al., 2015). Our groups did not vary in size. We did not assess predation risk, although we witnessed distress in response to raptors (mantled hawk Pseudastur polionotus) in G4 twice. We also did not analyse habitat quality or record phenology. However, food resources likely differ between the two groups’ home ranges. G3’s home range seems to have more fruit trees than that of G4, a suggestion that is supported by the data showing that G3 consumed more fruits than G4. The home ranges of the two groups also differed in landscape features, with G3 home range restricted by the forest edge and open grasslands, and composed of secondary forest, a habitat known for its lower quality (Ries & Sisk, 2004). Moreover, G4’s home range had more hilly areas, which could also affect its quality (Jung et al., 2015). Hence, G4’s home range may be both larger and lower quality. However, because we lack key data to clearly understand the factors underlying the difference in home range, this remains an open question.
The presence of neighbouring groups may also influence home range size. Although we recorded similar frequencies of vocalization from neighbouring groups for both focal groups, G3 vocalized three times more than G4. Moreover, encounters between neighbouring groups were rare, and there was no overlap in home ranges between any adjacent groups. These findings indicate that PECB howler monkeys might be territorial (Powell, 2000), a definition not regularly applied to howler monkeys due to the recurrent broad overlap between neighbouring groups (Cornick & Markowitz, 2002). The broad overlap frequently observed for the genus may be related to the small fragments in which they were studied, preventing groups from avoiding each other and leading to a large overlap in home ranges (Knowlton & Graham, 2010).
The presence of heterospecifics is not commonly reported as a mechanism influencing home range but spatial avoidance/segregation among sympatric species is a frequent outcome of interspecific competition (Houle et al., 2010). G4 had 2.5 times more encounters with other species than G3. Subordinate species, as seems to be the case of howlers (see below), usually avoid dominant ones (Creel & Creel, 1995). We need more spatial distribution estimates from multiple groups to assess whether spatial avoidance/segregation in response to conspecifics and heterospecifics influences home range.
Activity Budget and Feeding
The howlers activity budget matches those reported in the literature: howler monkeys spend a large proportion of their daytime resting (45-65% for A. guariba, Agostini et al., 2010a; Jung et al., 2015; Ferreguetti et al., 2020; or 64.7% for A. caraya, Rímoli et al., 2012). The large time spent resting is proposed as an energy optimisation strategy, because leaves provide a low energetic reward (Di Fiore et al., 2011; Milton, 1998). Moreover, mature leaves and unripe fruits are difficult to digest (Amato et al., 2015), requiring longer digestion time (Rosenberger & Strier, 1989) and consequently, a greater amount of time devoted to resting.
The two groups differed significantly in their activity budget, with G3 resting more and G4 moving more. Differences in locomotor behaviour (such as moving) can reflect distinct habitat structures (Prates & Bicca-Marques, 2008) or distinct levels of scramble competition (Clutton-Brock et al., 1997; Clutton-Brock & Harvey, 1977; Isbell, 1991). We also witnessed more aggression in G3 than in G4 (unpublished data). Given these differences, it is possible that G3 faces more contest competition (Isbell, 1991; Vogel & Janson, 2007), whereas G4 faces more scramble-like competition.
PECB howlers spent more feeding bouts eating leaves than fruits, a pattern already described for this folivorous primate (Agostini et al., 2010b; Miranda & Passos, 2004). Changing from a highly folivorous to a highly frugivorous diet is one explanation for why howlers are resistant to habitat fragmentation and reduction (Bicca-Marques, 2003; Bicca-Marques et al., 2020). Other primate species in PECB, such as the muriquis, tend to be more frugivorous than conspecifics inhabiting more fragmented forests (Carvalho Jr. et al., 2004; Talebi et al., 2005). We also found marked differences between groups in the proportion of leaves and fruits ingested, and we suggest future studies investigate these differences between neighbouring groups in a large continuous forest.
Daily Path Length
We found that G4 group not only spent more time moving but also moved greater distances. Such differences in ranging patterns between groups are important proxies for food competition (Clutton-Brock & Harvey, 1977; Isbell, 1991), meaning that G4 is potentially facing greater food competition compared to G3. Greater food competition is commonly found in larger group sizes (Isbell, 1991). However, both groups were the same size, so the observed variation in DPL can derive not from intragroup competition, but from intergroup competition or habitat qualities and resourced availability.
Overall mean DPL values were similar to those of other brown howler populations (Agostini et al., 2010a; Fortes et al., 2015). DPL is not very variable in Alouatta and seems to be independent of fragment size (Bicca-Marques, 2003). Colobus monkeys, an even more folivorous primate genus than howler monkeys, has a DPL that barely exceeds 450 m (Dasilva, 1992; Teichroeb & Sicotte, 2009). Highly folivorous animals show strategies to optimise digestion, either by reducing their activity time or their daily path length (Hladik, 1978).
Path analysis detected the influence of eating leaves and fruits on DPL, although these variables acted in opposite ways, with fruits negatively influencing DPL and leaves positively influencing it. This contrast may result from leaves requiring longer processing times than fruits, reducing DPL (Aristizabal et al., 2016; Nagy & Milton, 1979; Reynoso-Cruz et al., 2016). Similarly, large fruit patches may result in animals staying at those trees, without needing to travel further distances to find other patches (Jung et al., 2015; Strier, 1987). Considering that G3’s home range is influenced by edge effect, we can expect differences in habitat quality and structure (Ries & Sisk, 2004). Edge effects decrease the number of tree species and alter tree community composition (Benítez-Malvido & Martínez-Ramos, 2003). Larger trees with greater amounts of fruits and leaves are more common in forest interiors than at the edge (Chapman et al., 1992). Although edge effects may reduce the absolute number of fruits produced because large trees are absent, young leaves that are of high nutritional value and easy to digest are more common (Arroyo-Rodríguez & Mandujano, 2006). This trade-off seems interesting for a species like howler monkeys (Bolt et al., 2021) and might apply to G3, but further studies evaluating habitat quality and diet are needed in this population. If available food has a lower energetic return or is in smaller quantity, primates may reduce their daily range (energy minimizing strategy; Milton, 1980) or increase their daily range in search of higher quality food items (energy maximizing strategy; Terborgh, 2014).
Presence of Conspecifics
We recorded 116 calls during our study, with a rate of 1.17 calls per day, whereas intraspecific encounter rates were low (n = 8 in 100 days). Intergroup encounter rates were much lower in PECB population than reported for Alouatta pigra (neighbours meet once every 3 days; Van Belle et al., 2013) or A. caraya (5.7 encounters per hour; Kowalewski, 2007), the species with the highest encounter rates reported. One study found a high correlation between roaring sessions and intergroup encounters in our study species (Cunha & Jalles-Filho, 2007). Although visual encounters were rare, path analysis suggested that these calls were enough to cause variation in DPL.
Vocalisation is a striking feature of howler monkeys, with many species showing a “morning choir” (e.g., Alouatta seniculus, Sekulic, 1982). Resource defence is the most strongly supported hypothesis to explain such behaviour (de Cunha et al., 2015; Kitchen et al., 2015). We did not observe morning or dawn chorus in the PECB howlers, although we followed from before dusk and after dawn to ensure that animals were no longer moving. This routine also required us to use a thermal sensor to detect animals at pitch-black night, even detecting some nocturnal behaviour in this population, but never howling bouts (Sobral et al., 2022). Additionally, most of G3’s territory was near the dorms we slept in, so we would have heard calls if they happened.
G4 had several neighbours, unlike G3, and the presence of a greater number of neighbouring groups has the potential to increase scramble competition (Müller & Manser, 2007). However, G4 vocalised less than G3. Reduced aggression has also been observed among neighbours, the so-called “dear enemy effect” (Temeles, 1994). The population density at PECB appears to be lower than in other sites, and previous surveys did not find howler monkeys (González-Solís et al., 2001). We could not easily find groups during our study (even leading to an unsampled month). However, our population’s average group size and home range (six individuals in 31 ha) suggests a density of 1.86 ind/km2. This value is much lower than that found in island populations of A. guariba (1 group/km2, Ferreguetti et al., 2020) or southern South America (10 ind/km2, Agostini et al., 2010a). Given that local population density is low, intergroup encounters were rare and that home range overlap was minimal, we conclude that vocalizations alone can significantly influence DPL.
Presence of Heterospecifics
PECB howlers coexist with muriquis, large folivore-frugivore primates (Carvalho Jr. et al., 2004; Talebi et al., 2005) and black capuchins, medium-sized primates that feed on insects, leaves and fruits (Izar et al., 2012). Encounters with other species were common, particularly for the group from the forest interior (G4) and interactions with muriquis were more common than with black capuchins. Encounter rates per day (0.63/day) are still lower than encounters between neighbouring groups of any Alouatta population (Cunha & Jalles-Filho, 2007; Kowalewski, 2007; Van Belle et al., 2013).
In 75% of heterospecific encounters, the behaviour of howlers was disrupted by being displaced from their feeding/sleeping site, remaining alert or chasing. On three occasions, interactions became physical, including one long event with a muriqui group, although muriquis are called a friendly primate (Gross, 2017). PECB howlers never won (Cristóbal-Azkarate et al., 2015) these interactions, and both capuchins and muriquis seem to be dominant over howlers. Dominant species may limit the population growth of subordinate species (Rowley & Christian, 1976). Although howlers are considered specialized folivores, fruits are their preferred food (Behie & Pavelka, 2015). Encounters with syntopic species commonly derive from—or result in—competition for food, leading to reduced fitness for competitors, especially for losers (Janson, 1985; Pruetz & Isbell, 2000). Despite this disruption, path analysis did not detect the influence of heterospecific presence on DPL, supporting the pattern observed in earlier studies (Agostini et al., 2010a, 2010b, 2012).
Our analyses show the intricate effect of conspecifics and heterospecifics on ranging behaviour. Species interactions—and groups—vary in space and time (Laundré et al., 2010; Prugh et al., 2019). These interactions influence foraging success, with profound fitness consequences (survival, growth, and reproduction), possibly altering the behaviour and ecology of a species in a given community (Goodale et al., 2017). Because we detected such an influence in a continuous forest with low population density, stronger effects may be expected in smaller and more fragmented areas.
Conclusions
The two groups of howlers we studied were the same size, suggesting that the differences we observed in activity and ranging behaviour do not derive from group size. Our modelling revealed that the presence of conspecifics alters DPL but did not detect an influence of heterospecific presence on DPL, even though howlers frequently altered their behaviour after encountering other primate species. This suggests that intraspecific interactions influence ranging behaviours of howler monkeys more strongly than the presence of heteropecifics. Our two study groups exemplify two scenarios: one group (G4) lives in a large home range in the forest interior with primary forest (but an abundance of hillside habitats); their home range is not restrained in any direction; they have less contact with humans and more encounters with other species and more neighbouring groups. The other group (G3) resides in a secondary forest area at the edge of the forest, with frequent contact with humans and fewer encounters with other species and other groups but more frequent conspecific vocalizations. It is difficult to pinpoint which factor influences ranging behaviour the most. Small, fragmented, or highly anthropized environments can increase inter and intraspecific competition in howlers, and our study adds to the growing body of literature that shows how these interactions can disrupt their behaviour. Because moving is energetically costly, factors that lead to excessive movement may cause an energetic imbalance, compromising health and reproduction. Our study brings light to the intricate relationship that multiple species and multiple groups have on one another’s behaviour.
References
Agetsuma, N. (1995). Foraging strategies of Yakushima macaques (Macaca fuscata yakui). International Journal of Primatology, 16(4), 595–609.
Agostini, I. (2009). Ecology and behavior of two howler monkey species (Alouatta guariba clamitans and Alouatta caraya) living in sympatry in northeastern Argentina. PhD dissertation, Università degli Studi di Roma “La Sapienza”, Rome, Italy.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2010a). Ranging patterns of two syntopic howler monkey species (Alouatta guariba and A. caraya) in Northeastern Argentina. International Journal of Primatology, 31, 363–381. https://doi.org/10.1007/s10764-010-9390-x.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2010b). Are howler monkey species ecologically equivalent? Trophic niche overlap in syntopic Alouatta guariba clamitans and Alouatta caraya. American Journal of Primatology, 72(2), 173–186. https://doi.org/10.1002/ajp.20775.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2012). Influence of seasonality, group size, and presence of a congener on activity patterns of howler monkeys. Journal of Mammalogy, 93(3), 645–657. https://doi.org/10.1644/11-MAMM-A-070.1.
Alba-Mejia, L., Caillaud, D., Montenegro, O. L., Sánchez-Palomino, P., & Crofoot, M. C. (2013). Spatiotemporal interactions among three neighboring groups of free-ranging white-footed tamarins (Saguinus leucopus) in Colombia. International Journal of Primatology, 34(6), 1281–1297. https://doi.org/10.1007/s10764-013-9740-6.
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–267.
Amato, K. R., Leigh, S. R., Kent, A., Mackie, R. I., Yeoman, C. J., Stumpf, R. M., ... & Garber, P. A. (2015). The gut microbiota appears to compensate for seasonal diet variation in the wild black howler monkey (Alouatta pigra). Microbial Ecology, 69(2), 434-443.
Aristizabal, J. F., Rothman, J. M., García-Fería, L. M., & Serio-Silva, J. C. (2016). Contrasting time-based and weight-based estimates of protein and energy intake of black howler monkeys (Alouatta pigra). American Journal of Primatology, 79(4), e22611. https://doi.org/10.1002/ajp.22611.
Arroyo-Rodríguez, V., & Mandujano, S. (2006). Forest fragmentation modifies habitat quality for Alouatta palliata. International Journal of Primatology, 27(4), 1079–1096.
Back, J. P., & Bicca-Marques, J. C. (2019). Supplemented howler monkeys eat less wild fruits, but do not change their activity budgets. American Journal of Primatology, 81(9), e23051. https://doi.org/10.1002/ajp.23051.
Behie, A. M., & Pavelka, M. S. (2015). Fruit as a key factor in howler monkey population density: conservation implications. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys Behavior, Ecology, and Conservation (pp. 357–382). Springer.
Behie, A. M., Pavelka, M. S., & Chapman, C. A. (2010). Sources of variation in fecal cortisol levels in howler monkeys in Belize. American Journal of Primatology, 72(7), 600–606. https://doi.org/10.1002/ajp.20813.
Benítez-Malvido, J., & Martínez-Ramos, M. (2003). Impact of forest fragmentation on understory plant species richness in Amazonia. Conservation Biology, 17(2), 389–400.
Bersacola, E., Svensson, M. S., & Bearder, S. K. (2015). Niche partitioning and environmental factors affecting abundance of strepsirrhines in Angola. American Journal of Primatology, 77(11), 1179–1192. https://doi.org/10.1002/ajp.22457.
Bestley, S., Jonsen, I. D., Hindell, M. A., Harcourt, R. G., & Gales, N. J. (2015). Taking animal tracking to new depths: synthesizing horizontal–vertical movement relationships for four marine predators. Ecology, 96(2), 417–427. https://doi.org/10.1890/14-0469.1
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In M. K. Larsh (Ed.), Primates in Fragments: Ecology and Conservation (pp. 283–303). Springer.
Bicca-Marques, J. C., Chaves, Ó. M., & Hass, G. P. (2020). Howler monkey tolerance to habitat shrinking: Lifetime warranty or death sentence? American Journal of Primatology, 82(4), 1–9. https://doi.org/10.1002/ajp.23089.
Bolt, L. M., Russell, D. G., & Schreier, A. L. (2021). Anthropogenic edges impact howler monkey (Alouatta palliata) feeding behaviour in a Costa Rican rainforest. Primates, 62(4), 647–657.
Bonadonna, G., Zaccagno, M., Torti, V., Valente, D., De Gregorio, C., Randrianarison, R. M., Tan, C., Gamba, M., & Giacoma, C. (2020). Intra-and intergroup spatial dynamics of a pair-living singing primate, Indri indri: a multiannual study of three indri groups in Maromizaha Forest, Madagascar. International Journal of Primatology, 41(2), 224–245. https://doi.org/10.1007/s10764-019-00127-5
Bonvicino, C. R. (1989). Ecologia e comportamento de Alouatta belzebul (Primates: Cebidae) na mata atlântica. Revista Nordestina de Bilogia, 6, 149–179.
Bravo, S. P., & Sallenave, A. (2003). Foraging behavior and activity patterns of Alouatta caraya in the northeastern Argentinean flooded forest. International Journal of Primatology, 24, 825–846. https://doi.org/10.1023/A:1024680806342
Bryson-Morrison, N., Tzanopoulos, J., Matsuzawa, T., & Humle, T. (2017). Activity and habitat use of chimpanzees (Pan troglodytes versus) in the anthropogenic landscape of Bossou, Guinea, West Africa. American Journal of Primatology, 38(2), 282–302.
Buss G, Oklander LI, Bicca Marques JC, Hirano ZB, Chaves OM, Mendes SL et al., (2019). Brown howler monkey: Alouatta guariba Humboldt, 1812. In: Primates in Peril. The world’s 25 most endangered primates 2018-2020.
Caillaud, D., Crofoot, M. C., Scarpino, S. V., Jansen, P. A., Garzon-Lopez, C. X., et al (2010). Modelling the spatial distribution and fruiting pattern of a key tree species in a neotropical forest: methodology and potential applications. PLoS One, 5(11), e15002. https://doi.org/10.1371/journal.pone.0015002.
Carvalho Jr., O., Ferrari, S. F., & Strier, K. B. (2004). Diet of a muriqui group (Brachyteles arachnoides) in continuous primary forest. Primates, 45(3), 201–204. https://doi.org/10.1007/s10329-004-0079-7.
Ceccarelli, E., Negrín, A. R., Coyohua-Fuentes, A., Canales-Espinosa, D., & Dias, P. A. D. (2019). An exploration of the factors influencing the spatial behavior of mantled howler monkeys (Alouatta palliata). International Journal of Primatology, 40(2), 197–213. https://doi.org/10.1007/s10764-018-0075-1.
Chapman, C. A. (1990). Ecological constraints on group size in three species of neotropical primates. Folia Primatologica, 55, 1–9.
Chapman, C. A., Chapman, L. J., Wangham, R., Hunt, K., Gebo, D., & Gardner, L. (1992). Estimators of fruit abundance of tropical trees. Biotropica, 527–531.
Chapman, C. A., Wrangham, R. W., & Chapman, L. J. (1995). Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology, 36, 59–70.
Chaves, Ó. M., Fernandes, F. A., Oliveira, G. T., & Bicca-Marques, J. C. (2019). Assessing the influence of biotic, abiotic, and social factors on the physiological stress of a large Neotropical primate in Atlantic forest fragments. Science of the Total Environment, 690, 705–716. https://doi.org/10.1016/j.scitotenv.2019.07.033.
Chiarello, A. G. (1992). Dieta, padrão de atividades e área de vida de um grupo de bugios (Alouatta fusca) na Reserva de Santa Genebra, Campinas, SP. Masters' dissertation. Universidade Estadual de Campinas, Campinas, Brazil.
Clutton-Brock, T. H., & Harvey, P. H. (1977). Primate ecology and social organization. Journal of Zoology, 183(1), 1–39. https://doi.org/10.1111/j.1469-7998.1977.tb04171.x.
Clutton-Brock, T. H., Rose, K. E., & Guinness, F. E. (1997). Density–related changes in sexual selection in red deer. Proceedings of the Royal Society B: Biological Sciences, 264(1387), 1509–1516.
Cooke, S. J., Hinch, S. G., Wikelski, M., Andrews, R. D., Kuchel, L. J., Wolcott, T. G., & Butler, P. J. (2004). Biotelemetry: a mechanistic approach to ecology. Trends in Ecology & Evolution, 19(6), 334–343.
Cornick, L. A., & Markowitz, H. (2002). Diurnal vocal patterns of the black howler monkey (Alouatta pigra) at Lamanai, Belize. Journal of Mammalogy, 83(1), 159–166. https://doi.org/10.1644/1545-1542(2002)083<0159:DVPOTB>2.0.CO;2.
Cortés-Ortiz, L., Bermingham, E., Rico, C., Rodrıguez-Luna, E., Sampaio, I., & Ruiz-Garcıa, M. (2003). Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Molecular Phylogenetics and Evolution, 26(1), 64–81.
Courbin, N., Fortin, D., Dussault, C., & Courtois, R. (2014). Logging-induced changes in habitat network connectivity shape behavioral interactions in the wolf–caribou–moose system. Ecological Monographs, 84(2), 265–285. https://doi.org/10.1890/12-2118.1
Creel, S., & Creel, N. M. (1995). Communal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behaviour, 50(5), 1325–1339.
Cristóbal-Azkarate, J., Urbani, B., & Asensio, N. (2015). Interactions of howler monkeys with other vertebrates: a review. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler Monkeys: Behavior, Ecology, and Conservation (pp. 141–164). Springer.
Cunha, R. G. T., & Jalles-Filho, E. (2007). The roaring of southern brown howler monkeys (Alouatta guariba clamitans) as a mechanism of active defence of borders. Folia Primatologica, 78(4), 259–271. https://doi.org/10.1159/000105545.
Cunningham, E., & Janson, C. (2007). Integrating information about location and value of resources by white faced-saki monkeys (Pithecia pithecia). Animal Cognition, 10, 293–304.
Dasilva, G. L. (1992). The western black-and-white colobus as a low-energy strategist: activity budgets, energy expenditure and energy intake. Journal of Animal Ecology, 61, 79–91. https://doi.org/10.2307/5511.
de Cunha, R. G. T., de Oliveira, D. A. G., Holzmann, I., & Kitchen, D. M. (2015). Production of loud and quiet calls in howler monkeys. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: Adaptive radiation, systematics, and morphology (pp. 337–369). Springer.
Di Bitetti, M. S., Di Blanco, Y. E., Pereira, J. A., Paviolo, A., & Pírez, I. J. (2009). Time partitioning favors the coexistence of sympatric crab-eating foxes (Cerdocyon thous) and pampas foxes (Lycalopex gymnocercus). Journal of Mammalogy, 90(2), 479–490.
Di Fiore, A., Link, A., & Campbell, C. J. (2011). The atelines: behavioral and socioecological diversity in a New World monkey radiation. In C. J. Campbell, A. Fuentes, M. C. Mackinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in Perspective (pp. 155–188). Oxford University Press.
Dias, L. G., & Strier, K. B. (2000). Agonistic encounters between muriquis, Brachyteles arachnoides hypoxanthus (Primates, Cebidae), and other animals at the Estação Biológica de Caratinga, Minas Gerais, Brazil. Neotropical Primates, 8, 138–141.
dos Santos-Barnett, T. C., Cavalcante, T., Boyle, S. A., Matte, A. L., Bezerra, B. M., de Oliveira, T. G., & Barnett, A. A. (2022). Pulp fiction: why some populations of ripe-fruit specialists Ateles chamek and A. marginatus prefer insect-infested foods. International Journal of Primatology. https://doi.org/10.1007/s10764-022-00284-0.
Falótico, T., Mendonça-Furtado, O., Fogaça, M. D., Tokuda, M., Ottoni, E. B., & Verderane, M. P. (2021). Wild robust capuchin monkey interactions with sympatric primates. Primates, 62, 659–666. https://doi.org/10.1007/s10329-021-00913-x.
Ferreguetti, A. C., Oliveira, A., Pereira, B. C., Santori, R. T., Geise, L., & Bergallo, H. G. (2020). Encounter rate and behavior of Alouatta guariba clamitans in the Ilha Grande State Park, Rio de Janeiro State, Brazil. Zoologia (Curitiba), 37, e36846. https://doi.org/10.3897/zoologia.37.e36846.
Fortes, V. B., Bicca-Marques, J. C., Urbani, B., Fernández, V. A., & Da Silva Pereira, T. (2015). Ranging behavior and spatial cognition of howler monkeys. In M. M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler Monkeys: Behavior, Ecology, and Conservation (pp. 219–258). Springer.
Gibson, L., & Koenig, A. (2012). Neighboring groups and habitat edges modulate range use in Phayre’s leaf monkeys (Trachypithecus phayrei crepusculus). Behavioral Ecology and Sociobiology, 66(4), 633–643. https://doi.org/10.1007/s00265-011-1311-2.
Gómez-Posada, C., & Londoño, J. M. (2012). Alouatta seniculus: density, home range and group structure in a bamboo forest fragment in the Colombian Andes. Folia Primatologica, 83(1), 56–65. https://doi.org/10.1159/000339803
González-Solís, J., Guix, J. C., Mateos, E., & Llorens, L. (2001). Population density of primates in a large fragment of the Brazilian Atlantic rainforest. Biodiversity and Conservation, 10(8), 1267–1282. https://doi.org/10.1023/A:1016678126099.
Goodale, E., Beauchamp, G., & Ruxton, G. (2017). Mixed-species groups of animals: behavior, community structure, and conservation. Academic Press.
Gross, M. (2017). Primates in peril. Current Biology, 27(12), 573–576. https://doi.org/10.1016/j.cub.2017.06.002.
Harris, T. R., & Chapman, C. A. (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48(3), 208–221.
Hartig, F. (2017). DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. https://cran.r-project.org/package=DHARMa. Accessed Mar 2023
Heymann, E. W., & Buchanan-Smith, H. M. (2000). The behavioural ecology of mixed-species troops of callitrichine primates. Biological Reviews, 75(2), 169–190.
Hladik, C. M. (1978). Adaptive strategies of primates in relation to leaf eating. In G. G. Montgomery (Ed.), The Ecology of arboreal Folivores (pp. 373–395). Smithsonian Institution Press.
Hobson, K. A., Norris, D. R., Kardynal, K. J., & Yohannes, E. (2019). Animal migration: a context for using new techniques and approaches. In Tracking animal migration with stable isotopes (pp. 1–23). Academic Press.
Houle, A., Chapman, C. A., & Vickery, W. L. (2010). Intratree vertical variation of fruit density and the nature of contest competition in frugivores. Behavioral Ecology and Sociobiology, 64(3), 429–441.
Isbell, L. A. (1991). Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behavioral Ecology, 2(2), 143–155. https://doi.org/10.1093/beheco/2.2.143.
Izar, P., Verderane, M. P., Peternelli-Dos Santos, L., Mendonça-Furtado, O., Presotto, A., Tokuda, M., et al (2012). Flexible and conservative features of social systems in tufted capuchin monkeys: comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology, 74(4), 315–331. https://doi.org/10.1002/ajp.20968.
Janmaat, K. R., de Guinea, M., Collet, J., Byrne, R. W., Robira, B., van Loon, E., Jang, H., Biro, D., Ramos-Fernandez, G., Ross, C., Presotto, A., Allritz, M., Alavi, S., & van Belle, S. (2021). Using natural travel paths to infer and compare primate cognition in the wild. Iscience, 24(4), 102343.
Janson, C. (1985). Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behavioral Ecology and Sociobiology, 18(2), 125–138.
Janson, C. H., & Van Schaik, C. P. (1988). Recognizing the many faces of primate food competition: methods. Behaviour, 105(1/2), 165–186.
Jerusalinsky, L., Bicca-Marques, J.C., Neves, L.G., Alves, S.L., Ingberman, B., Buss, G., Fries, B.G., Alonso, A.C., da Cunha, R.G.T., Miranda, J.M.D., Talebi, M., de Melo, F.R., Mittermeier, R.A. & Cortes-Ortíz, L. (2021). Alouatta guariba (amended version of 2020 assessment). The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T39916A190417874.en
Jung, L., Mourthe, I., Grelle, C. E., Strier, K. B., & Boubli, J. P. (2015). Effects of local habitat variation on the behavioral ecology of two sympatric groups of brown howler monkey (Alouatta clamitans). PLoS One, 10(7), e0129789. https://doi.org/10.1371/journal.pone.0129789.
Kamilar, J. M., & Beaudrot, L. (2013). Understanding primate communities: Recent developments and future directions. Evolutionary Anthropology, 22(4), 174–185. https://doi.org/10.1002/evan.21361.
Kernohan, B. J., Gitzen, R. A., & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In J. J. Millspaugh & J. M. Marzluff (Eds.), Radio tracking and animal populations (pp. 125–166). Academic Press.
Kitchen, D. W., Cunha, R. G. T., Holzmann, I., & Oliveira, D. A. G. (2015). Function of loud calls in howler monkeys. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: adaptive radiation, systematics, and morphology (pp. 369–402). Springer.
Knowlton, J. L., & Graham, C. H. (2010). Using behavioral landscape ecology to predict species’ responses to land-use and climate change. Biological Conservation, 143(6), 1342–1354. https://doi.org/10.1016/j.biocon.2010.03.011.
Kowalewski, M. M. (2007). Patterns of affiliation and co-operation in howler monkeys: an alternative model to explain social organization in non-human primates. PhD dissertation, University of Illinois, Urbana, USA.
Kurihara, Y., & Muto, H. (2021). Behavioral responses of Japanese macaques to playback-simulated intergroup encounters. Behavioural Processes, 182, 104279.
Laundré, J. W., Hernández, L., & Ripple, W. J. (2010). The landscape of fear: ecological implications of being afraid. The Open Ecology Journal, 3, 1–7.
Markham, A. C., Alberts, S. C., & Altmann, J. (2012). Intergroup conflict: ecological predictors of winning and consequences of defeat in a wild primate population. Animal Behaviour, 84(2), 399–403. https://doi.org/10.1016/j.anbehav.2012.05.009.
Martins, M. M. (2008). Fruit diet of Alouatta guariba and Brachyteles arachnoides in Southeastern Brazil: comparison of fruit type, color, and seed size. Primates, 49(1), 1–8.
Matsuda, I., Clauss, M., Tuuga, A., Sugau, J., Hanya, G., Yumoto, T., ... & Hummel, J. (2017). Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Scientific Reports, 7(1), 1-10.
Mendes, S. L. (1989). Estudo ecológico de Alouatta fusca (Primates: Cebidae) na Estação Biológica de Caratinga, MG. Revista Nordestina de Biologia, 71–104.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. The American Naturalist, 114(3), 362–378.
Milton, K. (1980). The foraging strategy of howler monkeys: a study in primate economics. Columbia University Press.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): energetic and digestive considerations and comparison with the Colobine. International Journal of Primatology, 19, 513–548. https://doi.org/10.1023/A:1020364523213.
Miranda, J., & Passos, F. C. (2004). Hábito alimentar de Alouatta guariba (Humboldt)(Primates, Atelidae) em Floresta de Araucária, Paraná, Brasil. Revista Brasileira de Zoologia, 21(4), 821–826.
Morellato, L. P. C., Talora, D. C., Takahasi, A., Bencke, C. C., Romera, E. C., & Zipparro, V. B. (2000). Phenology of Atlantic rain forest trees: a comparative study. Biotropica, 32(4b), 811–823. https://doi.org/10.1111/j.1744-7429.2000.tb00620.x.
Müller, C. A., & Manser, M. B. (2007). ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proceedings of the Royal Society B: Biological Sciences, 274(1612), 959–965. https://doi.org/10.1098/rspb.2006.0222.
Nagy, K. A., & Milton, K. (1979). Energy metabolism and food consumption by wild howler monkeys (Alouatta palliata). Ecology, 60(3), 475–480.
Nathan, R., et al (2022). Big-data approaches lead to an increased understanding of the ecology of animal movement. Science, 375(6582), eabg1780.
Oklander L. I., Buss G., Bicca-Marques J.C., Hirano Z. B., Chaves O. M., Jardim M. M. A., Valença-Montenegro M. M., Mendes S. L., Neves L. G., Kowalewski M., Melo F. R., Rylands A. B., Jerusalinsky L. (2022). Brown howler monkey: Alouatta guariba Humboldt, 1812. In: Primates in Peril. The world’s 25 most endangered primates 2020-2022.
Pinto, L. P., Barnett, A. A., Bezerra, B. M., Boubli, J.-P., Bowler, M., Cardoso, N., Castelli, C., Rodriguez, M. J. O., Santos, R. R., Setz, E. Z., & Veiga, L. M. (2013). Why we know so little: the challenges of fieldwork on the pitheciines. In L. Veiga, A. Barnett, S. Ferrari, & M. Norconk (Eds.), Evolutionary Biology and Conservation of Titis, Sakis and Uacaris (pp. 145–150). Cambridge University Press.
Pisciotta, K. (2002). The Paranapiacaba forest fragment. In E. Mateos, J. C. Guix, A. Serra, & K. Pisciotta (Eds.), Censuses of vertebrates in a Brazilian Atlantic rainforest area: The Paranapiacaba fragment. University of Barcelona.
Plano de Manejo. (2015). Plano de Manejo Parque Estadual Carlos Botelho. Unidades de Terreno do Parque. Available in <http://observatorio.wwf.org.br/site_media/upload/gestao/planoManejo/PM_PECarlosBotelho_parte_002.pdf>. Accessed: August 2021.
Powell, R. A. (2000). Animal home ranges and territories and home range estimators. In L. Boitoni & T. K. Fuller (Eds.), Research techniques in animal ecology: controversies and consequences (pp. 65–110). Columbia University Press.
Prates, H. M., & Bicca-Marques, J. C. (2008). Age-sex analysis of activity budget, diet, and positional behavior in Alouatta caraya in an orchard forest. International Journal of Primatology, 29, 703–715. https://doi.org/10.1007/s10764-008-9257-6
Pruetz, J. D., & Isbell, L. A. (2000). Correlations of food distribution and patch size with agonistic interactions in female vervets (Chlorocebus aethiops) and patas monkeys (Erythrocebus patas) living in simple habitats. Behavioral Ecology and Sociobiology, 49(1), 38–47.
Prugh, L. R., Sivy, K. J., Mahoney, P. J., Ganz, T. R., Ditmer, M. A., van de Kerk, M., ... & Montgomery, R. A. (2019). Designing studies of predation risk for improved inference in carnivore-ungulate systems. Biological Conservation, 232, 194-207.
Pyke, G. (2019). Animal movements: an optimal foraging approach. In Encyclopedia of animal behavior (pp. 149–156). Elsevier Academic Press.
R Core Team. (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing https://www.R-project.org. Accessed Mar 2023
Raño, M., Kowalewski, M. M., Cerezo, A. M., & Garber, P. A. (2016). Determinants of daily path length in black and gold howler monkeys (Alouatta caraya) in northeastern Argentina. American Journal of Primatology, 78(8), 825–837. https://doi.org/10.1002/ajp.22548.
Reynoso-Cruz, J. E., Rangel-Negrín, A., Coyohua-Fuentes, A., Canales-Espinosa, D., & Dias, P. A. D. (2016). Measures of food intake in mantled howling monkeys. Primates, 57(2), 161–166. https://doi.org/10.1007/s10329-016-0513-7.
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6), 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021.
Ries, L., & Sisk, T. D. (2004). A predictive model of edge effects. Ecology, 85(11), 2917–2926.
Rímoli, J., Nantes, R. D. S., & Júnior, A. L. (2012). Diet and activity patterns of black howler monkeys Alouatta caraya (Humboldt, 1812, Primates, Atelidae) in ecotone Cerrado-Pantanal in the left bank of Aquidauana River, Mato Grosso do Sul, Brazil. Oecologia Australis, 16, 933–948. https://doi.org/10.4257/oeco.2012.1604.15.
Rosa, M. R., Brancalion, P. H., Crouzeilles, R., Tambosi, L. R., Piffer, P. R., Lenti, F. E., ... & Metzger, J. P. (2021). Hidden destruction of older forests threatens Brazil’s Atlantic Forest and challenges restoration programs. Science Advances, 7(4), eabc4547. https://doi.org/10.1126/sciadv.abc4547
Rose, L. M., Perry, S., Panger, M. A., Jack, K., Manson, J. H., Gros-Louis, J., Mackinnon, C. K., & Vogel, E. (2003). Interspecific interactions between Cebus capucinus and other species: data from three Costa Rican sites. International Journal of Primatology, 24(4), 759–796. https://doi.org/10.1023/A:1024624721363
Rosenberger, A. L., & Strier, K. B. (1989). Adaptive radiation of the ateline primates. Journal of Human Evolution, 18(7), 717–750.
Rowley, M. H., & Christian, J. J. (1976). Interspecific aggression between Peromyscus and Microtus females: A possible factor in competitive exclusion. Behavioral Biology, 16(4), 521–525.
Sanchez, G. (2013). PLS Path Modeling with R. Trowchez Editions. Berkeley, 2013. Available in: http://www.gastonsanchez.com/PLS Path Modeling with R.pdf. Accessed October 2022.
Sanz, C. M., Strait, D., Ayina, C. E., Massamba, J. M., Ebombi, T. F., Kialiema, S. N., ... & Morgan, D. B. (2022). Interspecific interactions between sympatric apes. Iscience, 25(10), 105059.
Scarry, C. J. (2013). Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Animal Behavior, 85(5), 931–939. https://doi.org/10.1016/j.anbehav.2013.02.013.
Schoener, T. W. (1974). Resource Partitioning in Ecological Communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science, 185(4145), 27–39.
Schreier, A. L., Bolt, L. M., Russell, D. G., Readyhough, T. S., Jacobson, Z. S., Merrigan-Johnson, C., & Coggeshall, E. M. (2021). Mantled howler monkeys (Alouatta palliata) in a Costa Rican forest fragment do not modify activity budgets or spatial cohesion in response to anthropogenic edges. Folia Primatologica, 92(1), 49–57. https://doi.org/10.1159/000511974.
Sekulic, R. (1982). The function of howling in red howler monkeys (Alouatta seniculus). Behaviour, 38-54. https://doi.org/10.1163/156853982X00517.
Sha, J. C. M., & Hanya, G. (2013). Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed macaques (Macaca fascicularis). American Journal of Primatology, 75(6), 581–592. https://doi.org/10.1002/ajp.22137.
Sih, A. (2005). Predator-prey space use as an emergent outcome of a behavioral response race. Ecology of Predator-Prey Interactions, 256, 78.
Singh, M. R., Singh, M., Ananada Kumar, M., Kumar, H. N., Sharma, A. K., & Sushma, H. S. (2000). Niche separation in sympatric lion-tailed macaques (Macaca silenus) and Nilgiri langur (Presbytis johnii) in an Indian tropical rain forest. Primate Report, 58, 83–95. http://repository.ias.ac.in/89653/1/13p.pdf. Accessed Mar 2023
Snaith, T. V., & Chapman, C. A. (2007). Primate group size and interpreting socioecological models: do folivores really play by different rules? Evolutionary Anthropology, 16(3), 94–106. https://doi.org/10.1002/evan.20132.
Sobral, G., Siqueira Martins, G., & Oliveira, C. A. (2022). Thermal imaging aids behavioural studies: the case of a diurnal neotropical primate. Mastozoologia Neotropical, 29(1), e0622. https://doi.org/10.31687/saremMN.22.29.1.07.e0622.
Sobroza, T. V., Pequeno, P. A. C. L., Gordo, M., Kinapp, N. M., Barnett, A. A., & Spironello, W. (2021). Does co-occurrence drive vertical niche partitioning in parapatric tamarins (Saguinus spp.)? Austral Ecology, 46, 1335–1342. https://doi.org/10.1111/aec.13085.
Strier, K. B. (1987). Ranging behavior of woolly spider monkeys, or muriquis, Brachyteles arachnoides. International Journal of Primatology, 8(6), 575–591.
Strier, K. B. (1989). Effects of patch size on feeding associations in muriquis (Brachyteles arachnoides). Folia Primatologica, 52, 70–77. https://doi.org/10.1159/000156383.
Talebi, M., Bastos, A., & Lee, P. C. (2005). Diet of southern muriquis in continuous Brazilian Atlantic Forest. International Journal of Primatology, 26(5), 1175–1187. https://doi.org/10.1007/s10764-005-6463-3.
Teichroeb, J. A., & Sicotte, P. (2009). Test of the ecological-constraints model on ursine colobus monkeys (Colobus vellerosus) in Ghana. American Journal of Primatology, 71(1), 49–59. https://doi.org/10.1002/ajp.20617.
Temeles, E. J. (1994). The role of neighbours in territorial systems: when are they'dear enemies'? Animal Behavior, 47(2), 339–350. https://doi.org/10.1006/anbe.1994.1047.
Terborgh, J. (2014). Five new world primates. Princeton University Press.
Van Belle, S., & Estrada, A. (2020). The influence of loud calls on intergroup spacing mechanism in black howler monkeys (Alouatta pigra). International Journal of Primatology, 41(2), 265–286.
Van Belle, S., Estrada, A., & Garber, P. A. (2013). Spatial and diurnal distribution of loud calling in black howlers (Alouatta pigra). International Journal of Primatology, 34(6), 1209–1224. https://doi.org/10.1007/s10764-013-9734-4.
Vogel, E. R., & Janson, C. H. (2007). Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. American Journal of Primatology, 69(5), 533–550. https://doi.org/10.1002/ajp.20368.
Walter, H. (1973). Vegetation of the Earth in Relation to Climate and the Eco-Physiological Conditions. Springer.
Worton, B. J. (1995). A convex hull-based estimator of home-range size. Biometrics, 51, 1206–1215. https://doi.org/10.2307/2533254.
Acknowledgments
A preliminary version of this paper was presented to a committee in partial fulfilment of the requirements for the PhD degree. Hence, we are indebted to committee members J.C. Bicca-Marques, P. Izar, R. G. Ferreira, and A. Araújo for their important suggestions, which greatly increased the quality increase of this paper. We are also thankful to A. Caccavo for producing the home range figure; I. Campell from Stackoverflow for enabling the calculation of DPL using a simple R code. We would also thank L. Wiseman-Jones for her help in proofreading the English language of this paper. Finally, the authors thank the anonymous referees, the Associate Editor and the Editor-in-Chief, for reviews that greatly enhanced the quality of the manuscript. Fieldwork was conducted within the current legal norms in Brazil (Instituto Chico Mendes de Conservação da Biodiversidade License number 55826-1; Secretaria do Meio Ambiente - Fundação Florestal authorisantion number 009.188/2016; SISGEN register number A165E11).
Inclusion and diversity statement
• One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
• One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community.
• While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 (to GS), and part by FAPESP (#2017/07954-0) (to LFF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Addisu Mekonnen
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sobral, G., Fuzessy, L.F. & de Oliveira, C.A. The Challenge of Coexistence: Changes in Activity Budget and Ranging Behaviour of Brown Howler Monkeys in Response to the Presence of Conspecifics and Heterospecifics. Int J Primatol 44, 558–580 (2023). https://doi.org/10.1007/s10764-023-00359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00359-6