Abstract
Forest fragmentation is viewed as a serious threat to primates, yet whether or not it can disrupt food resources and cause energetic stress remains largely untested. I present the results of a 12-mo study of the feeding ecology of Propithecus diadema in fragmented and continuous forest at Tsinjoarivo, eastern Madagascar. Two continuous forest groups had higher dietary diversity and ate more fleshy fruit, but during the dry season, diversity was reduced and they relied heavily on mistletoe (Bakerella clavata). In contrast, 2 groups in fragments employed the lean season strategy of eating mistletoe year-round; the fruiting tree species that sustain continuous forest groups through the rainy season were largely absent. As expected, intersite dietary overlap was highest in the dry season. The level of specialization was high: fragment groups devoted 30–40% of feeding time to Bakerella clavata, compared to 28–30% in continuous forest. The major characteristic of Bakerella clavata enabling it to be an important fallback or staple resource, or both, is its extended phenology. The difference in resource utilization between sites may have important implications for nutritional status, as well as ranging and social behavior, largely owing to the small size and high abundance of feeding patches of Bakerella. Understanding resource shifts in fragments can shed light on socioecological questions by providing comparisons between continuous forest and fragment populations with differing diets and resource distributions. In addition, understanding dietary shifts in fragments can aid in species-specific conservation efforts, while contributing to a better understanding of the considerable interspecific variability of primates in responses to fragmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat destruction is one of the most pervasive causes of biodiversity loss throughout the tropics (Laurance 1999). Researchers can easily and accurately assess habitat loss and fragmentation via satellite images (Green and Sussman 1990; Jorge and Garcia 1997), but the relationships between fragmentation and extinctions are complex; fragmentation has profound effects on biodiversity beyond habitat loss alone, but they remain poorly understood (Fahrig 2003; Laurance et al. 2002). One can sometimes predict losses of species based on the area of habitat lost (species-area relationships: Cowlishaw 1999; Ganzhorn et al. 2003), but such analyses do not help targeted conservation efforts because they do not identify taxa at risk or yield information about the processes leading to extinction.
Many researchers have shown that primate species vary in their ability to persist in fragments (Ganzhorn et al. 2000a, b; Gilbert and Setz 2001; Marsh 2003; Onderdonk and Chapman 2000; Tutin et al. 1997; Umapathy and Kumar 2000a). However, in most cases no simple variable such as diet, body size, or home range adequately explains this interspecific variation (Onderdonk and Chapman 2000). Ganzhorn et al. (2000a, b, 2003) showed that mammalian communities in Madagascar have a highly nested structure in fragmented landscapes, i.e., extinction proneness is consistent, and that estimated initial population size in a fragment tends to predict survival. However, much of the interspecific variation remains unexplained, probably because species react in different ways to the altered habitat and landscape. Behavioral plasticity (Dehgan 2003) may be more important than static measures of specific characteristics, e.g., body mass, abundance in nonfragmented forest, or fragment size.
One difficulty in investigating the impacts of fragmentation on primate populations is that it causes several different effects acting on different temporal scales. First, direct anthropogenic impacts, e.g., hunting (Cullen et al. 2000), caused by increased proximity to humans threaten most immediately. Second, on a longer time scale, habitat characteristics are altered in fragments; this includes all changes resulting from fragmentation, both natural, e.g., altered tree mortality, increased density of heliophilic trees (Laurance et al. 1998) and anthropogenic, e.g., tree extraction or introductions by humans. Third, on the longest time scale, habitat spatial discontinuity can affect population-level processes by constraining dispersal or necessitating travel through dangerous matrix habitats, or both (Peacock and Smith 1997).
Within the second category, an impact of fragmentation that can have a major effect on primate populations is a change in available food resources. Fragmentation has the potential to affect the feeding ecology of a species whenever habitat changes affect the presence, abundance, distribution, or phenology of food resources. Primates presumably can adjust to the altered conditions via ecological and behavioral shifts within limits, but face local extinction if changes are extreme.
Though several researchers have examined primate incidence patterns in fragments, few have examined dietary changes. Lion-tailed macaques (Macaca silenus) in fragments consume more flowers and less fruit and insects (Umapathy and Kumar 2000b), and many foods are introduced or cultivated species (Singh et al. 2001). Tutin (1999) found that mustached guenons (Cercopithecus cephus) in a fragment consumed fewer fruits, seeds, and flowers, and more insects and leaves, than continuous forest groups did. Onderdonk and Chapman (2000) found that dietary composition, in terms of plant parts, was similar for black-and-white colobus (Colobus guereza) in intact forest and fragments, but food species differed; fragment groups relied on species that were rarely eaten in continuous forest. Thus, researchers have documented dietary shifts in fragments, but without providing generalizations, and have not explored fitness consequences.
Understanding altered diet in fragments is important for conservation efforts because the shifts can impact many aspects of ecology and behavior and ultimately affect viability. The quality of food resources directly impacts health and body condition, which can affect birth and mortality rates, as well as susceptibility to predation and disease (Chapman et al. 2005; Milton 1996; Olupot 2000). Resource distributions can affect ranging, e.g., groups may range farther when food density is lower, intragroup spacing, and behavioral patterns, including optimum group size, time budgets, dominance relationships, and rates of social behaviors. Thus, local extinction can be a fairly direct result of fragmentation, e.g., altered food resources make it impossible to achieve minimum nutrient intakes in the short term, or a more indirect result, e.g., fragmentation’s effects on physiology or behavior eventually cause extinction.
I examine the effects of forest fragmentation on the feeding ecology of the diademed sifaka, Propithecus diadema. I consider only the effects of altered habitat characteristics on feeding ecology, and do not address direct anthropogenic impacts (the study groups are not hunted) or population-level processes (which may be present, but acting on a longer time scale). Specifically, I address the following questions:
-
1)
Does diet differ between fragment and continuous forest groups in terms of plant parts consumed?
-
2)
Which plant parts are preferred foods, i.e. consumed in proportion to their availability, and is preference different between fragmented and continuous sites?
-
3)
Do groups in fragments and continuous forest differ in terms of plant species consumed and patterns of dietary diversity, including seasonal changes, and to what degree does diet overlap between continuous forest and fragments?
-
4)
What consequences do dietary differences have for population viability?
Materials and Methods
Study Site
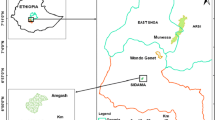
Tsinjoarivo Forest is located in eastern Madagascar, ca. 80 km SSE of the capital, Antananarivo, on the escarpment dividing the central plateau from the eastern lowlands (Fig. 1). The region contains an unprotected block of central domain mid-altitude rain forest, part of a corridor between Ranomafana National Park (150 km SSW) and Mantadia National Park (100 km NE). Settlers from the central plateau have fragmented and degraded the corridor’s western half, while the eastern half is minimally disturbed (Irwin 2006a).
Location of study site in Madagascar. Gray indicates extent of remaining rain forest vegetation (Green and Sussman 1990).
I established 2 research camps at Tsinjoarivo, separated by 12 km. Mahatsinjo (19°40.94′ S, 47°45.46′ E, 1590 m), in the corridor’s western half, contains a network of hill- and ridge-top forest fragments surrounded by settlements, cultivated land, grass, and secondary forest. Vatateza (19°43.25′ S, 47°51.41′ E, 1396 m) is within continuous forest in the corridor’s eastern half; human settlements are located nearby, near the Onive River to the south. Fragments at Mahatsinjo are disturbed by a combination of anthropogenic extraction and nonanthropogenic edge effects. They show reduced physical structure (tree density, crown volume, and basal area per ha) and tree diversity relative to Vatateza (Irwin 2006a). Habitat loss and fragmentation have occurred over ca. 50 yr at Mahatsinjo, and though there is no botanical datum before human settlement, locals attest that the forest at Mahatsinjo once resembled that at Vatateza in terms of specific composition and physical structure.
At both sites, there is a distinct rainy season between December and March and a dry season between April and November. Rainfall at Vatateza totals 2632 mm, of which 1697 mm (64.5%) falls during the rainy season. Rainfall at Mahatsinjo is lower, 2008 mm, with 1317 mm (65.6%) falling during the rainy season. Temperature is highest in December–January and lowest in June–August (Irwin 2006a).
Study Population
Tsinjoarivo sifakas are provisionally referred to Propithecus diadema (Mayor et al. 2004), though body mass and morphometric and pelage differences suggest they may be a distinct subspecies (Irwin 2006a; Glander and Irwin unpub. data). They live in small groups of 2–6 individuals (excluding infants < 1 yr). Group sizes and composition are similar to that of Propithecus edwardsi at Ranomafana (Pochron and Wright 2003; Pochron et al. 2004) and P. diadema at Mantadia (Powzyk 1997). Groups at Tsinjoarivo have 1 breeding adult male, 1–2 breeding adult females, and ≤4 immature offspring. At Mahatsinjo, groups live in fragments as small as 25 ha, but their long-term viability is unknown.
I studied 4 groups, 2 in continuous forest at Vatateza (CONT1, CONT2) and 2 in neighboring fragments at Mahatsinjo (FRAG1, FRAG2). CONT occupied large home ranges (70–80 ha) in the continuous forest corridor (Fig. 1). FRAG1 was the sole group in a 24-ha fragment, and FRAG2 used a 37-ha home range within 3 closely apposed fragments and had 1 neighboring group via a degraded forest corridor to the west (Irwin 2007b). Group size was similar (CONT1: 6–7, CONT2: 5–6, FRAG1: 4–5, FRAG2: 5), and all groups had 2 breeding adults (1 male, 1 female) except CONT2 (1 male, 2 females). As in other sifaka populations, females are dominant over males (Irwin 2006a). I captured individuals in all groups in November 2002 and 2003, using the Pneu-dart ™ system, via disposable darts loaded with Telazol® (in collaboration with K. Glander per Glander et al. 1992). I affixed radiocollars (Telonics, Mesa, AZ; mass ≤35 g) to 1 or 2 individuals/group, and olefin collars and metal pendants to all other captured individuals. I could reliably identify all individuals because no group had >1 uncollared individual per size class. Groups were habituated in December 2002, and I could observe them reliably at close distances. Based on capture data for 2002–2005, females at Vatateza and Mahatsinjo do not differ significantly in body mass, but males at Mahatsinjo are 13% lighter than those at Vatateza, and same-aged immatures are lighter at Mahatsinjo (Irwin 2006a; Irwin et al. 2007).
Behavioral Data Collection
I collected behavioral data between January and December 2003. I followed CONT1 and CONT2 during the first 2 wk of each mo and FRAG1 and FRAG2 during the last 2 wk. The single exception was June, when I followed CONT June 14–20, and FRAG June 24 to July 2. I compiled observations from day-long focal individual follows conducted by either myself with 1 local assistant, or a local assistant and 1–2 trained research assistants. Focal individuals included only individuals estimated to be >1.5 yr in January 2003, and were selected on a rotating basis to equalize sample size among individuals. I worked on a rotating basis with all assistants and standardized data collection and distance estimation with interobserver reliability checks.
Teams collected feeding data continuously. For each feeding bout, we recorded 1) start and stop times to the nearest second, 2) plant part consumed, and 3) food species. Bouts ended when the individual 1) stopped eating for >10 s, 2) changed from one feeding site (= individual plant) to another, or 3) continued feeding at the same site but on a different plant part. In some cases (6.0% of feeding bouts), subjects fed simultaneously on ≥2 plant parts at 1 site; when this occurred, I recorded a single bout with multiple plant parts. For analysis, I divided time spent in such bouts evenly among the plant parts.
Habitat Assessments and Phenology
I inventoried 10 1000-m2 (10 × 100 m) plots at each of 4 sites (total: 40 plots, 40,000 m2), corresponding to the 4 sifaka group home ranges. Within each plot, I collected the following data for each stem ≥5 cm diameter at breast height (DBH): specific identity, position relative to plot origin, DBH, height, crown height, 2 crown diameters (maximum and perpendicular), and the incidence of hemiparasites (Bakerella clavata, B. cf. hoyifolia, Viscum sp.) and hemiepiphytes (Medinilla humblotii, M. parvifolia). I estimated crown volume as an ellipsoid via the height and 2 diameters. I included lianas when their DBH was >5 cm but it proved too difficult to estimate their crown dimensions; therefore they are not represented in crown volumetric data (lianas represented only 0.19–0.97% of stems and 0.05–0.18% of basal area per site). I included 1 bamboo species >5 cm DBH (Arundinaria sp.) because it is an important component of the forest structure in some plots.
I monitored the phenology of 818 plants monthly between January and December 2003. The data set includes 638 canopy trees, 92 understory trees, 28 edge trees, and 60 nontrees (hemiepiphytes, hemiparasites, lianas, and stranglers). The species targeted—79 in continuous forest, 64 in fragments—were a mixture of common tree species in the environment (via data from previous botanical surveys) and common food resources of Propithecus diadema (based on preliminary observations and information from other sites). The plants were almost equally divided between CONT (434) and FRAG (384) study sites. I assigned each tree a percentage score for each of 4 reproductive categories—flower buds, flowers, unripe fruits, and ripe fruits—representing the estimated crown coverage. The measure of availability is volume-based rather than absolute, i.e. any given score represents a higher absolute availability in trees with larger crown sizes. Though the estimation is based on a subjective image of full capacity (100%) for a given item, it provides a rough separation based on abundance and avoids the problem of presence-absence scoring whereby trees with only 1 or 2 flowers or fruits are counted as equivalent to trees with large fruit or flower crops. Because all 4 reproductive categories occupy the same physical space within the crown, the sum of the 4 scores is ≤100%. I scored young and mature leaves separately, as estimated percentages of total leaves in the crown. Analyses are based on average percentage scores weighted by crown volumes, for 3 pooled categories: flower buds and flowers, fruits, and young leaves.
Data Analysis
I based analyses on 651 individual-days encompassing 6464 h of contact time (average 9.9 h/d). The data include 35,281 feeding bouts and 2001 hours of feeding time. I constructed monthly dietary profiles via the feeding time devoted to different plant parts and an overall profile for each group via an average of monthly values (to avoid bias toward months with greater observation time). Similarly, I constructed an overall dietary profile for Propithecus diadema at Tsinjoarivo by averaging the values for the 4 groups.
To investigate differences among groups, I analyzed monthly averages for individual subjects via a 2-factor repeated measures ANOVA, with months as blocks (von Ende 2001). The repeated measures approach was necessary because many data sets showed significant departures from sphericity. To assess correlations between monthly availability measures (plant parts), between resource availability and consumption of food items, and between dietary diversity and folivory, I used Spearman’s rank-order correlations (Siegel and Castellan 1988).
To determine the selectivity of sifaka groups for the top 10 foods in their diet, I constructed 2 indices. The first is the percentage of feeding time devoted to a plant species divided by its density (ind/ha). The second is the percentage of feeding time devoted to a species divided by its abundance in terms of crown volume (ind/ha × average crown volume [m3]). The distinction is important because some favored species have very small crown volumes. I derived frequency and average crown volume from botanical transects.
I assessed dietary diversity for both annual and monthly diets via Simpson’s diversity index (Begon et al. 1996):
wherein S = the total number of species in the diet and P i = proportion of feeding time devoted to species i. The value increases with both the specific richness of the diet (S) and the equitability of those species (rarely eaten species contribute less to D than commonly eaten species).
I examined dietary overlap among pairs of groups via Schoener’s Index (Schoener 1968):
wherein p ij is the proportional representation of food i in group j’s diet, p ik is the proportional representation of food i in group k’s diet, and n is the total number of foods both groups eat. D varies between 0 (no overlap) to 1 (complete overlap).
Results
Plant Parts Consumed
Tsinjoarivo sifakas spend 53.1% of their feeding time eating foliage, 23.9% on fruits (with or without seeds), 7.3% on seeds (without fruit), and 15.1% on flower buds and flowers. The remaining 0.60% is devoted to soil (0.35%), galls (0.11%), leaf petioles (0.01%), tree exudates (0.006%), bark (0.005%), insects (Homoptera, 0.003%), and undetermined items. Groups had similar dietary profiles in terms of the major food items (Fig. 2). Overall, the dietary profile is similar to that of Propithecus diadema at Mantadia (44.4% foliage, 30.9% seeds, 15.0% flower buds and flowers, and 6.2% fruits; Powzyk 1997), but with more fruit consumption and lower seed consumption.
All groups show marked seasonal variation (Fig. 3). They consume many fruits and seeds between January and March (rainy season), when they are most abundant (Fig. 4). In April–May, fruit/seed consumption declines, and leaves are the dominant food in June–July (early dry season). From July (FRAG) or August (CONT) through September (mid-late dry season), buds and flowers are the dominant food, though leaf consumption remains high. In October–November (late dry/early rainy season), leaves once again become important. Finally, in November–December (early rainy season), fruit/seed consumption increases in anticipation of the rainy season.
Using individual subjects’ dietary profiles, there are small but significant differences between groups and sites (Table I). FRAG1 shows the most striking differences, consuming fewer fruits and seeds and more buds, flowers, and foliage; FRAG2 profiles generally resemble those of CONT groups. However, it is important to note that individuals within groups may not constitute independent sampling points.
Correlations with Availability
Understanding the motivations behind the choice of plant parts requires an understanding of changes in resource availability. Phenological patterns in each habitat are in Fig. 4. For all groups, fruit/seed consumption correlates positively with fruit availability, while bud/flower consumption correlates negatively with fruit availability (Table II). Bud/flower consumption also tends to correlate positively with bud/flower availability and negatively with young leaf availability, but this is significant only for FRAG1. The result is consistent with dietary profiles (Fig. 3); buds and flowers are consumed mainly in the dry season, when fruit availability is lowest, suggesting that subjects prefer fruits to buds and flowers.
For 2 groups, fruit/seed consumption and young leaf availability correlate positively, which appears to be an artifact of the near-significant correlation between fruit availability and young leaf availability (Vatateza: r s = 0.510, p = 0.09, Mahatsinjo: r s = 0.559, p = 0.06). When I removed the effect of young leaf availability via Pearson’s partial correlation coefficients, correlations with fruit availability remained in all groups (p < 0.03), but when I removed the effect of fruit availability, no correlation with young leaf availability remained (0.13 < p < 0.72).
Finally, young leaf consumption is not significantly correlated with availability measures and in all cases the correlation coefficient is negative. This suggests that, despite the traditional classification of sifakas as folivores, leaves are the least preferred food. The results are in contrast with those of Meyers and Wright (1993), who suggested that sifakas track young leaves (cf. Wright et al. 2005).
Taxonomic Composition of Diet
I recorded 165 food species, and list the top 10 species for each group in terms of feeding time in Table III. Dietary diversity was highest in CONT groups; FRAG groups fed on fewer species and had lower Simpson’s diversity indices, and FRAG1 had fewer species contributing ≥1% of feeding time (Table IV). FRAG1 suffered the greatest reduction in diversity, which is consistent with habitat structure and diversity assessments that place FRAG2’s habitat intermediate between those of FRAG1 and CONT groups (Irwin 2006a). Trends are similar for diversity at the familial level.
Seasonal Variation in Dietary Diversity
For both CONT groups, dietary diversity shows a consistent pattern of seasonal change (Fig. 5). From January to March (peak fruit availability), diversity is low. During this time, CONT groups concentrated on a few tree species with large and reliable fruit crops, including Ocotea sp. (Lauraceae), Erythroxylum sp. (Erythroxylaceae), and Maintipototra (unknown). During January and February, these 3 species accounted for 60–70% of CONT groups’ feeding time. Diversity increased between March and June, as leaf consumption increased. After peaking in June, diversity was low between July and October, when plant reproductive parts are least abundant and CONT groups concentrated on buds and flowers. Finally, diversity is again high in November (the second leaf consumption peak) and declines in December, as larger fruit crops again made it possible to specialize on fewer species.
Seasonal patterns are more complex in FRAG groups. FRAG2 displays a pattern similar to that of CONT groups, while FRAG1 has low diversity throughout the year and exhibits much less seasonal variation.
Dietary composition (plant parts) had a strong effect on dietary diversity. For 3 groups, the percentage contribution of leaves to the diet correlates positively with monthly dietary diversity (CONT1: r s = 0.60, p = 0.04; CONT2: r s = 0.35, p = 0.27; FRAG1: r s = 0.68, p = 0.02; FRAG2: r s = 0.90, p < 0.001).
The Importance of Mistletoe, Bakerella clavata
Much of the seasonal variation in dietary diversity is due to a single food species, the hemiparasitic mistletoe Bakerella clavata (Loranthaceae). This species has a small crown size (1–2 m diameter), is common at both Vatateza (98.5 ind/ha) and Mahatsinjo (151.5 ind/ha), and is the most common food species for all groups (Table III).
Bakerella clavata consumption varied greatly between CONT and FRAG groups (Fig. 6). CONT groups ate Bakerella clavata at low levels (<25 % of feeding time) for most months of the year, but exhibited a heavy reliance (36–67%) during the season of lowest fruit availability (July – October). Monthly mistletoe consumption correlates negatively with overall fruit availability for CONT (CONT1: rs = −0.713, p = 0.009; CONT2: rs = −0.678, p = 0.015). Instead of ceasing reproduction during the dry season, as most trees do, Bakerella clavata exhibits extended phenology (Watson 2001), with 1 year’s fruiting season overlapping the beginning of the next year’s flowering (Fig. 7). Thus, Bakerella clavata produces buds and flowers when most trees are not producing any reproductive parts.
Among FRAG groups, a strikingly different pattern is evident: Bakerella clavata consumption is not limited to the dry season, but is more evenly distributed—or perhaps bimodal—throughout the year (Fig. 6). Consumption is low in January, when groups concentrate on seeds of Macaranga ankafinensis (Euphorbiaceae), but is then high (>45% of feeding time) in February–March. After a brief drop-off in April–June, consumption returns to high levels in July–September and intermediate levels in October–December. The sifakas consumed Bakerella clavata at high levels even during the season of fruit abundance (mistletoe consumption is not correlated with overall fruit availability; FRAG1: r s = −0.133, p = 0.68; FRAG2: r s = −0.098, p = 0.76), and it is the top-ranked food species in 10 mo for FRAG1, and 11 mo for FRAG2 (vs. 6 mo for CONT1 and 5 mo for CONT2). For FRAG groups, it is more accurately described as a staple resource, i.e., consumed at high levels in most months of the year.
Dietary Overlap
Monthly dietary overlap in terms of plant parts (Fig. 8a) is consistently high (0.68–0.95) for the 2 intrasite comparisons. In contrast, the 4 intersite comparisons show extreme variation, ranging from 0.33 to 0.93. Overlap between sites is lowest in January–February (rainy season), when CONT groups concentrated on fruit pulp (discarding seeds) while FRAG groups mostly ate fruit with seeds, or seeds alone. Overlap peaked in May–July, when values converged on intrasite comparisons, and remained high for the rest of the year.
Overlap in species consumed shows a similar pattern (Fig. 8b). Intrasite comparisons are high year-round (0.49–0.81), but each comparison has 2 troughs. The first is in the late wet/early dry season (May–June for CONT groups, April–May for FRAG), and corresponds very well to each site’s peaks in: 1) folivory and 2) dietary diversity. The second, smaller, divergence is in October–November (late dry/early wet season); it corresponds to the smaller peaks in folivory and dietary diversity. Thus, within sites, diet diverges when sifakas eat more leaves.
Intersite comparisons are more variable, ranging from 0.05 to 0.63. Values are low in January–February (rainy season), when CONT groups concentrated on large fruiting trees that are absent from fragments, then peaked in July–September (peak bud/flower consumption), when all groups relied on leaves, buds, and flowers of Bakerella clavata. Finally, overlap dropped during October–December (late dry/early wet season) when all groups diversified their diets, shifting back to foliage, and then to fruits and seeds.
Discussion
Despite extreme habitat differences (Irwin 2006a), CONT and FRAG groups have similar dietary profiles in terms of plant parts consumed. The diet is similar to those of other rain forest Propithecus (Irwin 2006b), with a few notable differences: Tsinjoarivo sifakas eat considerably fewer seeds, slightly less fruit, and considerably more leaves, buds, and flowers than P. edwardsi do at Ranomafana (Hemingway 1995), and considerably fewer seeds, and more fruits and leaves than P. diadema do at Mantadia (Powzyk 1997). CONT and FRAG groups also have similar patterns of seasonal change. For all groups, the diet shows strong seasonal variation, with a rainy season peak in fruit and seeds, a dry season peak in buds and flowers, and 2 major peaks in foliage consumption at the transitions between seasons.
However, CONT and FRAG groups differ in dietary diversity. Both FRAG groups fed on a lower overall number of species, but in terms of species contributing >1% of feeding time, only FRAG1 shows lower diversity than CONT groups. FRAG1 has also largely lost the seasonal variation in diversity of other groups. The dietary diversity of CONT groups and FRAG2 is comparable to those of Propithecus edwardsi at Ranomafana (Hemingway 1995) and P. tattersalli at Daraina (Meyers 1993): 2 groups of P. edwardsi fed on 22 and 16 species that contributed >1% of feeding time, while groups of P. tattersalli fed on 23–29 such species.
Seasonal changes in diversity are linked to folivory: diversity is highest in months of high leaf consumption. There are several possible explanations for this relationship. First, a single tree might present a richer food source (in terms of calories or nutritional gain) in fruits than in young leaves; sifakas can thus afford to be more selective, because fewer food patches must be visited in any given day. Second, intake rates (mass and/or calories) may be higher for fruit than for leaves, leaving more time for travel and allowing sifakas to seek out distant individuals of preferred species. Third, sifakas may diversify to avoid effects of secondary compounds in young leaves. If toxins and digestion inhibitors can be tolerated in small quantities but are detrimental in large quantities, i.e., if processed by enzymes in finite supply, one would expect a complementarity strategy (Glander 1982): because most compounds are unique to a species or group, animals can limit the intake of any single compound by increasing dietary diversity.
The most striking difference between CONT and FRAG groups is in the use of Bakerella clavata. CONT groups use it as a fallback resource (Terborgh 1986), relying on it heavily during the dry season when fruits and other reproductive parts are rare. During this time, Bakerella clavata produces large crops of buds and flowers: because of its extended phenology, it produces reproductive parts for almost the entire year (Watson 2001). Mistletoe is a reliable fallback resource: unlike many tree species at Tsinjoarivo, which do not reproduce each year, Bakerella clavata had high reproductive effort each year from 2000 to 2006 (local assistants corroborate the reliability). FRAG groups have elevated this fallback resource to staple status, relying on Bakerella clavata during the dry season, consuming buds, flowers, and leaves, and during much of the wet season, consuming fruits and leaves. The species accounts for 30–40% of FRAG groups’ feeding time, and is the top resource in 10–11 mo of the year.
This level of devotion to a single food resource is much higher than reported in other studies of eastern sifakas. For 2 groups of Propithecus edwardsi at Ranomafana, Hemingway (1995) reported 10.04% and 9.99% of feeding time devoted to the top-ranked species; Bakerella clavata was only the eighth- and fifth-ranked item (3.4% and 8.5% of feeding time). Similarly, for 2 groups of Propithecus diadema at Mantadia, 10.5% of feeding time, averaged over 2 groups, was spent on the top-ranked food species; Bakerella clavata contributed only 3.4% of feeding time (Powzyk 1997). Finally, 3 groups of Propithecus tattersalli at Daraina spent 6.3–15.7% of feeding time on the top-ranked resource and Bakerella spp. contributed ≤ 2.0% of feeding time (Meyers 1993).
Mistletoes are thus a critical resource for sifakas at Tsinjoarivo, despite representing a small fraction of the landscape’s biomass. Even assuming a generous average crown diameter of 2 m, Bakerella clavata presents only 410–750 m3/ha, as compared to ca. 21,000–50,000 m3/ha presented by trees >5 cm DBH. This is consistent with other studies showing that mistletoes are crucial for the survival of animal populations (Atsalis 1999; Watson 2001). Indeed, because they are easily overlooked—being small and difficult to spot within tree crowns—future research may demonstrate their importance for more species. Moreover, their importance might not be fully appreciated when studying primates in pristine forest, because there they may be important only in years when fruit crops fail or during brief periods of low resource availability. It is crucial for researchers to investigate the less synchronized or extended phenologies of nontree resources (lianas, epiphytes, hemiparasites; Morellato and Leitão-Filho 1996) to understand the mechanisms allowing them to be important food resources.
Analyses of the nutritional characteristics of Bakerella clavata and other foods are necessary to understand fully the physiological consequences of sifaka dietary differences and their implications for viability. However, indirect evidence suggests that FRAG groups’ diet is lower quality than that of CONT groups. During the rainy season when FRAG groups concentrated on fruits of Bakerella clavata, CONT groups ignored them (though they were abundant) in favor of the fruiting trees available in their habitat but absent in fragments, This is also consistent with 2 other, independent lines of evidence. First, FRAG groups spent more time feeding each day, suggesting that either resources are poorer quality and/or intake rates are lower, and they spent considerably less time grooming and playing. The difference decreased in the lean season when CONT groups ate Bakerella clavata (Irwin 2006a). Second, preliminary data suggest that adult males and immatures in FRAG groups are lighter than counterparts in CONT groups (Irwin 2006a; Irwin et al. 2007). The reduction does not occur for adult females, possibly because their dominance buffers them from the effects of reduced food quality; they can exclude subordinates from food patches. Determining the nutritional status of FRAG groups is important because if they are nutritionally stressed they may be vulnerable to longer-term threats such as predation, disease, and parasitism.
It is important to note that because this study is cross-sectional rather than longitudinal, i.e. groups were studied simultaneously in fragmented and continuous habitat separated by 12 km, naturally occurring geographic variation may have contributed to the patterns observed. Indeed, the sites differ slightly in altitude and more substantially in rainfall. However, it seems most likely that the observed differences are largely caused by fragmentation, for 2 reasons. First, conversations with local residents revealed that Mahatsinjo’s forest was previously very similar to Vatateza in specific composition and physical structure. Two of the main tree species whose fruit CONT groups consumed (Ocotea sp. 1 and Cryptocarya sp.) were common at Mahatsinjo, but have been targeted for extraction because they are preferred trees for house construction and are now largely extirpated. Because CONT groups chose the fruits over mistletoe fruits, Mahatsinjo groups likely did the same in the past. Second, the difference in rainfall between sites (recorded in 2002–2007) may be deceiving because fragmentation and habitat loss are known to affect water cycling and precipitation (Laurance 2004). Thus, rainfall at Mahatsinjo may have been higher before habitat loss and fragmentation. Nevertheless, to examine the effects of fragmentation most directly, future studies taking a longitudinal approach (following primate populations before and after fragmentation) would be invaluable.
The results have important implications for conservation of sifakas and other primates. Understanding sifaka responses to fragmentation will allow better informed management, and will aid in the selection and management of protected areas. This study has demonstrated dietary differences between fragments and continuous forest, but longer-term monitoring is necessary to assess the direct and indirect consequences of the shifts. Most importantly, planners must decide whether the conservation value of small fragments is high enough to warrant inclusion in protected areas; this is not a trivial concern in Madagascar, where fragmentation is widespread (Irwin et al. 2005). More broadly, this study will contribute to an understanding of why certain primate species tolerate fragmentation better than others, because documenting the reactions of individual species is a necessary first step to meta-analyses of variation among species.
In addition, from a theoretical perspective, the Tsinjoarivo sifakas offer an opportunity to test aspects of socioecological theory. The differing resource characteristics in fragmented and continuous forest present a natural experiment: 2 conspecific populations with differing resource distributions and too little time since fragmentation to expect evolutionary adaptations to the 2 habitats. The distribution of Bakerella clavata is different than that of most trees: mistletoes are more abundant, but exist as much smaller, more dispersed, and more easily monopolizable patches. Further study is underway to relate shifts in social behavior, ranging, and interindividual spacing to the differing resource distributions (Irwin 2007a).
In summary, I have shown that primate populations in fragments may be sustained, at least in the short term, by increasing reliance on fallback resources. The possibility for generalizations across primate species and habitats is currently limited by the paucity of detailed studies of primates in fragments. However, there are 2 reasons to assume the result may apply to other species and habitats. First, when preferred resources are rare, the probability of capturing sufficient individuals of that species in a fragment is reduced, especially when home range is limited by fragment size. Second, when preferred resources are targeted by human extraction, their density will decrease in fragments; this is likely for many frugivores that rely on large, mature fruit trees because they are often harvested for timber, fueling brewing stills, or charcoal (Medley 1993). In either case, when preferred resources are diminished, primates are forced to switch to more common or less preferred species or both, and the shifts may have important consequences for physiology, ecology, and behavior, and ultimately impact population viability. Understanding the varying responses of different primate species will be an important first step in efforts to prevent future population declines and extinctions in increasingly fragmented forests worldwide.
References
Atsalis, S. (1999). Diet of the brown mouse lemur (Microcebus rufus) in Ranomafana National Park, Madagascar. International Journal of Primatology, 20, 193–229.
Begon, M., Harper, J. L., & Townsend, C. R. (1996). Ecology (3rd ed.) Oxford: Blackwell Sciences.
Chapman, C. A., Gillespie, T. R., & Goldberg, T. L. (2005). Primates and the ecology of their infectious diseases: How will anthropogenic change affect host-parasite interactions? Evolutionary Anthropology, 14, 134–144.
Cowlishaw, G. (1999). Predicting the pattern of decline of African primate diversity: An extinction debt from historical deforestation. Conservation Biology, 13, 1183–1193.
Cullen, L., Jr., Bodmer, R. E., & Valladares Pádua, C. (2000). Effects of hunting in habitat fragments of the Atlantic forests, Brazil. Biological Conservation, 95, 49–56.
Dehgan, A. (2003). The Behavior of Extinction: Predicting the Incidence and Local Extinction of Lemurs in Fragmented Habitats of Southeastern Madagascar. Ph.D. dissertation. Chicago: University of Chicago.
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34, 487–515.
Ganzhorn, J. U., Goodman, S. M., & Dehgan, A. (2003). Effects of forest fragmentation on small mammals and lemurs. In Goodman, S. M., & Benstead, J. P. (Eds.), The natural history of Madagascar (pp. 1228–1234). Chicago: University of Chicago Press.
Ganzhorn, J. U., Goodman, S. M., Ramanamanjato, J.-B., Rakotondravony, D., Rakotosamimanana, B., & Vallan, D. (2000a). Vertebrate species in fragmented littoral forests of Madagascar. In Lourenço, W. R. & Goodman, S. M. (Eds.), Diversité et Endémisme à Madagascar (pp. 155–164). Paris: Societé de Biogéographie.
Ganzhorn, J. U., Goodman, S. M., Ramanamanjato, J.-B., Ralison, J., Rakotondravony, D., & Rakotosamimanana, B. (2000b). Effects of fragmentation and assessing minimum viable populations of lemurs in Madagascar. In Rheinwald, G. (Ed.), Isolated Vertebrate Communities in the Tropics (pp. 265–272). 4th International Symposium, Bonn.
Gilbert, K. A., & Setz, E. Z. F. (2001). Primates in a fragmented landscape: Six species in central Amazonia. In Bierregaard, R. O., Jr., Gascon, C., Lovejoy, T. E., & Mesquita, R. C. G. (Eds.), Lessons from Amazonia: The ecology and conservation of a fragmented forest (pp. 262–270). New Haven: Yale University Press.
Glander, K. E. (1982). The impact of plant secondary compounds on primate feeding behavior. Yearbook of Physical Anthropology, 25, 1–18.
Glander, K. E., Wright P. C., Daniels, P. S. & Merenlender, A. M. (1992). Morphometrics and testicle size of rain forest lemur species from southeastern Madagascar. Journal of Human Evolution, 22, 1–17.
Green, G. M., & Sussman, R. W. (1990). Deforestation history of the eastern rain forests of Madagascar from satellite images. Science, 248, 212–215.
Hemingway, C. A. (1995). Feeding and Reproductive Strategies of the Milne-Edwards’ Sifaka, Propithecus diadema edwardsi, Ph.D. dissertation, Durham, North Carolina: Duke University.
Irwin, M. T. (2006a). Ecological Impacts of Forest Fragmentation on Diademed Sifakas (Propithecus diadema) at Tsinjoarivo, Eastern Madagascar: Implications for Conservation in Fragmented Landscapes. Ph.D. dissertation, New York: Stony Brook University, Stony Brook.
Irwin, M. T. (2006b). Ecologically enigmatic lemurs: The sifakas of the eastern forests (Propithecus candidus, P. diadema, P. edwardsi, P. perrieri and P. tattersalli). In Gould, L., & Sauther, M. (Eds.), Lemurs: Ecology and adaptation (pp. 305–326). New York: Springer.
Irwin, M. T. (2007a). Living in forest fragments reduces group cohesion in diademed sifakas (Propithecus diadema) in eastern Madagascar, by reducing food patch size. American Journal of Primatology, 69, 434–447.
Irwin, M. T. (2007b) Diademed sifaka (Propithecus diadema) ranging and habitat use in continuous and fragmented forest: Higher density but lower viability in fragments? Biotropica DOI 10.1111/j.1744-7429.2007.00368.x.
Irwin, M. T., Glander, K. E., Raharison, J.-L., Samonds, K. E. (2007). Effect of habitat and sex on body mass and morphometrics of diademed sifakas (Propithecus diadema). American Journal of Physical Anthropology ,132, (S44):134.
Irwin, M. T., Johnson, S. E., & Wright, P. C. (2005). The state of lemur conservation in southeastern Madagascar: Population and habitat assessments for diurnal and cathemeral lemurs using surveys, satellite imagery and GIS. Oryx, 39, 204–218.
Jorge, L. A. B., & Garcia, G. J. (1997). A study of habitat fragmentation in Southeastern Brazil using remote sensing and geographic information systems (GIS). Forest Ecology and Management, 98, 35–47.
Laurance, W. F. (1999). Reflections on the tropical deforestation crisis. Biological Conservation, 91, 109–117.
Laurance, W. F. (2004). Forest-climate interactions in fragmented tropical landscapes. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences, 359, 345–352.
Laurance, W. F., Ferreira, L. V., Rankin-de Merona, J., & Laurance, S. G. (1998). Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology, 79, 2032–2040.
Laurance, W. F., Lovejoy, T. E., Vasconcelos, H. L., Bruna, E. M., Didham, R. K., Stouffer, P. C., et al. (2002). Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conservation Biology, 16, 605–618.
Marsh, L. K. (2003). Primates in fragments: Ecology and conservation. New York: Kluwer.
Mayor, M. I., Sommer, J. A., Houck, M. L., Zaonarivelo, J. R., Wright, P. C., Ingram, C. et al. (2004). Specific status of Propithecus spp. International Journal of Primatology, 25, 875–900.
Medley, K. E. (1993). Extractive forest resources of the Tana River National Primate Reserve, Kenya. Economic Botany, 47, 171–183.
Meyers, D. M. (1993). The Effects of Resource Seasonality on Behavior and Reproduction in the Golden-Crowned Sifaka (Propithecus tattersalli, Simons, 1988) in Three Malagasy Forests. Ph.D. dissertation, Durham, North Carolina: Duke University.
Meyers, D. M., & Wright, P. C. (1993). Resource tracking: Food availability and Propithecus seasonal reproduction. In Kappeler, P. M., & Ganzhorn, J. U. (Eds.), Lemur social systems and their ecological basis (pp. 179–192). New York: Plenum Press.
Milton, K. (1996). Interactions between a host-specific bot fly, Alouattamyia baeri, and a free-ranging howler monkey (Alouatta palliata) population in Panama. Journal of Zoology (London), 239, 39–63.
Morellato, P. C., & Leitão-Filho, H. F. (1996). Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica, 28, 180–191.
Olupot, W. (2000). Mass differences among male mangabey monkeys inhabiting logged and unlogged forest compartments. Conservation Biology, 14, 833–843.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology, 21, 587–611.
Peacock, M. M., & Smith, A. T. (1997). The effect of habitat fragmentation on dispersal patterns, mating behavior, and genetic variation in a pika (Ochotona princeps) metapopulation. Oecologia, 112, 524–533.
Pochron, S. T., Tucker, W. T., & Wright, P. C. (2004). Demography, life history, and social structure in Propithecus diadema edwardsi from 1986–2000 in Ranomafana National Park, Madagascar. American Journal of Physical Anthropology, 125, 6172.
Pochron, S. T., & Wright, P. C. (2003). Variability in adult group compositions of a prosimian primate. Behavioral Ecology and Sociobiology, 54, 285–293.
Powzyk, J. A. (1997). The Socio-ecology of Two Sympatric Indriids: Propithecus diadema diadema and Indri indri, a Comparison of Feeding Strategies and Their Possible Repercussions on Species-Specific Behaviors. PhD dissertation, Durham, North Carolina: Duke University.
Schoener, T. W. (1968). The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology, 49, 704–726.
Siegel, S., & Castellan, N. J., Jr., (1988). Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill.
Singh, M., Kumara, H. N., Ananda Kumar, M., & Sharma, A. K. (2001). Behavioural responses of lion-tailed macaques (Macaca silenus) to a changing habitat in a tropical rain forest fragment in the Western Ghats, India. Folia Primatologica, 72, 278–291.
Terborgh, J. (1986). Keystone plant resources in the tropical forest. In Soulé, M. E. (Ed.), Conservation biology: The science of scarcity and diversity (pp. 330–344). Sunderland: Sinauer.
Tutin, C. E. G. (1999). Fragmented living: Behavioural ecology of primates in a forest fragment in the Lopé Reserve, Gabon. Primates, 40, 249–265.
Tutin, C. E. G., White, L. J. T., & Mackanga-Missandzou, A (1997). The use by rain forest mammals of natural forest fragments in an equatorial African savanna. Conservation Biology, 11, 1190–1203.
Umapathy, G., & Kumar, A. (2000a). The occurrence of arboreal mammals in the rain forest fragments in the Anamalai Hills, south India. Biological Conservation, 92, 311–319.
Umapathy, G., & Kumar, A. (2000b). Impacts of the habitat fragmentation on time budget and feeding ecology of lion-tailed macaque (Macaca silenus) in rain forest fragments of Anamalai Hills, south India. Primate Report, 58, 67–82.
von Ende, C. N. (2001). Repeated-measures analysis: Growth and other time-dependent measures. In Scheiner, S. M. & Gurevitch, J. (Eds.), Design and analysis of ecological experiments (pp. 134–157). Oxford: Oxford University Press.
Watson, D. M. (2001). Mistletoe: A keystone resource in forests and woodlands worldwide. Annual Review of Ecology and Systematics, 32, 219–249.
Wright, P. C., Razafindratsita, V. R., Pochron, S. T., & Jernvall, J. (2005). The key to Malagasy frugivores. In Dew, J. L., & Boubli, J. P. (Eds.), Tropical fruits and frugivores: The search for strong interactors (pp. 121–138). New York: Springer.
Acknowledgments
I thank the Government of the Democratic Republic of Madagascar, CAFF/CORE and the Direction des Eaux et Forêts (DEF) for research authorization. Stony Brook University IACUC approved the animal capture methods. For research facilitation I thank P. Wright, B. Andriamihaja, the Malagasy Institute for the Conservation of Tropical Environments (MICET, Antananarivo), and the Institute for the Conservation of Tropical Environments (ICTE, Stony Brook: L. Donovan, F. van Berkum). I thank Margot Marsh Biodiversity Foundation, Primate Conservation, Inc., IPPL, Stony Brook University, the Earth and Space Foundation, and the National Geographic Society for providing funding and NSERC for a postdoctoral fellowship that supported manuscript preparation. For data collection assistance, I thank E. Razanadrakoto, H. Rakotoarimanana, E. Ranaivoson, J. Rakotofanala, C. Randrianarimanana, F. Ranaivomanana, J.-C. Rakotoniaina, P. Rasabo, K. Parks, T. Anderson, N. Melaschenko, J. Lapoint, E. Hatton, J. Mitchell, J. Anderson, J. Tardi, and M. Ali. Student/colleague Jean-Luc Raharison provided invaluable assistance in Madagascar, Ken Glander did the initial animal capture and training, and Karen Samonds aided throughout. Discussions with P. Wright, C. Janson, J. Fleagle, D. Doran-Sheehy, and J. Ganzhorn greatly improved the project, and the comments of C. Chapman, T. Snaith, and 3 anonymous reviewers greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Irwin, M.T. Feeding Ecology of Propithecus diadema in Forest Fragments and Continuous Forest. Int J Primatol 29, 95–115 (2008). https://doi.org/10.1007/s10764-007-9222-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-007-9222-9