Abstract
Curcumin is well known for possessing anti-inflammatory properties and for its beneficial effects in the treatment of asthma. Current study investigates the immunomodulatory and anti-inflammatory effects of curcumin using mouse model of ovalbumin-induced allergic asthma. BALB/c mice were immunized with ovalbumin on day 0 and 14 to induce allergic asthma. Animals were treated with two different doses of curcumin (20 mg/kg and 100 mg/kg) and methylprednisolone from day 21 to 28. Mice were also daily challenged intranasally with ovalbumin during treatment period, and all groups were sacrificed at day 28. Histopathological examination showed amelioration of allergic asthma in treated groups as evident by the attenuation of infiltration of inflammatory cells, goblet cell hyperplasia, alveolar thickening, and edema and vascular congestion. Curcumin significantly reduced total and differential leukocyte counts in both bronchoalveolar lavage fluid and blood. Reverse transcription polymerase chain reaction analysis showed significantly suppressed mRNA expression levels of IL-4 and IL-5 (pro-inflammatory cytokines), TNF-α, TGF-β (pro-fibrotic cytokines), eotaxin (chemokine), and heat shock protein 70 (marker of airway obstruction) in treated groups. Attenuation of these pro-inflammatory markers might have led to the suppression of airway inflammation. The expression levels of aquaporin-1 (AQP) and AQP-5 were found significantly elevated in experimental groups which might be responsible for reduction of pulmonary edema. In conclusion, curcumin significantly ameliorated allergic asthma. The anti-asthmatic effect might be attributed to the suppression of pro-inflammatory cytokines, and elevation of aquaporin expression levels, suggesting further studies and clinical trials to establish its candidature in the treatment of allergic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Asthma is characterized by complex interplay of inflammation, pulmonary edema, airflow obstruction, and other environmental factors [1]. Approximately, 25.7 million persons suffered from asthma in 2010. An increasing trend was found in the prevalence of asthma between 2001 (7.3%) and 2010 (8.4%). It was found higher in females, children, and low-income families [2]. In recent years, the number of person suffering from asthma has reached over 300 million worldwide. It is expected that asthma might affect 100 million more people by 2025. Thus, asthma has the tendency to become one of the most prevalent chronic disorders in the world [3].
The role of T-helper cells type-2 (Th2) and their cytokines is well established in the pathophysiology of allergic asthma. Cytokines, such as IL-4 and IL-5, are known to orchestrate the scope of allergic response [4]. Eotaxin coordinates with IL-5 and mediates the chemotaxis and degranulation of eosinophils. They also play role in recruitment of eosinophils at the site of inflammation [5,6,7]. TNF-α and TGF-β are released from macrophages and epithelial cells and play pro-inflammatory and pro-fibrotic roles in the pathogenesis of asthma [1]. Aquaporins (AQPs) regulate water permeability in lungs, and their reduced expression levels are associated with the progression of pulmonary edema [8]. It was proposed that anti-asthmatic therapies can reduce the pulmonary edema by increasing the levels of AQP-1 and AQP-5 [9]. The role of heat shock protein 70 (HSP70) as a marker of degree of airway obstruction in asthmatic patients is well established [10].
Oral and inhaled corticosteroids are frequently being used for the treatment of asthma; however, a large number of patients failed to respond to even high doses of corticosteroids [11]. These uncontrolled patients approximately account for 50% healthcare cost of asthma [12]. Many adverse effects are also associated with the use of corticosteroids, such as adrenal suppression, decreased bone metabolism, and suppression of growth in children [13]. Due to inadequate asthma control and adverse effects associated with asthma therapy, both patients and physicians are vastly considering plant extract and their constituents as a source of alternative medicine [14].
Curcumin is the most abundantly found curcuminoid of Indian spice turmeric and is obtained from the rhizomes of the Curcuma longa [15]. It is known to possess both anti-inflammatory and anti-oxidant properties [16]. Clinical studies have also indicated the anti-inflammatory potential of curcumin in inflammatory disorders [17]. Previously, it was demonstrated that curcumin significantly improved the mean values of forced expiratory volume 1 s and could be used for the treatment of bronchial asthma as an effective and safe add-on-therapy [18]. Curcumin is also known to inhibit Th1 cytokine profile by attenuation of IL-12 [19]. Another study displayed that curcumin prevented acute airway inflammation by inhibiting Notch1-GATA3 signaling pathway [20]. Current study investigates the anti-inflammatory and immunomodulatory effects of curcumin using mouse model of ovalbumin-induced allergic asthma.
MATERIAL AND METHODS
Experimental Animal
Thirty male BALB/c mice, weighing 28–33 g, were kept in the experimental research laboratory, University of Health Sciences, Lahore, under 12-h light/dark cycles. The standard humidity (45–65%) and temperature (22–24 °C) conditions were maintained. All the mice were provided with water and standard pallet diet ad libitum [21]. Approvals of all the experimental protocols were taken from the Ethical Review Committee, University of Health Sciences, Lahore, and were performed in accordance to the declaratoin of Helsinki developed by World Medical Association.
Induction of Allergic Asthma
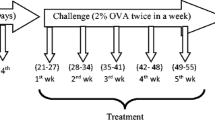
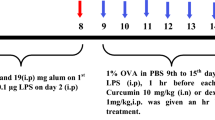
Allergic asthma was induced by intraperitoneal sensitization and subsequent intranasal airway challenge with ovalbumin. On days 0 and 14, all the groups except the control group were intraperitoneally administered with 20 μg of OVA dissolved in aluminum hydroxide solution (2 mg dissolved in 0.1 ml phosphate buffer saline). Intranasal challenge with OVA (1 mg/ml phosphate buffer saline) was given 1 week after the second sensitization to the mice for seven consecutive days (from day 21 to day 27). Control group animals were sham-sensitized as well as challenged with PBS solution only [22].
Treatment Protocol
Commercially available curcumin was purchased from Sigma-Aldrich. The animals of group III (Cur20) and group IV (Cur100) were intraperitoneally administered with curcumin at a dose of 20 mg/kg b.w. and 100 mg/kg b.w., respectively. The treatment continued daily for seven consecutive days and was scheduled 1 h before the intranasal challenge. Similarly, group V (MP) received methylprednisolone (15 mg/kg) and followed the same protocol as described for curcumin. Only normal saline was administered to group I and group II [8, 23].
Inflammatory Cell Count in Blood and BALF
TLC and DLC in both blood and BALF were evaluated using automated hemocytometer [21, 24]. The trachea with intact lungs was extracted out and subsequently lavaged for the collection of BALF. Ice-cold PBS (2 ml) was instilled and withdrawn using blunt needle [25, 26].
Histopathological Evaluation
A lobe of the lungs was fixed in 10% neutral buffered formalin and then processed for dehydration by passing them through pools of ethanol having different concentrations. Then, paraffin blocks were prepared and 5-μm-thick sections were cut for staining with hematoxylin and eosin (H&E), and periodic acid-Schiff (PAS) stains. Parameters like inflammatory cell infiltration, goblet cell hyperplasia, alveolar thickening, and edema and vascular congestion scores were determined by a histopathologist in a blinded fashion. The scoring was conducted using same criteria as mentioned in our previous publications [8, 22].
Determination of mRNA Expression Levels of IL-4, IL-5, TGF-β, TNF-α, Eotaxin, AQP-1, AQP-5, and HSP-70
Lung tissues were subjected to TRIzol method for the isolation of total RNA. The NanoDrop spectrophotometer was used for the quantification of yield and purity of total RNA. One thousand nanograms/reaction total RNA was used for the synthesis of cDNA via reverse transcription process using kit manufacturer’s protocol (Enzynomics). The reaction agents and their concentrations used for RNA isolation and reverse transcription were same as mentioned in our previous publications [8, 25]. The final mixture for cDNA synthesis was incubated at 42 °C for 60 min, and resultantly produced cDNA was stored at − 20 °C. The cDNA was used as a template for amplification by polymerase chain reaction using gene-specific primers. The primers sequences of IL-4, IL-5, TNF-α, AQP-1, AQP-5, and eotaxin were picked from our previous publications and same annealing temperatures were used as mentioned there [8, 22]. The primers of TGF-β and HSP-70 were manually designed using gene sequences picked from Ensembl Genome Browser (Table 1). The reaction mixture for PCR included 10 μl (2×) master mix (Thermo Scientific, America), 02 μl template (cDNA), and 0.5 μl forward and reverse primers (10 μM) each. Temperatures for denaturation, annealing, and elongation steps were set at 95 °C (10 s), 58 °C (20 s), and 72 °C (30 s), respectively, for a total of 30 cycles.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism v.5 software and by applying one-way ANOVA followed by Tukey’s test. The data were represented with mean ± standard deviation (SD). A P value ≤ 0.05 was considered statistically significant.
RESULTS
Curcumin Significantly Attenuated TLC and DLC in Blood
A significant elevation in total leucocyte count of diseased group was observed as compared to control which meant that ovalbumin challenge in diseased group resulted in the airway recruitment of leukocytes. Both curcumin-treated groups showed a significant decrease in TLC as compared to diseased group (all P < 0.001). Similarly, MP-treated group also showed a significant decrease in TLC as compared to diseased group (all P < 0.001).
The data showed significant increase (all P < 0.001) in neutrophil, lymphocyte, and eosinophil levels in blood of mice of diseased group as compared with control group. Treatment with both curcumin and MP caused a significant reduction (all P < 0.001) in DLC as compared with diseased group (Table 2).
Treatment with Curcumin Significantly Lowered TLC and DLC in BALF
TLC in BALF was found significantly increased in diseased group as compared to control. Treatment with curcumin and MP significantly alleviated TLC as compared to diseased group. Likewise, a significant increase (all P < 0.001) in neutrophil, lymphocyte, and eosinophil levels in BALF of mice of diseased group were found as compared to control group. Treatment with curcumin and MP significantly attenuated (all P < 0.001) DLC as compared to diseased group (Table 3).
Curcumin Significantly Attenuated Airway Inflammation
The data showed significantly higher airway inflammation in diseased group as compared to control group (all P < 0.001). Treatment with curcumin and MP resulted in significant attenuation of inflammation, when compared to diseased group (all P < 0.001) (Figs. 1a and 2).
Histopathological evaluation showed that curcumin significantly attenuated infiltration of inflammatory cells (a), goblet cell hyperplasia (b), alveolar thickening (c), and edema and vascular congestion (d). Mean ± SD values presented the data. ***P < 0.001 as compared to diseased group. ###P < 0.01 as compared to control group.
H&E staining; red arrows are showing normal lung tissue, normal alveoli, and no inflammation in control group (a). Black arrows are showing severe inflammation in peribronchial and perivascular areas. Red arrows are showing alveolar thickening in diseased group (b). In c–e, red arrows are showing resolution of alveolar thickening, while black arrows are indicating resolution of inflammation after treatment with curcumin 20 mg, curcumin 100 mg, and methylprednisolone, respectively.
Treatment with Curcumin Caused Significant Reduction in Goblet Cell Hyperplasia
The current study showed a significant increase in mucous-producing goblet cells of diseased group as compared to control (all P < 0.001). Treatment with curcumin and MP significantly inhibited goblet cells hyperplasia as compared to diseased group (all P < 0.001) (Figs. 1b and 3).
PAS staining; green arrows are showing goblet cell hyperplasia in diseased group (a). Resolution of alveolar thickening is shown with red arrows and reduction in goblet cell hyperplasia with green arrows after treatment with curcumin 20 mg (b), curcumin 100 mg (c), and methylprednisolone (d). Black arrows are indicating resolution of inflammation (c).
Curcumin Therapy Significantly Alleviated Alveolar Thickening
Alveolar thickening was also found significantly increased in diseased group as compared to control (all P < 0.001). Treatment with curcumin and MP significantly alleviated thickening of alveolar walls as compared to diseased group (all P < 0.001) (Figs. 1c, 2, and 3).
Curcumin Significantly Inhibited Edema and Vascular Congestion
The results showed a significant increase in edema and vascular congestion of diseased group as compared to control (all P < 0.001). Treatment with both curcumin and MP significantly inhibited edema and vascular congestion as compared to diseased group (all P < 0.001) (Fig. 1d).
Curcumin Significantly Reduced the mRNA Expression Levels of IL-4, IL-5, TNF-α, TGF-β, and Eotaxin
IL-4 is responsible for driving the differentiation of Th0 cells towards Th2 cells, while IL-5 is associated with eosinophilic migration and recruitment [25]. The data showed elevated mRNA expression levels of IL-4 and IL-5 in diseased group as compared to control group. Treatment with both curcumin and MP significantly reduced the mRNA expression levels of IL-4 and IL-5 as compared to diseased group (Fig. 4a, b).
Eotaxin is known to further promote the mediation of IL-5 and coordinate with later in eosinophilic migration and recruitment. Eotaxin levels were also found up-regulated in diseased group as compared to control group. A significant down-regulation was determined in eotaxin levels after treatment with both curcumin and MP (Fig. 4c).
Similarly, expression levels of TNF-α and TGF-β were also found increased in diseased group as compared to control group. TNF-α and TGF-β are both considered as fibrogenic agents and are known to induce pro-fibrotic mechanisms [27, 28]. Treatment with both low and high doses of curcumin, and MP resulted in significant attenuation of expression levels of TNF-α (Fig. 4d) and TGF-β (Fig. 5a).
Treatment with curcumin significantly reduced mRNA expression levels of TGF-β (a) and HSP-70 (d) as compared with diseased group. The expression levels of AQP-1 (b) and AQP-5 (c) were found increased after treatment with curcumin and MP. Mean ± SD values presented the data. ***P < 0.001 as compared to diseased group. ###P < 0.01 as compared to control group.
Treatment with Curcumin Significantly Elevated the mRNA Expression Levels of AQP-1 and AQP-5
Decreased expression levels of AQP-1 and AQP-5 were associated with pulmonary edema and inflammation [29, 30]. Current study also found significant down-regulation in the mRNA expression levels of AQP-1 and AQP-5 in the diseased group as compared to control group. Treatment with both curcumin and MP caused significant up-regulation of the mRNA expression levels of AQP-1 and AQP-5 as compared to diseased group (Fig. 5b, c).
Treatment with Curcumin Significantly Decreased mRNA Expression Levels of HSP-70
HSP-70 is considered as an important marker of airway obstruction in asthmatic patients. The results showed significantly increased expression levels of HSP-70 in diseased group as compared to control group. Treatment with both curcumin and MP significantly decreased the expression levels of HSP-70 (Fig. 5d).
DISCUSSION
Around 300 million people around the world suffer from asthma and the disease burden to patients, families, governments, and healthcare systems is increasing globally. This higher prevalence has been associated with elevated atopic sensitization [31]. Currently, corticosteroids and beta agonists are being used primarily as agents of choice for the treatment of uncontrolled and controlled asthma [32]. However, affordability of corticosteroids, their adverse effects due to long-term usage, and steroid insensitivity does pose a significant barrier in the management of disease, especially in developing countries [33]. So, to combat all these challenges, complexity and heterogeneity of asthma, treatment approaches that focus the single mechanism are uncertain to provide significant clinical benefit; the most effective treatment are those that target multiple mediators.
Histopathological evaluation during current study showed increased severity of inflammation, bronchial epithelial cell hyperplasia, alveolar thickening, goblet cells hyperplasia, and vascular edema in diseased group as compared with control group. These inferences are in line with the previously known pathophysiology of asthma which describes bronchoconstriction and increased mucus production as characteristic indicators of asthma. Inflammatory and structural changes in the airway wall contribute to bronchial thickening and edema formation which lead to the development of these characteristic indicators [34]. Blood counts are considered as valuable support for the monitoring of asthma [35]. Especially eosinophil counts, which are found in high concentration in peripheral blood and BALF; this is helpful in the diagnosis of asthma. Eosinophilic asthma has been a classical feature associated with Th2 mediated immune response and allergic sensitization [34]. We evaluated TLC, eosinophil counts, and neutrophil counts in both blood and BALF, and their levels were found significantly elevated in diseased group, which is in line with the results of current histopathological evaluation and our previous publication [8]. Eosinophils play an important role to develop airway hyper responsiveness by the release of inflammatory proteins and free oxidative radicals [36]. Treatment with curcumin significantly ameliorated histopathological parameters of asthma and eosinophil counts in both blood and BALF.
Eosinophils require facilitation/mediation from cytokines, such as IL-5, to get matured from precursor cells and for the purpose of activation and infiltration into tissues. Eotaxin, a chemokine, further promotes the mediation of IL-5 and also possibly contributes in the survival of eosinophils after infiltration into the tissues [37]. Appearance of mature and immature eosinophils into peripheral circulation by mobilizing them from the bone marrow sinuses is an important step in the infiltration of eosinophils into the airways in allergic asthma. IL-5 and eotaxin coordinate each other to mediate this migratory response and recruitment of eosinophils to the place of allergic inflammation, by acting systemically and locally, respectively [7]. Eotaxin binds with CCR3 which leads to the chemotaxis and degranulation of eosinophils through a downstream molecule called ERK-2 and p38 MAPK [5, 6]. We found elevation in the expression levels of IL-5 and eotaxin in diseased group which was significantly attenuated after treatment with curcumin.
IL-4 is another important cytokine which drives the T cell lineage towards Th2 cells. These activated Th2 cells then leave the lymph nodes and enter the airways, where they further release cytokines, including IL-13, IL-9, IL-6, IL-5, IL-4, IL-3, and GM-CSF [38]. Various important characteristics steps which lead to asthma development are mediated by IL-4, including switching of B-lymphocytes to IgE synthesis, activation of basophils and eosinophils, and formation of mast cells and mucus metaplasia [39]. In the present study, treatment with curcumin significantly alleviated IL-4 expression levels. These results are in line with the previous studies which emphasized on inhibition of eosinophilic function by blocking IL-4 and IL-5 or their receptors [38]. Current study also demonstrated significant increase in the expression levels of both TGF-β and TNF-α in untreated group. Both TGF-β and TNF-α has interesting implications in asthma. TGF-β can either perform the function of pro-inflammatory or anti-inflammatory cytokine [40]. Similarly, although TNF-α is largely considered as Th1 type cytokine, yet various studies have described its pro-inflammatory role in asthmatic airway inflammation [41]. The pro-inflammatory function of TGF-β is believed to be a fibrogenic agent and a mediator of leukocyte chemotaxis causing accumulation of granulocytes and macrophages to pulmonary tissue [28]. TNF-α is also known to induce the pro-fibrotic mechanisms in the sub-epithelium and influence the recruitment of eosinophils and neutrophils in asthma [27]. In addition, TGF-β is also capable of disseminating an acute inflammatory process through induction of Th17 cell differentiation and generation of IL-17 in large quantities [28]. The data showed that treatment with curcumin significantly suppressed TGF-β and TNF-α expression levels.
Pulmonary edema is another characteristic feature of asthma. Previous studies have shown markedly decreased expression levels of AQP-1 and AQP-5 associated with pulmonary edema and inflammation [29, 30]. AQP-5 and AQP-1 are expressed in the apical membrane of the respiratory epithelial cells and microvascular endothelial cells, respectively. They maintain water homeostasis and their dysfunction leads to the development of asthma [42]. Histopathological evaluation showed the development of edema and vascular changes in diseased group, which were ameliorated after treatment with curcumin. Current study also found significant reduction in the expression levels of AQP-1 and AQP-5 in diseased group. Treatment with both curcumin and methylprednisolone caused the up-regulation of aquaporin levels, which might led to the amelioration of pulmonary edema.
The airway obstruction in asthmatic patients is assessed by the levels of HSP70. HSPs are released outside the cell in response to infection, exercise, and temperature, where they convey a partial maturation signal to dendritic cells. These events lead to the pathogenesis of asthma, activation of NF-ĸB pathway, and stimulation of pro-inflammatory cytokine production [10]. Previous study demonstrated an elevation in the levels of HSP70 in the patients suffering from allergic asthma and suggested it as a potential therapeutic target [43]. We also found significantly increased levels of HSP70 in diseased group, which were found attenuated after treatment with curcumin. The attenuation of HSP70 expression levels is in line with the inferences of Liu et al. [44] who demonstrated amelioration of airway hyperresponsiveness by curcumin.
CONCLUSION
Treatment with curcumin ameliorated OVA-induced allergic asthma, which was evident by the reduction in airway inflammation and pulmonary edema in histopathological analysis. The inferences of hematological analysis in both blood and BALF further endorsed the reduction in inflammation as shown by histopathological analysis. The reduction in airway inflammation may be attributed to the attenuation of IL-4, IL-5, TNF-α, TGF-β, and eotaxin expression levels. The suppression of pulmonary edema may be ascribed to the elevation of AQP-1 and AQP-5 expression levels.
References
Ishmael, F.T. 2011. The inflammatory response in the pathogenesis of asthma. The Journal of the American Osteopathic Association 111: S11–S17.
Akinbami, L.J., J.E. Moorman, C. Bailey, H.S. Zahran, M. King, C.A. Johnson, and X. Liu. 2012. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief 94: 1–8.
Dhami, S., A. Kakourou, F. Asamoah, I. Agache, S. Lau, M. Jutel, A. Muraro, G. Roberts, C.A. Akdis, M. Bonini, O. Cavkaytar, B. Flood, P. Gajdanowicz, K. Izuhara, Ö. Kalayci, R. Mosges, O. Palomares, O. Pfaar, S. Smolinska, M. Sokolowska, M. Asaria, G. Netuveli, H. Zaman, A. Akhlaq, and A. Sheikh. 2017. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy. 72 (12): 1825–1848.
Li, Y., and S. Hua. 2014. Mechanisms of pathogenesis in allergic asthma: Role of interleukin-23. Respirology. 19 (5): 663–669.
Kampen, G.T., S. Stafford, T. Adachi, T. Jinquan, S. Quan, J.A. Grant, P.S. Skov, L.K. Poulsen, and R. Alam. 2000. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 95: 1911–1917.
Oliveira, S.H.P., and N.W. Lukacs. 2003. The role of chemokines and chemokine receptors in eosinophil activation during inflammatory allergic reactions. Brazilian Journal of Medical and Biological Research 36 (11): 1455–1463.
Sehmi, R., S. Dorman, and A. Baatjes. 2003. Allergen-induced fluctuation in CC chemokine receptor 3 expression on bone marrow CD341 cells from asthmatic subjects: Significance for mobilization of haemopoietic progenitor cells in allergic inflammation. Immunology. 109: 536–546.
Rana, S., M. Shahzad, and A. Shabbir. 2016. Pistacia integerrima ameliorates airway inflammation by attenuation of TNF- α, IL-4, and IL-5 expression levels, and pulmonary edema by elevation of AQP1 and AQP5 expression levels in mouse model of ovalbumin-induced allergic asthma. Phytomedicine. 23: 838–845.
Dong, C., G. Wanga, B. Li, K. Xiaoa, Z. Mab, H. Huangd, X. Wanga, and C. Chunxue Baia. 2012. Anti-asthmatic agents alleviate pulmonary edema by upregulating AQP1 and AQP5 expression in the lungs of mice with OVA-induced asthma. J. Resp. 181: 21–28.
Changchun, H., Z. Haijin, Li Wenjun, L. Zhenyu, Z. Dan, L. Laiyu, T. Wancheng, C. Shao-xi, and Z. Fei. Increased heat shock protein 70 levels in induced sputum and plasma correlate with severity of asthma patients. Cell Stress & Chaperones 16 (6): 663–671.
Ito, K., K.F. Chung, and I.M. Adcock. 2006. Update on glucocorticoid action and resistance. The Journal of Allergy and Clinical Immunology 117 (3): 522–543.
D'Amato, G., A. Stanziola, A. Sanduzzi, G. Liccardi, A. Salzillo, C. Vitale, A. Molino, A. Vatrella, and M. D'Amato. 2014. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): A review. Multidiscip. Respir. Med. 9 (1): 23.
Abbas, A.T., M.M. Abdel-Aziz, K.R. Zalata, and D. Abd Al-Galel Tel. 2005. Effect of dexamethasone and Nigella sativa on peripheral blood eosinophil count, IgG1 and IgG2a, cytokine profiles and lung inflammation in murine model of allergic asthma. The Egyptian Journal of Immunology 12: 95–102.
Markham, A.W., and J.M. Wilkinson. 2004. Complementary and alternative medicines (CAM) in the management of asthma: An examination of the evidence. The Journal of Asthma 41: 131–139.
Pawar, R.S., F.A. Toppo, A.S. Mandloi, and S. Shaikh. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian. J. Pharmacol 47 (2): 160–166.
Menon, V.P., and A.R. Sudheer. 2007. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 595: 105–125.
Jurenka, J.S. 2009. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alternative Medicine Review 14 (2): 141–153.
Abidi, A., S. Gupta, M. Agarwal, H.L. Bhalla, and M. Saluja. 2014. Evaluation of efficacy of curcumin as an add-on therapy in patients of bronchial asthma. Journal of Clinical and Diagnostic Research 8 (8): HC19–HC24.
Kang, B.Y., Y.J. Song, K.M. Kim, Y.K. Choe, S.Y. Hwang, and T.S. Kim. 1999. Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin-12 production in macrophages. British Journal of Pharmacology 128 (2): 380–384.
Chong, L., W. Zhang, Y. Nie, G. Yu, L. Liu, L. Lin, S. Wen, L. Zhu, and C. Li. 2014. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 37 (5): 1476–1485.
Shabbir, A., M. Shahzad, A. Ali, and M. Zia-ur-Rehman. 2014. Anti-arthritic activity of N'-[(2,4-dihydroxyphenyl)methylidene]-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-1(4H)-yl)acetohydrazide. European Journal of Pharmacology 738: 263–272.
Inam, A., M. Shahzad, A. Shabbir, H. Shahid, K. Shahid, and A. Javeed. 2017. Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-ĸB, and iNOS levels. Phytomedicine. 32: 1–7.
Zhu, T., Z. Chen, G. Chen, D. Wang, S. Tang, H. Deng, J. Wang, S. Li, J. Lan, J. Tong, H. Li, X. Deng, W. Zhang, J. Sun, Y. Tu, W. Luo, and C. Li. 2019 Apr 3. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPARγ-dependent NF-κB signaling pathway in vivo and in vitro. Mediators of Inflammation 2019: 4927430–4927415. https://doi.org/10.1155/2019/4927430.
Shabbir, A., M. Shahzad, A. Ali, and M. Zia-ur-Rehman. 2016. Discovery of new benzothiazine derivative as modulator of pro- and anti-inflammatory cytokines in rheumatoid arthritis. Inflammation. 39 (6): 1918–1929.
Ashraf, M.I., M. Shahzad, and A. Shabbir. 2015. Oxyresveratrol ameliorates allergic airway inflammation via attenuation of IL-4, IL-5, and IL-13 expression levels. Cytokine. 76 (2): 375–381.
Khan, M.A., M. Shahzad, M.B. Raza Asim, M. Imran, and A. Shabbir. 2015. Zingiber officinale ameliorates allergic asthma via suppression of Th2-mediated immune response. Pharmaceutical Biology 53 (3): 359–367.
Thomas, P.S. 2001. Tumour necrosis factor-alpha: The role of this multifunctional cytokine in asthma. Immunology and Cell Biology 79 (2): 132–140.
Tirado-Rodriguez, B., E. Ortega, P. Segura-Medina, and S. Huerta-Yepez. 2014. TGF-β: An important mediator of allergic disease and a molecule with dual activity in cancer development. J. Immunol. Res. Article ID 318481.
Krane, C.M., B. Deng, V. Mutyam, C.A. McDonald, S. Pazdziorko, L. Mason, S. Goldman, M. Kasaian, D. Chaudhary, C. Williams, and M.W.Y. Ho. 2009. Altered regulation of aquaporin gene expression in allergen and IL-13-induced mouse models of asthma. Cytokine. 46: 111–118.
Li, Z., C. Gao, and Y. Wang. 2011. Reducing pulmonary injury by hyperbaric oxygen preconditioning during stimulated high altitude exposure in rats. The Journal of Trauma 71: 673–679.
Masoli, M., D. Fabian, S. Holt, and R. Beasley. 2004. Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 59 (5): 469–478.
Bateman, E.D., S.S. Hurd, and P.J. Barnes. 2008. Global strategy for asthma management and prevention: GINA executive summary. The European Respiratory Journal 31: 143–178.
Aït-Khaled, N., G. Auregan, N. Bencharif, L.M. Camara, E. Dagli, K. Djankine, B. Keita, C. Ky, S. Mahi, K. Ngoran, D.L. Pham, O. Sow, M. Yousser, N. Zidouni, and D.A. Enarson. 2000. Affordability of inhaled corticosteroids as a potential barrier to treatment of asthma in some developing countries. The International Journal of Tuberculosis and Lung Disease 4 (3): 268–271.
Doeing, D.C., and J. Solway. 2013. Airway smooth muscle in the pathophysiology and treatment of asthma. Journal of Applied Physiology 114 (7): 834–843.
Zhang, X.Y., J.L. Simpson, H. Powell, I.A. Yang, J.W. Upham, P.N. Reynolds, S. Hodge, A.L. James, C. Jenkins, M.J. Peters, J.T. Lin, and P.G. Gibson. 2014. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clinical and Experimental Allergy 44 (9): 1137–1145.
Robinson, D.S., A.B. Kay, and A.J. Wardlaw. 2002. Eosinophils. Clinical Allergy and Immunology 16: 43–75.
Walford, H.H., and T.A. Doherty. 2014. Diagnosis and management of eosinophilic asthma: A US perspective. Journal Asthma Allergy 7: 53–65.
Gallelli, L., M.T. Busceti, A. Vatrella, R. Maselli, and G. Pelaia. 2013. Update on anticytokine treatment for asthma. BioMedical Research International 2013: 104315.
Larché, M., D.S. Robinson, and A.B. Kay. 2003. The role of T lymphocytes in the pathogenesis of asthma. The Journal of Allergy and Clinical Immunology 111 (3): 450–463.
Duvernelle, C., V. Freund, and N. Frossard. 2003. Transforming growth factor-beta and its role in asthma. Pulmonary Pharmacology & Therapeutics 16 (4): 181–196.
Babu, K.S., D.E. Davies, and T.S. Holgate. 2004. Role of tumor necrosis factor alpha in asthma. Immunology and Allergy Clinics of North America 24: 583–597.
Zhang, J., L. Gong, B. Hasan, J. Wang, J. Luo, H. Ma, and F. Li. 2015. Use of aquaporins 1 and 5 levels as a diagnostic marker in mild-to-moderate adult-onset asthma. International Journal of Clinical and Experimental Pathology 8 (11): 14206–14213.
Jassim, A.N., S.A. Brakhas, and A.J. Hassan. 2015. Study of serum interleukin 33 and heat shock protein70 in allergic disease. International Journal of Advanced Research 3 (2): 715–719.
Liu, L., Y. Shang, M. Li, X. Han, J. Wang, and J. Wang. 2015. Curcumin ameliorates asthmatic airway inflammation by activating nuclear factor-E2-related factor 2/haem oxygenase (HO)-1 signalling pathway. Clinical and Experimental Pharmacology & Physiology 42 (5): 520–529.
Acknowledgments
The authors would like to thank Department of Hematology, Department of Biochemistry, Department of Morbid Anatomy and Histopathology, Department of Immunology, and Resource Lab for providing technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approvals of all the experimental protocols were taken from the Ethical Review Committee, University of Health Sciences, Lahore.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahid, H., Shahzad, M., Shabbir, A. et al. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-inflammatory Cytokines and Aquaporins. Inflammation 42, 2037–2047 (2019). https://doi.org/10.1007/s10753-019-01066-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01066-2