Abstract
Allergic asthma is an inflammatory condition accompanied by inflammation as well as oxidative stress. Supplementation of an anti-inflammatory agent having antioxidant properties may have therapeutic effects against this disease. Over the recent decades, the interest in combination therapy as new alternative medication has increased and it offers numerous benefits along with noticeable lack of toxicity as well as side effects. In this study, protective effects of curcumin alone and in combination with piperine were evaluated in mouse model of allergic asthma. Balb/c mice were sensitized on days 0, 7, and 14 and challenged from days 16–30 on alternate days with ovalbumin (OVA). Mice were pretreated with curcumin (Cur; 10 and 20 mg/kg) and piperine (Pip; 5 mg/kg) alone and in combination via the intraperitoneal route on days 16–30 and compared with intranasal curcumin (5 mg/kg) treatment. Blood, bronchoalveolar lavage fluid (BALF), and lungs were collected after mice were sacrificed on day 31st. Mice immunized with OVA have shown significant increase in airway inflammation and oxidative stress as determined by oxidative stress markers. A significant suppression was observed with all the treatments, but intranasal curcumin treatment group has shown maximum suppression. So, among all the treatment strategies utilized, intranasal curcumin administration was most appropriate in reducing inflammation and oxidative stress and possesses therapeutic potential against allergic asthma. Present study may prove the possibility of development of curcumin nasal drops towards treatment of allergic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Asthma is emerging as one of the most common chronic respiratory disease affecting a large human population. World Health Organization (WHO) estimated about 300 million people suffering from asthma and over 80% of asthma deaths are reported from low and lower-middle income countries creating a burden on society [1]. This disease is characterized by a major condition of inflammation predominantly in the airways. There is increasing evidence that inflammation is main characteristic of asthma, which results in increased oxidative stress in the airways [2]. Inflammatory cells in the airways such as macrophages, neutrophils, and eosinophils release increased amounts of reactive oxygen species in asthmatic patients [3,4,5]. The increased release of reactive oxygen species can result in direct oxidative damage to epithelial cells and lead to cell shedding [6]. Studies have suggested that the preventive treatment of asthma is cost-effective, especially in more severe and uncontrolled cases [7].
Nowadays, herbal therapies have gained great area of interest because of their versatile applications. The use of traditional medicine is found to be cost-effective and could lead to better success [8]. Natural products derived from herbal plants have received attention for disease prevention due to their various health benefits and noticeable lack of toxicity as well as side effects [9]. About 80% populations of Asian and African countries rely on traditional medicines for primary health care [10]. Recently, the application of phytochemicals and their combination therapies, to treat various complex diseases including asthma, is emerging as a new area of interest. Combination therapies also limit their side effects and enhance their effectiveness. These therapies may serve as alternative therapies that are needed to reduce the need for continuous oral corticosteroids. The limited efficacy of therapies and side effects associated with conventional therapies has led to the introduction of better safe alternative therapy and especially for those patients in whom symptoms are not improved with current available therapies. In an attempt to develop more effective therapeutic strategies, the interest has been directed towards application of herbal remedies and their combination as better choice to treat asthma. In present study, curcumin and piperine have been used alone and in combination to examine their effects in OVA-induced chronic asthma Figs. 1, 2, and 3.
Structure of curcumin and piperine (commons.wikimedia.org and drugfuture.com).
Cytospin preparations of BALF cells after Giemsa staining. Nor group has very few number of inflammatory cells. Asth, group has large number of inflammatory cells; Veh Asth, group has similar accumulation of cells as observed in Asth group. All treated groups have reduced number of inflammatory cells (A-× 100 image of cytospin slide and in B-× 400 images of cytospin slide).
CURCUMIN
Curcumin is a naturally occurring polyphenolic phytochemical, isolated from the rhizomes of Curcuma longa (turmeric), which has been widely used for centuries as a potential candidate for the treatment of many diseases [11]. It has been shown to have a wide spectrum of biological activities, including anti-inflammatory, antioxidant, anti-proliferative, anti-carcinogenic, immunomodulatory, and anti-mutagenic [12,13,14,15].
Pharmacokinetic properties of curcumin indicate that following oral administration, it is poorly absorbed with only traces appearing in blood, while most of it is excreted in feces [16]. The absorption, biodistribution, metabolism, and elimination studies of curcumin have confirmed its poor absorption, rapid metabolism, and elimination of curcumin through oral administration as major reasons for poor bioavailability. Promises like adjuvants, which can block metabolic pathways of curcumin, different routes of administration with better absorption, complexes like nanoparticles, liposomes, micelles, and phospholipids are other emerging strategies, which appear to provide longer circulation, better permeability and resistance to metabolic pathways. However, curcumin could not achieve its expected therapeutic outcome in clinical trials due to its poor bioavailability.
PIPERINE
Piperine is main component of Piper longum Linn. and Piper nigrum Linn. is an alkaloid with a long history of medicinal value. It is known to exhibit variety of biological activities which include antipyretic, antioxidant, anti-depressant, hepatoprotective, anti-metastatic, anti-thyroid, immunomodulatory, and anti-tumor [17,18,19,20]. It is a powerful inhibitor of hepatic and intestinal glucuronidation, also increases the bioavailability of many drugs including curcumin. It has been reported to inhibit glucuronidation activity in rats and guinea pigs [21, 22]. Co-administration of piperine and curcumin in humans and rats has been shown to enhance the bioavailability of curcumin by 200 and 154%, respectively. Earlier investigations have also reported that increased serum concentration and bioavailability of curcumin in rats and men is probably due to increased absorption and reduced metabolism [21, 23].
As curcumin and piperine both possess anti-inflammatory and antioxidant activities, they may be effective in combination and could prove to be a better approach for the treatment of allergic asthma where inflammation and oxidative stress play important role in disease development. So in view of the above facts, the present study was undertaken to explore the efficacy of combined treatment of curcumin with piperine and it is compared with intranasal curcumin administration in mice model of asthma. As curcumin has poor absorption rate and undergoes rapid metabolism, which severely curtail its bioavailability, thus curcumin and its combination with piperine through intraperitoneal route and also intranasal administration of curcumin for comparative study have been tried as drug strategy. In previous findings, we found that intranasal administration of curcumin is effective in ameliorating asthmatic characteristics in acute as well as chronic asthma [24,25,26]. So, present study was planned to investigate the effects of curcumin and piperine alone and in combination against chronic asthma model in mice.
MATERIALS AND METHODS
Animals
Four- to 6-week-old pathogen-free inbred Balb/c mice were purchased from CDRI (Central Drug Research Institute, India) and housed in an animal facility under standard laboratory conditions for 1 week prior to experiments, and were provided water and standard mice feed. The study design was approved by the Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi.
Sensitization and Challenge Protocol
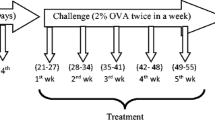
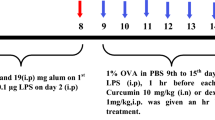
Mice were randomly divided into ten groups containing six animals in each. They were sensitized with 200 μl solution of OVA with alum (50 μg OVA adsorbed in 4 mg aluminum hydroxide) given on days 0, 7, and 14 (i.p.). They were challenged with 2% OVA aerosol for 30 min from days 16 to 30 alternatively (8 booster challenges). Curcumin and other drugs to be tested were given according to experimental protocol. Cur 10 (Curcumin 10 Mg/Kg, bw, i.p.), Cur 20 (Curcumin 20 mg/kg, bw, i.p.), Pip 5 (Piperine 5 mg/kg, bw, i.p.), Cur 10 + Pip 5 (Curcumin 10 + Piperine 5 mg/kg, bw, i.p.), Cur 20 + Pip 5 (Curcumin 20 + Piperine 5 mg/kg, bw, i.p.), Cur i.n. (Curcumin 5 mg/kg, bw, i.n.), and Sd (dexamethasone 1 mg/kg, bw, i.p.) were administered from the first day of OVA challenge (1 h prior to challenge) to last day of challenge regularly. Mice were sacrificed on 31st day, after 24 h of last aerosol challenge to obtain BALF (bronchoalveolar lavage fluid), blood, and lungs. The treatment doses were selected according to previous reports [24, 27,28,29]. The detailed experimental design for chronic asthma model development is shown in Fig. 4.
Grouping of Animals
Balb/c mice were divided into ten groups (6 mice/group), Table 1.
Collection of BALF, Blood and Lungs
Lungs were lavaged with 1 ml of chilled PBS and collected fluids were spun down at 3000 rpm for 10 min at 4 °C. BALF supernatants were stored at − 80 °C and the cell pellet was used for total cell enumeration by trypan blue dye exclusion test. Cytospin preparations were stained with Giemsa for inflammatory cells count. Serum was isolated from blood and stored at − 80 °C. Lungs were removed, washed with chilled PBS, and stored in 10% neutral-buffered formalin for histopathological analysis.
Histopathology
Lungs were fixed in 10% neutral-buffered formalin and paraffin-embedded 5-μm thin sections were cut. These sections were stained with hematoxylin and eosin (H&E) for inflammatory cells evaluation along with structural alterations and Masson’s trichrome stain was used for collagen deposition in lungs.
Determination of Eosinophil Peroxidase Activity
Eosinophil peroxidase (EPO) levels in BALF were determined [25].Substrate solution (110 ul) containing 0.1 mM o-phenylenediaminedihydrochloride (OPD), 0.1% Triton X-100, and 1 mM hydrogen peroxide in 0.05 M Tris–HCl was added to 100 μl of BALF supernatant in microtiter plates and incubated for 30 min at 37 °C. Reaction was stopped by adding 50 μl of 4 M sulfuric acid and absorbance was taken at 492 nm.
Serum IgE Level
OVA-specific IgE was measured in serum [30]. Microtiter plates were coated with 20 μg OVA in 100 μl coating buffer (sodium carbonate and sodium bicarbonate, pH 9.6) and incubated at 4 °C overnight. Plate was washed with PBST (0.05% Tween-20) and blocked with 1% BSA at 37 °C for 2 h, followed by washing with PBST. Immunoglobulin-containing serum (1:9) was incubated for 2 h at 37 °C, and washed thrice with PBST. HRP-conjugated anti-mouse IgE (1:1000) was added to the wells and incubated for 2 h at 37 °C. OVA-specific serum IgE was detected using TMB. Reaction was stopped by using 100 μl of 2.4 M sulfuric acid and absorbance was taken at 450 nm.
Measurement of Reactive Oxygen Species
Reactive oxygen species (ROS) was measured in BALF cells isolated from different experimental groups. Cells were washed with chilled PBS, counted for detection of ROS. Cells (1X105) were incubated with 20 mM DCF-DA, 100 μl for 45 min at 37 °C in the dark. After incubation, fluorescence intensity was monitored by using a spectrofluorometer (λex 490 nm; λem 515 nm). Values were normalized by the fluorescence intensity of the control cells.
Nitrite Level Estimation
Nitrite level in serum was detected as a measure of nitric oxide [26]. Briefly, 100 μl of serum was taken in 96 well plates and VCl3 (100 μl) was added for the release of nitric oxide. Griess reagent (100 μl) was added and plate was incubated for 45 min until pink color appeared. Plate was read at 540 nm in ELISA plate reader.
Statistical Analyses
All the values were expressed as mean ± SEM. Results were analyzed statistically by applying student’s t test for comparison of two means and one way ANOVA followed by Tukey’s test for more than two groups. Statistical significance was considered at P < 0.05 by using SPSS 16 software.
RESULTS
Development of Mice Model of Asthma
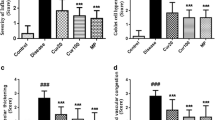
Asthmatic mice model was observed with elevated levels of IgE, EPO, ROS, and inflammatory cell recruitment in OVA-sensitized and OVA-challenged mice (Asth and Veh Asth) as compared to normal (Nor). After histological analysis, morphological destruction of bronchioles and alveolar region is clearly marked (Fig. 5) Large number of inflammatory cell recruitment was observed in lumen of asthmatic lungs (Asth and Veh Asth) with mucus accumulation (Fig. 5). Presence of collagen deposition was determined by Masson’s trichrome staining and blue colored band was also seen in asthmatic group as marker of collagen accumulation (Fig. 8).
Reduction in Airway Inflammation after Treatment
Total cells were counted in BALF to evaluate cellular infiltration into lungs. Remarkable increase in the number of leukocyte recruitments was observed in Asth and Veh Asth groups as compared to normal group (Fig. 4). Total number of inflammatory cells were significantly reduced in all treated groups but any remarkable reduction was not seen in curcumin and piperine combination (Fig. 4). Intranasal administration of curcumin has shown significant reduction in inflammatory cell accumulation, while reduction in inflammatory cell infiltration of all other groups were significant (Figs. 3a, b and 6).
Effect of curcumin, piperin, their combination, and intranasal curcumin on EPO level. EPO activity was found significantly higher in Asth and Veh asth groups while all treatments except cur10 decreased the level of EPO in BALF as compared to Asth group. The results are shown in mean ± SEM (*P < 0.05 as compared to Nor, #P < 0.05 as compared to Asth and Veh Asth).
Histological Analysis for Structural Alterations in Lungs
H&E staining was done to reveal the structural integrity as well as inflammatory cell recruitment analysis in the lungs. Large number of inflammatory cells could be seen in alveolar space in almost all the asthmatic groups and epithelial layer of bronchioles appear distorted in asthmatic groups as compared to normal. No differences were observed in all the treatment groups except curcumin (i.n.), which has shown the better structural integrity as compared to asthmatic group (Fig. 5). In terms of mucus secretion, bronchial lumen was filled with mucus in Asth and Veh Asth (Vehicle) groups but less accumulation was noted after treatments (Fig. 5). In terms of collagen deposition also, no difference was noted in treatment groups (Fig. 8).
Effect of Different Treatments on EPO Activity
Eosinophil peroxidase (EPO) activity in BALF was measured as marker of eosinophil recruitment to the lungs and it was significantly elevated in asthmatic groups (Asth and Veh Asth) as compared to normal. Elevated EPO activity was significantly ameliorated after treatment. Pip 5 and Cur 5 (i.n.) treatments were the most effective in inhibiting the level of EPO as compared to other treatment strategies (Fig. 6).
Effect of Different Treatments on IgE Level
Immunoglobulin E (IgE) levels in serum were detected and found significantly higher in asthmatic groups (Asth and Veh Asth) as compared to normal. IgE level was enhanced in OVA and vehicle asthmatic group but was significantly inhibited in all treatment groups excluding Curcumin 10 (10 mg/kg, i.p.) group. Intranasal curcumin was found better in significantly suppressing the enhanced IgE level as compared to other treatment groups (Fig. 7).
Effect of curcumin, piperine, their combination, and intranasal curcumin on IgE level. Maximum reduction in IgE level was seen in Cur (i.n.), whereas Cur 10 (i.p.) has shown least reduction. The results are shown in mean ± SEM (*P < 0.05 as compared to Nor, #P < 0.05 as compared to Asth and Veh Asth).
Effect of curcumin, piperine, their combination, and intranasal curcumin on ROS level. BALF was centrifuged and the pellet was assessed for total cell count followed by measurement of ROS which was done through spectroflurometric analysis. The results are shown in mean ± SEM (*P < 0.05 as compared to Nor, #P < 0.05 as compared to Asth and Veh Asth).
Effect of treatments on nitric oxide level. NO level was measured by Griess reagent in serum. No significant differences were observed in all the treatment groups while only intranasal curcumin administration has inhibited the nitrite level. The results are shown in mean ± SEM (*P < 0.05 as compared to Nor, #P < 0.05 as compared to Asth and Veh Asth).
Effect on Oxidative Stress Parameters After Treatments
The ROS and NO levels were measured to evaluate the oxidative stress. Significant increase in the level of ROS in asthmatic groups (Asth and Veh Asth) was observed as compared to normal. There was marked decrease in ROS level in the groups including the dexamethsone group (Fig. 8). The cur (i.n.) group has shown the maximum treatment reduction in ROS level (Fig. 9). All treatment groups have shown almost similar levels of ROS. No significant change was noted in nitrite level in serum after treatments, only cur (i.n.) group has shown some effect in reducing the level (Fig. 10).
DISCUSSION
In the present study, an attempt was made to explore the effects of curcumin, piperine, their combination, and comparison with intranasal curcumin to find out better treatment strategy in mice model of asthma. On the basis of the results of all parameters employed in this study, the asthma model was successfully developed.
Inflammation and oxidative stress are two important aspects of allergic asthma as both are involved in the progression of the disease [31, 32]. Thus, some parameters of inflammation and oxidative stress are selected for analysis. Since bioavailability of curcumin is very low; therefore, intranasal route has been selected to compare its effect with other treatment strategies. So, presently designed study is helpful to assess the possible therapeutic effects of piperine and curcumin alone and in combination against murine model of chronic asthma.
It has been reported earlier that curcumin and piperine both phytochemicals are able to attenuate inflammation. In the present study, asthmatic mice showed characteristic features of asthma and all treatment groups have shown inhibition in inflammatory parameters, whereas curcumin through intranasal route has shown the most promising effects in attenuating allergic asthma parameters at lower doses as compared to the intraperitoneal route of administration and combination therapy.
It is clear that airway inflammation is a fundamental characteristic of asthma, and persistent inflammation may lead to airway remodeling. Following sensitization and challenges, the numbers of total leukocytes in the bronchoalveolar lavage fluid (BALF) in asthmatics were apparently increased as compared to the control (Fig. 4). The BALF of the asthmatic animals showed increased number of total inflammatory cells, which is considered to be the main marker of inflammation. Higher dose of curcumin (20 mg/ kg, i.p.) has reduced the total inflammatory cell count by 38% as compared to asthmatic (P < 0.05) in dose-dependent manner. However, the combination therapy (Curcumin 10 + Piperine 5 and Curcumin 20 + Piperine 5 mg/kg, i.p.) did not show significant difference. Piperine alone (5 mg/kg, i.p.) has also decreased the total inflammatory cell count by 35%, whereas intranasal curcumin administration was found effective as 40% inhibition was noted.
Haematoxyline and eosin-stained lung sections revealed structural alterations in airways. Asthmatic lungs which have undergone changes like airway narrowing, mucus plug formation, thickened airway wall, shedding, and inflammatory cell infiltration into submucosa of bronchioles. However, all above changes were reduced in intranasal curcumin treatment groups as compared to other treatment groups which proved better effective route of administration because of its ability to maintain the structural integrity of lungs. Subepithelial extracellular matrix thickness was lower in Cur and dexamethasone groups as compared to asthmatic mice. Higher matrix deposition in Masson’s trichome-stained lung sections revealed structural changes in the lungs.
EPO released from the eosinophils being one of the important mediators, involved in the allergic reactions, are responsible for initiation leading to bronchoconstriction and difficulty in breathing [33]. Curcumin (i.p.) has inhibited EPO release in dose-dependent manner which was equivalent to intranasal administration (5 mg/kg, i.n.) and piperine (5 mg/kg, i.p.).
A key feature of bronchial asthma is elevated level of IgE [34]. After OVA sensitization and challenge, the level of OVA-specific IgE in serum gets significantly elevated both in the Asth and Veh Asth groups as compared to the Nor by 5.85 folds and 5.83 folds, respectively; whereas, this elevation was abolished in all treatment groups to some extent (Fig. 7). Cur 20, Pip 5, and Cur 5 (i.n.) treatments have decreased IgE level by 1.53, 1.64, and 1.95 folds as compared to Asth group. Elevated level of IgE in serum correlates with the severity of asthma and is the common inducer of AHR, which plays a crucial role in the pathogenesis of atopic asthma. Reducing IgE synthesis or its actions may be a key goal of asthma therapy. Intranasal curcumin has inhibited the production of IgE in the serum significantly as compared to other treatment groups.
Oxidative stress has been suggested to play an important role in the pathophysiology of asthma [35]. Allergens cause the recruitment and activation of inflammatory cells in asthmatic airways. The activated inflammatory cells generate ROS and release them into surrounding cells. When ROS levels overwhelm host antioxidant defenses, oxidative stress causes many detrimental effects on airway functions, including airway smooth muscle contraction, induction of AHR, mucus hypersecretion, epithelial shedding, and vascular exudation [36]. Our findings also demonstrate that higher dose of Cur 20, Pip 5, and lower dose of intranasal curcumin attenuated OVA-induced production of ROS by 60, 59, and 70%, respectively, as compared to Asth group. These results suggest that protective effects of these phytochemicals are related to oxidative stress inhibition, while enhanced nitrite level shows only effectiveness of intranasal curcumin (i.n.) (inhibition by 15% as compared to Asth).
DMSO was used as vehicle (curcumin solvent) and did not show anti-inflammatory effects in any of the observations including histology. So, we can suggest that curcumin itself is effective in inhibiting allergic airway inflammation. Standard drug (Sd) dexamethasone treatment has also significantly attenuated all the parameters studied except nitrite level and found effective in murine model of chronic asthma.
Intraperitoneal administration of curcumin was found effective in dose-dependent manner while its combination with piperine is not much effective. Piperine (alone) was also found effective except in suppressing nitrite level. Intranasal administration of curcumin was better at lower dose (5 mg/kg) which is equivalent to curcumin (20 mg/kg) administered through intraperitoneal route.
So, the present study suggests that intranasal treatment of curcumin has therapeutic potential to treat allergic asthma by regulating inflammation and oxidative stress via inhibiting inflammation, ROS, IgE, EPO, and nitrite levels in murine model of asthma.
References
Braman, S.S. 2006. The global burden of asthma. Chest 130: 4–12.
Dworski, R. 2000. Oxidant stress in asthma. Thorax 55: 51–53.
Calhoun, W.J., H.E. Reed, D.R. Moest, and C.A. Stevens. 1992. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. The American Review of Respiratory Research 145: 317–325.
Sedgwick, J.B., K.M. Geiger, and W.W. Busse. 1990. Superoxide generation by hypodense eosinophils from patients with asthma. American Review of Respiratory Disease 142: 120–125.
Vachier, I., M. Damon, C. Le Doucen, A.C. De Paulet, P. Chanez, F.B. Michel, and P. Godard. 1992. Increased oxygen species generation in blood monocytes of asthmatic patients. American Review of Respiratory Disease 146: 1161–1168.
Hulsmann, A.R., H.R. Raatgeep, J.C. Den Hollander, T.H.E.O. Stijnen, P.R. Saxena, K.F. Kerrebijn, and J.C. de Jongste. 1994. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. American Journal of Respiratory and Critical Care Medicine 149: 519–525.
Franco, R., A.C. Santos, H.F. do Nascimento, C. Souza-Machado, E. Ponte, A. Souza-Machado, S. Loureiro, M.L. Barreto, L.C. Rodrigues, and A.A. Cruz. 2007. Cost-effectiveness analysis of a state funded programme for control of severe asthma. Bio Med Central Public Health 7: 82.
Kumar, D., V. Arya, R. Kaur, Z.A. Bhat, V.K. Gupta, and V. Kumar. 2012. A review of immunomodulators in the Indian traditional health care system. Journal of Microbiology, Immunology and Infection 45: 165–184.
Manson, M. M., P. B. Farmer, A. Gescher, and W. P. Steward. 2005. Innovative agents in cancer prevention. In Tumor Prevention and Genetics III. Springer, Berlin, Heidelberg 257–275.
World Health Organization. 2008. Traditional Medicine Fact Sheet No. 134.
Aggarwal, B. B., Y. J. Surh, and S. Shishodia. 2007.Curcumin: The indian solid gold. The molecular targets and therapeutic uses of curcumin in health and disease. Springer Science & Business Media 595
Sandur, S.K., H. Ichikawa, M.K. Pandey, A.B. Kunnumakkara, B. Sung, G. Sethi, and B.B. Aggarwal. 2007. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radical Biology and Medicine 43: 568–580.
Reyes-Gordillo, K., J. Segovia, M. Shibayama, P. Vergara, M.G. Moreno, and P. Muriel. 2007. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress. Biochimica et Biophysica Acta (BBA)-General Subjects 1770: 989–996.
Xia, Y., L. Jin, B. Zhang, H. Xue, Q. Li, and Y. Xu. 2007. The potentiation of curcumin on insulin-like growth factor-1 action in MCF-7 human breast carcinoma cells. Life Sciences 80: 2161–2169.
Polasa, K., A.N. Naidu, I. Ravindranath, and K. Krishnaswamy. 2004. Inhibition of B (a) P induced strand breaks in presence of curcumin. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 557: 203–213.
Ammon, H.P., and M.A. Wahl. 1991. Pharmacology of Curcuma longa. Planta Medica 57: 1–7.
Lee, S.A., S.S. Hong, X.H. Han, J.S. Hwang, G.J. Oh, K.S. Lee, M.K. Lee, B.Y. Hwang, and J.S. Ro. 2005. Piperine from the fruits of piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chemical and Pharmaceutical Bulletin 53: 832–835.
Pradeep, C.R., and G. Kuttan. 2002. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clinical & Experimental Metastasis 19: 703–708.
Panda, S., and A. Kar. 2003. Piperine lowers the serum concentrations of thyroid hormones, glucose and hepatic 5′ D activity in adult male mice. Hormone and Metabolic Research 35: 523–526.
Koul, I.B., and A. Kapil. 1993. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Medica 59: 413–417.
Atal, C.K., R.K. Dubey, and J. Singh. 1985. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Journal of Pharmacology and Experimental Therapeutics 232: 258–262.
Reen, R.K., D.S. Jamwal, S.C. Taneja, J.L. Koul, R.K. Dubey, F.J. Wiebel, and J. Singh. 1993. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochemical Pharmacology 46: 229–238.
Shoba, G., D. Joy, T. Joseph, M. Majeed, R. Rajendran, and P.S.S.R. Srinivas. 1998. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica 64: 353–356.
Subhashini, P., S. Chauhan, S. Kumari, J.P. Kumar, R. Chawla, D. Dash, M. Singh, and R. Singh. 2013. Intranasal curcumin and its evaluation in murine model of asthma. International Immunopharmacology 17: 733–743.
Chauhan, P.S., D. Dash, and R. Singh. 2014. Intranasal curcumin attenuates airway remodeling in murine model of chronic asthma. International Immunopharmacology 21: 63–75.
Kumari, A., D. Dash, and R. Singh. 2015. Lipopolysaccharide (LPS) exposure differently affects allergic asthma exacerbations and its amelioration by intranasal curcumin in mice. Cytokine 76: 334–342.
Karaman, M., F. Firinci, S. Cilaker, P. Uysal, K. Tugyan, O. Yilmaz, N. Uzuner, and O. Karaman. 2012. Anti-inflammatory effects of curcumin in a murine model of chronic asthma. Allergologia et Immunopathologia 40: 210–214.
Bishnoi, M., K. Chopra, L. Rongzhu, and S.K. Kulkarni. 2011. Protective effect of curcumin and its combination with piperine (bioavailability enhancer) against haloperidol-associated neurotoxicity: cellular and neurochemical evidence. Neurotoxicity Research 20: 215–225.
Kim, S.H., and Y.C. Lee. 2009. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. Journal of Pharmacy and Pharmacology 61: 353–359.
Ram, A., M. Das, and B. Ghosh. 2003. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biological and Pharmaceutical Bulletin 26: 1021–1024.
Sahiner, U.M., E. Birben, S. Erzurum, C. Sackesen, and O. Kalayci. 2011. Oxidative stress in asthma. World Allergy Organization Journal 4: 151–158.
Kirkham, P., and I. Rahman. 2006. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacology & Therapeutics 111: 476–494.
Parra, A., M.L. Sanz, L. Vila, I. Prieto, I. Dieguez, and A.K. Oehling. 1999. Eosinophil soluble protein levels, eosinophil peroxidase and eosinophil cationic protein in asthmatic patients. Journal of Investigational Allergology & Clinical Immunology 9: 27.
Platts-Mills, T.A. 2001. The role of immunoglobulin E in allergy and asthma. American Journal of Respiratory and Critical Care Medicine 164: 1–5.
Cho, Y.S., and H.B. Moon. 2010. The role of oxidative stress in the pathogenesis of asthma. Allergy, Asthma & Immunology Research 2: 183–187.
Wood, L.G., P.G. Gibson, and M.L. Garg. 2003. Biomarkers of lipid peroxidation, airway inflammation and asthma. European Respiratory Journal 21: 177–186.
Acknowledgements
The authors are thankful to Prof. Dash for providing spectrofluorometer facility to analyze reactive oxygen species (ROS) by using DCF-DA fluorescence at the Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi. We are also thankful to University Grants Commission (UGC), DST-Science and Engineering Research Board (DST-SERB), and Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study design was approved by the Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi.
Rights and permissions
About this article
Cite this article
Chauhan, P.S., Jaiswal, A., Subhashini et al. Combination Therapy with Curcumin Alone Plus Piperine Ameliorates Ovalbumin-Induced Chronic Asthma in Mice. Inflammation 41, 1922–1933 (2018). https://doi.org/10.1007/s10753-018-0836-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0836-1