Abstract
Objective
Bacterial infections can exacerbate asthmatic inflammation depending on lipopolysaccharide (LPS) composition, the outermost component of cell wall, its exposure timings as well as host’s immune status. In present study, Balb/c mice were exposed to antigen (ovalbumin) and LPS simultaneously to establish an asthmatic model. Curcumin (diferuloylmethane), well known for its anti-inflammatory potential, was administered through intranasal route 1 h before LPS and OVA (ovalbumin) exposure to evaluate its efficacy against airway structural changes.
Methods
Inflammatory cell infiltration in lungs was measured by flow cytometry and further eosinophils were especially measured by immunofluorescence detection of major basic protein (MBP) as marker of eosinophilc granule protein. We also measured reactive oxygen species (ROS) in BALF by spectrofluorometry. MMP-9 activity was evaluated by gelatin zymography and mRNA expressions of MMP-9, TIMP-1, TGF-β1, IL-13, Collagen-1 and TLR-4 were measured in lungs. Protein expression of MAP kinases (P-ERK, P-JNK, P-p38), TLR-4, Cox-2, Lox-5 and Eotaxin was measured by western blotting. Hydroxyproline level and masson’s trichrome staining were used to evaluate collagen deposition in lung.
Results
Exposure to LPS (0.1 µg) exacerbates airway inflammation and induces structural changes in lungs by enhanced ROS production, collagen deposition, expression of genes involved in airway remodeling and activation of MAP kinases pathway enzymes. Intranasal curcumin pretreatment had significantly suppressed inflammatory mediators and airway remodeling proteins.
Conclusion
Our results strongly suggest that intranasal curcumin effectively protects LPS-induced airway inflammation and structural changes by modulating genes involved in airway remodeling in safer way; hence, it can be considered as supplementary alternative towards asthma treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiratory infections mostly bacterial have dual effect on the asthma pathology as they have been reported to exacerbate as well as protect from ongoing asthmatic inflammation. The hallmark of such exacerbated asthma involves hyperresponsiveness, increased bronchoconstriction and mucous secretion mediated by elevated levels of histamine, platelet activating factors, lipid-derived molecules like prostanoids and leukotrienes C4 (Dong et al. 2009). We reported earlier that innate immunity plays a central role in deciding phenotype of asthmatic inflammatory response, which was significantly modulated by curcumin. LPS modulates asthmatic responses differently when given at different doses to mice along with antigen. Lower dose (0.1 µg) was more effective in modulating the asthmatic response with high serum IgE, Th2 cytokine (IL-4 and IL-5) levels, eosinophil peroxidase and myeloperoxidase activities and oxidative damage in lungs (Kumari et al. 2015). Acute and chronic infective model with Chlamydia pneumonia and Mycoplasma pneumonia has significant modulation to allergic response on antigen exposure (Sutherland and Martin 2007). However, the detailed mechanism and molecules involved in modulation of innate immunity due to bacterial infection are yet to be studied. Toll-like receptor-4 (TLR-4) is an integral part of mammalian innate immune system, which recognizes LPS present on Gram-negative bacterial species. LPS binding activates multiple signaling cascades such as MAP kinases and recruitment of interleukin (IL)-1 receptor signaling complex, which involve Myd88 and IRAK (Perros et al. 2011).
MAP kinase proteins (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 stimulate the induction of multiple genes encoding inflammatory mediators and co-stimulatory molecules (Alam and Gorska 2011). The ERK1/2 kinase induces gene expression responsible for mucin production via producing MUC5AC in inflammatory cells resulting in mucus production in asthmatic model (Yang et al. 2011). JNK kinase has been reported to be associated with T cell activation and maturation, whereas p38 plays an important role in inducing expression of proinflammatory mediators IL-1β, TNF-α, IL-8, IL-6, and IL-3, especially in the macrophages (Choudhury et al. 2002; Yang et al. 2014). The current treatment regime available for asthma involves inhaled corticosteroids with several serious side effects on long-term uses, such as disruption in calcium and phosphate metabolism with subsequent risk of osteoporosis, adrenocortical suppression, bruising, skin thinning, posterior sub capsular cataracts and glaucoma (Roland et al. 2004). Arachidonic acid derivatives mainly prostaglandins and leukotrienes are hallmark mediators of asthma. Cycloxygenase-2 (Cox-2) and Lipoxygenase-5 (Lox-5) are key enzymes involved in their synthesis resulting in bronchoconstriction, airway hyperresponsiveness and mucus production (Martel-Pelletier et al. 2003).
Prostaglandins are potent chemotactic factor in asthma responsible for recruiting cells mainly mast cells and eosinophils to lungs (Arima and Fukuda 2011). Eosinophil-derived peroxidases and cationic protein cause an extensive tissue injury in the later phase of asthmatic response (McBrien and Menzies-Gow 2017). LPS alone has been reported to induce recruitment of eosinophils in nasal airways with increased release of granulocyte colony stimulating factor (GM-CSF) and interleukin-5 (IL-5) (Doreswamy et al. 2011). Recent studies have reported that neutrophils are also prime effector cells in severe form of asthma as only 50% of patients show eosinophilic dominated inflammation in their airways. These neutrophil dominated asthmatics represent newer asthmatic phenotype forms which respond poorly to inhaled corticosteroids and other anti-eosinophilic treatment strategies (Douwes et al. 2002; Green et al. 2002). Extensive infiltration of lungs with neutrophils as well as macrophages often leads to major damage in asthma as these cells contribute to release of reactive oxygen species (ROS) and other inflammatory mediators, interleukin-6, interleukin-1, tumor necrosis factor-α (Caramori and Papi 2004).
The initial inflammatory phase is followed by airway wall thickening and narrowing, edema and mucous hypersecretion referred to as airway remodeling which involves sub-epithelial fibrosis, mucous hyperplasia, neovascularization, epithelial alterations and epithelial hypertrophy (Bergeron et al. 2009). Transforming growth factor-beta (TGF-β1) is a multifactorial cytokine which acts as fibrogenic and immunomodulatory factor in asthma and secreted primarily from eosinophils, neutrophils and mast cells as well as from resident cells of lungs such as epithelial, endothelial and fibroblasts (Makinde et al. 2007). Single gene polymorphisms in TGF-β1 promoter have been reported to be associated with asthma predisposition in the Chinese population (Xue-Xi et al. 2011). IL-13, a Th2 cytokine, plays a central role in airway remodeling as it activates mucin secretion, airway reactivity, IgE synthesis and chitinase regulation. Further, in vitro studies suggest that blocking of IL-13 leads to decrease in PAS positive cells in asthma (Munitz et al. 2008; Danahay et al. 2002). The initial episodes of acute inflammation promote airway remodeling by altering the homeostasis of extracellular matrix in lungs. Matrix metalloproteinase (MMPs) and their tissue inhibitors changing ratio in lungs are two important remodeling markers in asthma. The balance between these two factors contributes to the asthma pathogenesis, as they regulate migration of inflammatory cells to lungs as well as extracellular matrix deposition and degradation (Mohamed et al. 2012). They help in maintaining homeostasis in basement membrane degeneration, epithelial repair and angiogenesis at the early phase of asthma exacerbation (Ohbayashi and Shimokata 2005). The level of MMP-9 and TIMP-1 has been reported to be increased in sputum and BALF of asthmatic patients as compared to control (Kim et al. 2013). We hypothesized here that curcumin can also modulate signaling cascades and airway remodeling changes involved in LPS-induced asthmatic inflammation with immunomodulatory actions.
Materials and methods
Experimental animals
Balb/c mice (6–8 weeks old, 20–24 g) were purchased from central animal facility of Central Drug Research Institute, Lucknow, India. Animals were kept in a well-maintained animal house with 25 °C temperature and 12 h light:dark cycle. Experimental animal handling and killing procedures were approved by the Institutional Animal Ethical Committee Banaras Hindu University, Varanasi, India.
Reagents
LPS (Escherichia coli serotype 0111:B4), ovalbumin (OVA, grade V) and curcumin (diferuloylmethane), DCFDA (Dichlorflourodiacetate) were purchased from Sigma-Aldrich (St Louis, MO, USA). The Toxin Sensor™ chromogenic LAL endotoxin assay and Toxin Eraser™ endotoxin removal kit were purchased from Genscript (Piscataway, NJ, USA). Anti-Gr1 FITC, Anti-CD4 PE, and anti-CD11b PE were purchased from Biolegend (San Diego, USA). Antibodies for P-ERK, P-JNK and P-p38 were purchased from cell signaling technology, whereas cyclooxygenase-2 and lipooxygenease-5 and HRP-conjugated secondary antibody were purchased from Cayman chemicals (Michigan, USA). Goat-anti mouse β-actin antibody was purchased from Genescript (Piscataway, NJ, USA), goat-anti-mouse anti-TLR-4 antibody was purchased from Santa Cruz biotechnology (Delaware Ave., USA) and goat-anti-rat IgG FITC was purchased from Invitrogen, whereas rat anti-mouse major basic protein antibody was kindly gifted by Mayo Clinic (Scottsdale, AZ, USA). Polyvinylidenedifluoride (PVDF) membrane was purchased from Genetix Biotech Asia Pvt. Ltd. and Immobilon western chemiluminescent HRP substrate kit was purchased from Merck (Darmstadt, Germany).
Preparation of LPS free OVA
LPS content in OVA solution was detected by limulus ameobocyte lysate assay using Toxin Sensor™ chromogenic LAL endotoxin assay kit. Up to 0.4 ng/ml of LPS contamination was detected in 1% OVA solution, which was purified by using Toxin Eraser™ endotoxin removal kit as described earlier (Kumari et al. 2015).
Development of LPS-induced mouse model of asthma
LPS-induced mouse model of asthma was established as reported earlier (Kumari et al. 2015). Briefly, mice were randomly divided into six groups (n = 5), Control, OVA, OVA + LPS, OVA + LPS + DM, OVA + LPS + CU, OVA + LPS + DE and were sensitized with 100 µg OVA and 1 mg aluminum hydroxide (i.p) on day 1 and 8 in 0.25 ml phosphate buffer saline (PBS), followed by exposure to 1% aerosolized LPS free OVA for 7 consecutive days from day 9 to 15 in a close plexiglass chamber. Control group was sensitized and challenged with PBS alone (Table 1) (Fig. 1).
Treatment schedule and sample collection
Mice were exposed to LPS (0.1 µg) in 100 µl sterile saline on day 2 of sensitization phase an hour before antigen administration through intraperitoneal route and on each day of OVA aerosol challenge. Control group was given LPS-free saline. Curcumin was administered 2 h before antigen challenge. Briefly, curcumin was dissolved in dimethylsulphoxide (DMSO) and administered (2.5 µl at 10 mg/kg) to each nostril and was kept in a supine position for few minutes (Fig. 1). Mice were killed after 24 h of last OVA—aerosol challenge, serum and lungs were stored at − 20 °C and − 80 °C, respectively, for further analysis. Right lung lobe was fixed in 10% neutral buffered formalin (NBF) for 24 h for histopathological analysis. Bronchoalveolar lavage fluid (BALF) was collected as described earlier and was stored at − 80 °C (Kumari et al. 2015).
Inflammatory cells analysis in BALF
Inflammatory cell population was measured in BALF cell pellet lungs by flow cytometry as described earlier (Chauhan et al. 2014). In brief, BALF from each group was collected and centrifuged at 3000 rpm at 4 °C for 5 min and cell pellet was collected. RBC was lysed with RBC lysis buffer and 1 × 104 cells were suspended in PBS containing 0.1% sodium azide and stained with antibodies (Anti-Gr1 FITC, Anti-CD4 PE and Anti-CD11b PE) against cell surfaces antigen on neutrophils, T cell and macrophages for 30 min. Cells were washed with PBS in dark and immediately acquired in flow cytometer (FACS caliber, BD Biosciences) and analyzed with Cell Quest Pro software.
Reactive oxygen species (ROS) measurement in BALF
Reactive oxygen species was measured in BALF (Eruslanov and Kusmartsev 2010). Briefly, pooled BALF cell pellet from each group was washed with PBS and 1 × 106 cells were plated in a flat bottomed black plate. A freshly prepared DCFDA (10 µm) in DMSO was added and the plate was incubated for 30 min at 37 °C in dark. Fluorescence was measured at excitation (485 nm) and emission (530 nm) wavelengths in a microplate fluorescence reader (Bio-Tek instruments Inc., 9 Winooski, VT, USA). ROS level in each group was represented as fluorescence intensity in arbitrary units.
Immunofluorescence detection of eosinophils
The major basic protein (MBP) was detected in lung section as an indirect measurement of eosinophil recruitment using immunofluorescence as described earlier (Stelts et al. 1998). In brief, paraffin-embedded lung sections (5 µm) were deparaffinized and dehydrated. The epitopes were kept free in 1% trypsin and 0.1% CaCl2 (pH-7.6) at 37 °C for 30 min and washed with phosphate buffer saline 0.05% tween 20 (PBST). Sections were blocked with 10% normal serum containing 0.1% bovine serum albumin (BSA) for 2 h followed by washing with PBST. Sections were incubated in rat anti-mouse MBP antibody (1:500) overnight at room temperature and blocked in 1% chromotrope for 1 h. Further sections were washed with PBST and incubated in fluorescein-tagged secondary antibody (1:500) for 1 h and mounted in 50% glycerol and observed under fluorescence microscope.
MMP-9 activity by gelatin zymography
Enzyme activity of MMP-9 was checked in lung tissue by gelatin zymography with some modifications (Kupai 2011). Lungs were homogenized (10%) in Tris buffer (pH 7.8) and centrifuged (12,000 rpm at 4 °C) for 20 min. The supernatant was collected and protein concentration was measured by Lowry’s method using bovine serum albumin (BSA) as standard (Lowry et al. 1951). Proteins (50 µg) from each group were mixed with loading buffer containing Tris buffer 0.5 M (pH 6.8) 1.25 ml, 1.00 ml glycerol, 2 ml 10% SDS and 0.1% bromophenol blue in 1:1 ratio. Gelatin (10 mg/ml) dissolved in distilled water at 60 °C was incorporated in 10% SDS-PAGE gel as a substrate. Prepared protein sample was electrophoresed at 4 °C for 3 h and gel was washed in distilled water once, followed by washing in 2.5% Triton X-100 for 30 min on a rocking platform. Gel was again washed in distilled water and incubated in the buffer containing 0.02% NaN3, 0.15 M NaCl, 10 mM CaCl2 and tris buffer, pH 7.6 at 37° for 48 h. Gel was stained with 2.5% coomassie brilliant blue for 30 min and destained in solution containing acetic acid and methanol to observe clear white bands against blue background. Bands were observed under gel documentation system and analyzed using alpha imager 4.0 software.
Immunoblotting
Lung homogenate (10%) was prepared in a homogenizing buffer containing phenylmethane sulfonyl fluoride, 1 mM sodium orthovanadate, 2.0 µg/ml lipoprotinin, 2.0 µg/ml aprotinin, and 1 mM dithiothreitol and centrifuged at 12,000 rpm for 20 min. Protein content in supernatant was quantified using Folin’s Ciocalteu reagent. Proteins (50 µg) were electrophoresed on 12% SDS-PAGE and transferred on polyvinylidene difluoride (PVDF) membrane in semidry transfer (Bio-Rad trans-Blot SD Semi-dry electrophoretic transfer cell) followed by blocking in 4% BSA. Blot was probed with mouse primary antibodies against MMP-9 (1:2000), TIMP-1 (1:2000), LOX-5 (1:2000), COX-2 (1:2000), TLR-4 (1:4000), P-ERK (1:4000), P-JNK (1:4000), p-p38 (1:2000) and eotaxin (1:4000) in different experimental sets. Proteins were detected using HRP-linked mouse anti-IgG secondary antibody (1:10,000) and enhanced chemiluminscence kit. Gene expression was analyzed after normalizing β-actin expression using image J software.
Quantitative real-time PCR for mRNA expression
Total cellular RNA was isolated from lungs using Trizol reagent followed by DNAase treatment. RNA (2 μg) was converted into cDNA and gene-specific primers for TLR-4, TGF-β1, IL-13, MMP-9, TIMP-1 and Collagen-1 were used to amplify in ABI 7500 with condition (Table 2). Data were presented as fold change in mRNA expression as compared to GAPDH using ΔΔCt (double delta Ct) method. In brief, differences between test gene being tested, experimental (TE) and housekeeping gene experimental HE (TE–HE) and gene being tested control (TC) and housekeeping gene control (HC) (TC–HC) were calculated to get ΔCt (Delta Ct values) for the experimental (ΔCTE) and control (ΔCTC) conditions, respectively. ΔΔCt was calculated by subtracting ΔCTE and ΔCTC (ΔCTE–ΔCTC). Finally, fold change was calculated using the formula 2−ΔΔCt.
Hydroxyproline level measurement in lungs
LPS-induced collagen content was quantified by measuring hydroxyproline as described earlier (Kumari et al. 2017). In brief, lung homogenate (10%) in PBS was mixed with 12 N HCL (1:1) and hydrolyzed for 110 °C for 16 h. Following hydrolysis samples were centrifuged at 13,000 rpm for 15 min at 4 °C and 50 µl of the supernatant was mixed with citrate acetate buffer (pH 6). Further, freshly prepared 1 ml chloramine-T solution was mixed and incubated for 20 min at room temperature. Ehrlich’s solution (1 ml) was mixed with samples and incubated for 65 °C for 30 min. The absorbance of samples was read at 550 nm in a spectrophotometer and hydroxyproline content was calculated using standard curves. Hydroxyproline concentration in lungs was expressed in µg/mg of lung tissue.
Masson’s trichrome staining
Formalin fixed lungs were paraffin embedded and cut into 5 μm thin sections and subjected to Masson’s trichrome stain to detect collagen deposition as a measurement of airway remodeling. Sections were observed under light microscope (40×) and analyzed by a blind observer.
Liver and kidney function test
Liver function was assessed by measuring aspartate transaminase (AST) and alanine transaminase (ALT) activities using Autospan Liquid Gold AST Kinetic assay and Autospan Liquid Gold ALT Kinetic assay kits, respectively, whereas kidney function was assessed by measuring creatinine level in serum using Creatinine measuring kit (Beacon) as per manufacturer’s instructions.
Statistical analysis
All the values were expressed as mean ± SEM. Significant difference between the groups was analyzed statistically by applying student’s t test and one-way ANOVA using SPSS 16 followed by Tukey’s test. Statistical significance was considered at p < 0.05. The experiments were repeated three times and one representative set of data has been presented here.
Results
Curcumin suppresses inflammatory cells recruitment and ROS production in lungs
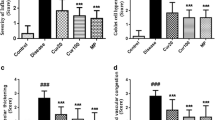
LPS exposure during sensitization and antigen challenge had caused significant increase in lung inflammation. Flow cytometry analysis of BALF cells had shown significant increase in neutrophils and macrophages as compared to only OVA-sensitized groups; however, CD4 cell count was lowered in LPS-exposed groups as compared to OVA. There was significant suppression in cell infiltration in curcumin-pretreated groups and dexamethasone-treated groups, which had probably lead to reduction in ROS production as compared to LPS-exposed groups (Figs. 2 a–c and 3).
Inflammatory cells were analyzed in BALF by flow cytometry (a–c). Unstained cells are shown here as control cell population. Neutrophils and macrophages were remarkably high in response to LPS administration as compared to OVA-treated andcontrol group, however T-helper cells were lowered in LPS-exposed group cells, which was suppressed by intranasal curcumin treatment resulted in suppression of neutrophils, T cell number and macrophage numbers
Effect of intranasal curcumin on ROS generation LPS exposure had significantly increased ROS production as compared to OVA alone. Intranasal curcumin treatment has lowered ROS level as compared to LPS-exposed groups. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, p < 0.05 (n = 5)
Curcumin suppresses eosinophils recruitment in lungs
MBP expression was checked as an indirect measurement of eosinophil infiltration in lungs, which was significantly higher near large and small airways in LPS-induced groups as compared to OVA-alone group; similar results were observed in the peribrochiolar space. Intranasal curcumin-treated groups had significantly low expression of MBP as compared to LPS-induced and OVA-sensitized groups (Fig. 4).
MBP detected in the lung section by immunohistochemistry. LPS treatment had resulted in high number of eosinophil recruitment in lungs near bronchioles and the peribrochiolar space as well. However, there was a marked suppression in the number of cells in the curcumin- and dexamethasone-treated groups
Curcumin suppresses MMP-9 activity in lungs
MMP-9 activity was checked in gelatin-incorporated polyacrylamide gel, which was observed in inactive and active forms. It was significantly higher in LPS-induced groups as compared to OVA alone. Intranasal curcumin pretreatment had caused significant decrease in MMP-9 activity in curcumin-treated groups as compared to other groups (p < 0.05) (Fig. 5).
Gelatin zymography in lung tissue showing MMP-9 activity. The level of active MMP-9 was higher in LPS exposed group as compared to OVA and control groups. The curcumin-treated group had significantly suppressed enzyme activity. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, **OVA + L vs. OVA + L + DEXA, p < 0.05 (n = 5)
Curcumin modulates inflammatory enzymes and MAP kinase gene expression
Protein expression of Cox-2, Lox-5 and Eotaxin was measured in lungs by western blotting. We observed that there was significant elevation in Cox-2 and Eotaxin level in lungs, whereas there was no change in Lox-5 expression. Further, a significant suppression in expression of these proteins was observed in curcumin-treated groups. Expression of MAP kinase and TLR4 was also measured in the lungs. Expression of TLR-4, P-p38, P-ERK and P-JNK was higher in LPS-exposed groups as compared to OVA alone and control groups. Intranasal curcumin pretreatment had resulted in significant suppression of these factors in lungs as compared to other groups (p < 0.05) (Figs. 6 and 7).
Protein expression of eotaxin, Cox-2 and Lox-5 were measured by western blotting in lung tissue. Marked increase in expressions of Eotaxin and Cox-2 in LPS-exposed groups was observed as compared to OVA and control, whereas Lox-5 expression was not changed. Curcumin administered group had resulted in significant suppression of these proteins. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, **OVA + L vs. OVA + L + DEXA, p < 0.05 (n = 5)
Protein expression of TLR-4, p-ERK, p-JNK and P-p38 was measured by western blotting in lung tissue (a–d). Marked increase in the expression of theses in LPS-treated group was observed as compared to OVA and control groups. Curcumin administered group had resulted in a significant suppression in these proteins. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, **OVA + L vs. OVA + L + DEXA, p < 0.05 (n = 5)
Curcumin suppresses mRNA expression of TLR-4 and genes involved in airway remodeling
We measured mRNA expression of MMP-9, TIMP-1, Collagen-1 and TGF-β1 in lungs as these are important genes involved in airway remodeling and asthma pathogenesis. The levels of these genes were significantly higher in LPS-induced group as compared to OVA alone suggesting their active role in airway remodeling. mRNA expressions were significantly suppressed in intranasal curcumin-treated groups. The mRNA of TLR4 in lungs was measured to evaluate the possible role of innate immunity in asthma. The level of TLR4 was significantly higher in LPS-induced group which was significantly suppressed after curcumin treatment as well as dexamethasone (p < 0.05) (Figs. 8, 9).
mRNA expression of MMP-9 and TIMP-1 was measured in lung tissue. The level of MMP-9 was higher in LPS-induced asthmatic group as compared to control and OVA groups; however, TIMP-1 level was not significantly high. Intranasal curcumin-treated group had lower mRNA expression of TIMP-1 and MMP-9. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, **OVA + L vs. OVA + L + DEXA, p < 0.05 (n = 5)
mRNA expression of IL-13, TGF-β1, Collagen-1 and TLR-4 were measured in lung tissue. The mRNA expression of these genes was higher in LPS-induced asthmatic group as compared to control and OVA group. Intranasal curcumin pretreatment had suppressed mRNA level of IL-13, TGF-β1 and Collagen-1 as compared to LPS-treated group. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, **OVA + L vs. OVA + L + DEXA, p < 0.05 (n = 5). # vs. OVA group * vs. OVA + L, **vs. OVA + L, p< 0.05 (n = 5)
Curcumin suppresses collagen deposition in asthmatic lungs
The level of hrdroxyproline in lungs was measured as an indicator of collagen deposition, which was further confirmed by Masson’s trichrome staining of paraffin-embedded lung sections. We observed that LPS exposure had significantly modulated airway architecture of lungs as higher hydroxyproline level and collagen deposition near bronchioles were observed in LPS-exposed groups as compared to OVA alone. They were significantly ameliorated in curcumin-treated groups (Figs. 10, 11).
Masson’s trichrome stained lung sections. Control had lowered collagen and inflammation in alveolar spaces whereas OVA, OVA + LPS and LPS + LPS + DM groups had inflammation, bronchoconstriction and collagen deposition in the alveolar space near bronchioles. Curcumin-treated group OVA + LPS + CU (10 mg/kg, i.n) was effective in reducing fibrosis as compared to dexamethasone
Effect of curcumin on kidney and liver function test
AST, ALT activities and creatinine levels were measured in serum to evaluate alterations in liver and kidney function due to LPS exposure and intranasal curcumin treatment. We observed that these markers were significantly suppressed in curcumin-pretreated animals, which was not altered due to LPS-treated groups (Fig. 12).
Effect of curcumin on the ALT (alanine aminotransferase), AST (aspartate aminotransferase), and creatinine level in serum. Curcumin administered through intranasal route had no significant effects on the level of these markers. The values are mean ± SEM, # OVA vs. OVA + L group, *OVA + L vs. OVA + L + CU, p < 0.05 (n = 5)
Discussion
The initial inflammatory processes in asthma involve recruitment of inflammatory cells and release of proinflammatory cytokines stimulating apoptosis and oxidative damages resulting in tissue injury. To restore tissue integrity and normal lung function, the repair processes start at later stages, whereas the onset of structural changes leads to increased vascularity, airway edema, basement membrane thickening, mucus gland hyperplasia, changes in the extracellular matrix and lining of airway passage (Sumi and Hamid 2007; Lloyd and Hawrylowicz 2009). LPS has been shown to exacerbate asthmatic inflammation; hence, the effect of LPS was studied on structural changes in lungs and immunodulatory effects of intranasal curcumin were evaluated. We measured neutrophils, macrophages and CD4 T-helper cell population in BALF cells using flow cytometry, as earlier measured by Geimsa staining (Kumari et al. 2015). Enhanced recruitments of neutrophils and macrophages in LPS-induced groups were noted as compared to OVA-alone group suggesting active role of LPS in lung damage as we had reported earlier (Kumari et al. 2015). Asthmatic inflammation has been characterized by higher Th2 and lower Th1 populations. Lowered CD4 cells in BALF may be due to the presence of reduced Th1 cells after LPS exposure as compared to only OVA-exposed groups responsible for asthmatic exacerbations (Nelson et al. 2003). Intranasal curcumin had actively suppressed inflammatory cell recruitment in lungs. Lower inflammatory cell recruitment in lungs also resulted in less ROS production in intranasal curcumin-treated groups suggesting that activated neutrophils and macrophages are major sources of oxidative damage in asthma (Figs. 2, 3).
Role of eosinophils in LPS-induced asthmatic model has been reported with increased eosinophil peroxidase level in BALF as well as in the lung tissue; hence, we measured MBP in paraffin-embedded lung sections to evaluate eosinophil recruitment. MBP is a potent cytotoxic enzyme present in the eosinophil and it is a potent inducer of histamine release from mast cells and basophils. It can also activate neutrophils as well as macrophages and induce airway bronchoconstriction and hyperesponsiveness (Gundel et al. 1991). Elevated EPO (eosinophils peroxidases level) was observed in our earlier study suggesting high eosinophils count in lungs. In the present study, we observed that there was an increase in MBP expression in peribrochiolar space as well as near bronchioles in LPS-treated animal lungs, which was effectively suppressed in the intranasal curcumin-treated groups as compared with other groups (Fig. 4). Eotaxin is a major chemokine factor mediating recruitment of eosinophils in asthma through CCR3, a single chemokine receptor. It mediates eosinophil recruitment as deletion of these receptors impairs eosinophil recruitment in acute experimental models (Fulkerson et al. 2006). Eotaxin released from epithelial and smooth cells of lungs plays an important role in asthmatic inflammation as it mediates early Th2 cell recruitment in asthma (Conroy and Williams 2001). We have reported for the first time that eotxain expression was significantly increased in LPS-induced asthmatic mice and intranasal curcumin pretreatment was effective in suppressing eotaxin expression. Inflammatory enzymes, Cox-2 and Lox-5, involved in prostaglandin and leukotriene production are extensively studied as participation inflammatory mediators responsible for the hallmark features of asthma such as bronchoconstriction and mucus production (Peebles and Sheller 2002; Claar et al. 2015). Leukotrienes are potent stimulator of leukocyte activation and release of proinflammatory cytokines including macrophages (Martel-Pelletier et al. 2003). Cox-2 expression was significantly higher, whereas Lox-5 expression was unaffected after LPS treatment and both the mediators were significantly suppressed in intranasal curcumin-treated groups (Fig. 6). TLR4 has been reported to play an important role in asthma as polymorphism on TLR4 locus and TLR4 promoter has been associated with asthma susceptibility (Bottcher et al. 2004; Al-Alawi et al. 2014). We measured mRNA and protein expression of TLR4 in lungs, which were significantly higher in LPS-induced groups as compared to the OVA-alone groups, suggesting cross-talk between innate and adaptive immunity in asthmatic exacerbation.
In previous studies, curcumin has been reported to attenuate several inflammatory diseases by inhibiting TLR4/MyD88/NF-kβ pathways, thus preventing the secretion of proinflammatory cytokines. It has also been reported that curcumin prevents ligand-induced and ligand-independent dimerization of TLR4 and, thus, can inhibit TLR4-mediated signaling pathway at the receptor level (Zhu et al. 2014; Sackesen et al. 2005). Intranasal curcumin was able to suppress protein as well as mRNA expression of TLR4 in lungs to a significant level and, hence, can protect from asthmatic exacerbations to a great extent. LPS binding to the TLR-four activates downstream MAP kinase proteins p38/ERK/JNK which regulates several characteristic features of asthma pathogenesis in airways such as epithelial cell damage, airway remodeling and inflammation (Chopra et al. 2008). Protein expression of phosphorylated forms of p38, ERK and JNK in lungs was evaluated. Significantly increased expressions of these molecules in LPS-induced groups were noted which were significantly ameliorated by intranasal curcumin treatment (Fig. 7). MMP-9 is most important among all matrix metalloproteinases as it regulates recruitment of inflammatory cells to lungs and induces cytokine release from them. The ratio of MMP-9 and its inhibitor (TIMP-1) has been reported to be associated with the asthma severity (Oshita et al. 2003; Belleguic et al. 2002). We measured MMP-nine activity by gelatin zymography and mRNA expression of MMP-9 and TIMP-1 which was increased markedly in LPS-treated animals as compared to the control and it was significantly suppressed by intranasal curcumin treatment (Figs. 5 and 8).
IL-13 is the most potent inducer of human epithelial cell proliferation, which has been reported to be higher in BALF of asthmatic with increased diseases severity (Shim et al. 2001). Therefore, mRNA expression of IL-13 was measured which was found much higher in LPS-induced group as compared to the OVA-alone group. Curcumin treatment had significantly ameliorated IL-13 expression in lungs. TGF-β1 is multifunctional regulators in asthma and can activate multiple signaling cascades responsible for inducing Fas-dependent apoptosis in the epithelial cells and fibrotic changes in alveolar spaces. It also induces smooth muscle cells to express more extracellular matrix proteins and inflammatory factors like plasminogen activator inhibitors (PAI-1), Cox-2 synthesis and interleukin-β1 (IL-β1). TGF-β1 plays an important role in asthmatic exacerbations by modulating inflammation, bronchoconstriction and fibrosis and, thus, can be a better therapeutic target. LPS was able to enhance mRNA expression of TGF-β1 and collagen-1 by synergtically acting with antigen in the murine model; further expression of these two mediators was significantly suppressed by intranasal curcumin (Fig. 9).
Prominent structural changes in lungs were correlated by collagen deposition. Increased hydroxyproline level and collagen deposition in lungs were detected by masson’s trichrome staining in LPS-exposed lungs. Curcumin treatment had significantly lowered collagen deposition in lungs which can be correlated with gene expression results (Figs. 10, 11). The present study suggests that intranasal curcumin treatment protects against detrimental remodeling changes in the lungs by modulating key signaling pathways and release of inflammatory mediators. Curcumin treatment did not alter liver and kidney functions as compared to other corticosteroid drugs (Fig. 12). Intranasal curcumin can be used as adjunct medication for asthma exacerbations caused by bacterial endotoxin exposure.
References
Al-Alawi M, Hassan T, Chotirmall SH (2014) Transforming growth factor β and severe asthma: a perfect storm. Respir Med 108:1409–1423
Alam R, Gorska MM (2011) Mitogen-activated protein kinase signaling and ERK1/2 bistability in asthma. Clin Exp Allergy 41:149–159
Arima M, Fukuda T (2011) Prostaglandin D2 and TH2 inflammation in the pathogenesis of bronchial asthma. Korean J Intern Med 26:1–8
Belleguic C, Corbel M, Germain N, Lena H, Boichot E, Delaval PH, Lagente V (2002) Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy 32:217–223
Bergeron C, Al-Ramli W, Hamid Q (2009) Remodeling in asthma. Am Thorac Soc 6:301–305
Bottcher MF, Hmani-Aifa M, Lindstrom A, Jenmalm MC, Mai XM, Nilsson L, Zdolsek HA, Bjorksten B, Soderkvist P, Vaarala O (2004) A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12 (p70) responses in Swedish children. J Allergy Clin Immunol 114:561–567
Caramori G, Papi A (2004) Oxidants and asthma. Thorax 59:170–173
Chauhan PS, Dash D, Singh R (2014) Intranasal curcumin attenuates airway remodeling in murine model of chronic asthma. Int Immunopharmacol 21:63–75
Chopra P, Kanoje V, Semwal A, Ray A (2008) Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin Investig Drugs 17:1411–1425
Choudhury BK, Wild JS, Alam R, Klinman DM, Boldogh I, Dharajiya N, Mileski WJ, Sur S (2002) In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma. J Immunol 169:5955–5961
Claar D, Hartert TV, Peebles RS Jr (2015) The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med 9:55–72
Conroy DM, Williams TJ (2001) Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res 2:150–156
Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT (2002) Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 282:226–236
Dong L, Li H, Wang S, Li Y (2009) Different doses of lipopolysaccharides regulate the lung inflammation of asthmatic mice via TLR4 pathway in alveolar macrophages. J Asthma 46:229–233
Doreswamy V, Alexis NE, Zhou H, Peden DB (2011) Nasal PMN response to repeated challenge with endotoxin in healthy volunteers. Inhal Toxicol 23:142–147
Douwes J, Gibson P, Pekkanen J, Pearce N (2002) Non-eosinophilic asthma: importance and possible mechanisms. Thorax 57:643–648
Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Adv Protoc Oxidative Stress 594:57–72
Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME (2006) A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci 103:16418–16423
Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID (2002) Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 57:875–879
Gundel RH, Letts LG, Gleich GJ (1991) Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest 87:1470–1473
Kim JS, Kang JY, Ha JH, Lee HY, Kim SJ, Kim SC, Ahn JH, Kwon SS, Kim YK, Lee SY (2013) Expression of nerve growth factor and matrix metallopeptidase-9/tissue inhibitor of metalloproteinase-1 in asthmatic patients. J Asthma 50:712–717
Kumari A, Dash D, Singh R (2015) Lipopolysaccharide (LPS) exposure differently affects allergic asthma exacerbations and its amelioration by intranasal curcumin in mice. Cytokine 76:334–342
Kumari A, Dash D, Singh R (2017) Curcumin inhibits lipopolysaccharide (LPS)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (TNF-α and TGF-β1) in murine model. Inflammopharmacology 25:329–341
Kupai K (2011) Gelatin zymography for detection of matrixmetalloproteinase-2 and-9 (MMP-2, MMP-9) from myocardium samples. In a practical manual p 29
Lloyd CM, Hawrylowicz CM (2009) Regulatory T cells in asthma. Immunity 31:438–449
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Makinde T, Murphy RF, Aggarwal DK (2007) The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol 2007:85–348
Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP (2003) Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 62:501–509
McBrien CN, Menzies-Gow A (2017) The biology of eosinophils and their role in asthma. Front Med 4:93
Mohamed GM, Farres MN, Mahmoud H (2012) Interplay between matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in acute asthma exacerbation and airway remodeling. Egypt J Chest Dis Tuberc 61:35–39
Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME (2008) Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci 105:7240–7245
Nelson HS, Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST (2003) Airway remodeling in asthma: new insights. J Allergy Clin Immunol 111:215–225
Ohbayashi H, Shimokata K (2005) Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy 4:177–181
Oshita Y, KogaT Kamimura T, Matsuo K, Rikimaru T, Aizawa H (2003) Increased circulating 92 kDa matrix metalloproteinase (MMP-9) activity in exacerbations of asthma. Thorax 58:757–760
Peebles RS, Sheller JR (2002) Role of prostaglandins in asthma. Immunol Allergy Clin 22:827–844
Perros F, Lambrecht BN, Hammad H (2011) TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res 12:125
Roland NJ, Bhalla RK, Earis J (2004) The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest 126:213–219
Sackesen C, Karaaslan C, Keskin O, Tokol N, Tahan F, Civelek E, Soyer OU, Adalıoglu G, Uncer A, Birben E, Oner C (2005) The effect of polymorphisms at the CD14 promoter and the TLR4 gene on asthma phenotypes in Turkish children with asthma. Allergy 60:1485–1492
Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, Tam DCW, Nadel JA (2001) IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol 280:134–140
Stelts D, Egan RW, Falcone A, Garlisi CG, Gleich GJ, Kreutner W, Kung TT, Nahrebne DK, Chapman RW, Minnicozzi M (1998) Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am J Respir Cell Mol Biol 18:463–470
Sumi Y, Hamid Q (2007) Airway remodeling in asthma. Allergol Int 56:341–348
Sutherland ER, Martin RJ (2007) Asthma and atypical bacterial infection. Chest 132:1962–1966
Xue-Xi Y, Fen-Xia L, Wu YS, Wu D, Jia-Yu T, Li M (2011) Association of TGF-[beta] 1, IL-4 and IL-13 gene polymorphisms with asthma in a Chinese population. Asian Pac J Allergy Immunol 29:273
Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM (2011) Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3 K-Akt/ERK MAP kinase signaling in human airway epithelial cells. Mol Cell Biochem 351:29–40
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY (2014) Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat Inflamm 20:2014
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK (2014) Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J neuroinflamm 11:59
Acknowledgements
This work was supported by Department of Science and Technology-Science and Engineering Research Board, New Delhi, India (DST-SERB). Real time PCR (ABI 7500) and flow cytometer (FACS Calibur, BD Biosciences) studies were performed at Central facility of Interdisciplinary Institute of Life Sciences (ISLS), Banaras Hindu University, Varanasi India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumari, A., Singh, D.K., Dash, D. et al. Intranasal curcumin protects against LPS-induced airway remodeling by modulating toll-like receptor-4 (TLR-4) and matrixmetalloproteinase-9 (MMP-9) expression via affecting MAP kinases in mouse model. Inflammopharmacol 27, 731–748 (2019). https://doi.org/10.1007/s10787-018-0544-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0544-3