Abstract
Pulmonary fibrosis is associated with irreversible, or partially reversible, airflow obstruction and ultimately unresponsiveness to asthma therapies such as corticosteroids. Intranasal curcumin, an anti-inflammatory molecule, has been found effective in allergic asthma. To study the effect of intranasal curcumin on airway remodeling and fibrosis in murine model of chronic asthma, BALB/c mice were sensitized to ovalbumin (OVA) and exposed to OVA aerosol (2%) from day 21 (after sensitization) for 5 weeks (twice/week). Curcumin (intranasal) was administered during the OVA aerosol challenge. Mice exposed to OVA developed inflammation dominated by eosinophils which lead to fibrosis and airway remodeling. Intranasal administration of curcumin significantly inhibited airway inflammation and pulmonary fibrosis, where MMP-9 activities were decreased along with α-smooth muscle actin (α-SMA), MMP-9, TIMP-1, and eotaxin expressions. These results suggest that intranasal curcumin regulates airway inflammation and remodeling in chronic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Structural alterations in asthmatic patients are related to the severity of the disease and results into airway remodeling which is a central pathophysiological feature of chronic asthma. It can be observed from early onset of the disease and therefore thought to be an important characteristic [1]. Activated resident cells recruit inflammatory cells to the tissue site where mediators like IL-4, IL-13, and TNF-α stimulate the generation of eotaxin (a chemokine for eosinophils) from lung epithelial cells, fibroblast, and smooth muscle cells. Several studies suggest an association between eosinophils and fibrosis, as they release cytotoxic mediators, pro-inflammatory cytokines, and TGF-β which has well-known fibrotic effects [2]. Eosinophil-derived TGF-β induces fibroblast activation and their differentiation to myofibroblasts [3]. Eosinophils also secrete factors involved in tissue remodeling and fibrosis like granule proteins, major basic protein (MBP), lysosomal hydrolytic enzymes, and peroxidases. Inflammatory mediators ultimately damage the parenchyma and degrade the extracellular matrix (ECM) components.
Extracellular matrix is a dynamic structure playing mechanical role in supporting and maintaining tissue architect. ECM is a scaffold structure where equilibrium is maintained between its synthesis and degradation [4]. The main ECM elements include collagen, elastic fibers, fibronectin, and members of the MMP family (MMP-1, MMP-2, MMP-9, and MMP-12) in addition to TIMP-1 and TIMP-2 (tissue inhibitors of MMPs). Inflammatory cell- and structural cell-derived MMPs also contribute to AHR and remodeling by altering ECM turnover, which affects smooth muscle contraction, airway fibroblast invasion, and accumulation of collagen. Alpha-smooth muscle actin (α-SMA) expression acts as a marker of fibroblast differentiation into its activated state, the myofibroblast. The α-SMA-positive myofibroblasts actively synthesize ECM components. Once fibroblasts become activated, they transform into alpha-smooth muscle actin expressing myofibroblasts that secrete ECM components. Myofibroblasts are responsible for the deposition of a dense, fibrotic collagen matrix [5].
Any imbalance between equilibrium of the normal processes of synthesis and degradation of ECM components make disease fatal. Investigations suggest that accelerated proliferation of fibroblasts lead to their accumulation [6].
It is refractory to treatments and carries a high mortality rate characterized by progressive and irreversible destruction of lung architecture caused by scar formation that ultimately leads to organ malfunction, disruption of gas exchanged, and death from respiratory failure and have limited therapeutic treatment [7].
MMPs are implicated in airway remodeling not only by matrix reorganization (ECM degradation) but also by alteration of angiogenesis and smooth muscle hyperplasia [8, 9]. They are involved in inflammation through their influence of inflammatory cell migration through ECM, basement membrane, and endothelial layer [10, 11]. It has been reported that MMP-9 levels are found significantly high in asthmatic patients as compared to normal subjects. MMP-9 is able to degrade ECM components such as laminin, entactin, and fibronectin involved in tissue remodeling processes expressed by variety of cells like macrophages, lymphocytes, mast cells, and eosinophils. Hydroxyproline is a non-proteinogenic amino acid, which is constituent of elastin and collagen. In tissue hydrolysates, it is a direct measurement of the amount of collagen present and responsible for fibrotic factors involved in fibrosis.
Once fibrotic changes make their impression in asthmatic airways, the survival rate decreases and till date no proven effective therapies exist to treat it completely. The prevalence of asthma has rapidly increased over the last few decades to epidemic proportions and around 300 million sufferers worldwide, a total that is expected to rise dramatically over the next 15–20 years [12]. In developed countries, prevalence of asthma in adult is about 10% and even higher in children, while in developing countries, it is low but growing rapidly, imposing enormous burden on health care [13].
Despite lack of effective treatments to reverse the fibrosis, a potential therapeutic intervention to inhibit or control airway remodeling is needed to be explored. The chemical constituents present in herbal remedies are a part of the physiological functions of living flora and hence they are believed to have better compatibility with the human body [14]. Curcumin derived from Curcuma longa (Turmeric) has shown its effectiveness in the treatment of asthma. However, poor bioavailability limits its therapeutic approval. Enhanced bioavailability of curcumin in the near future is likely to bring this promising natural product to the forefront of therapeutic agents for the treatment of allergic disease [15].
Recently, we have reported better efficacy of curcumin in OVA-induced airway inflammation after intranasal administration at lower doses without any side effect than currently used corticosteroid, dexamethasone, and disodium cromoglycate, a known mast cell stabilizer [16, 17]. Therefore, intranasal administration of curcumin might be effective in attenuating MMP activities and pulmonary fibrosis in OVA-induced mouse model of chronic asthma.
MATERIALS AND METHODS
Animals
Four- to six-week-old balb/c mice (22–26 g) were obtained from Central Drug Research Institute, Lucknow, India, and acclimatized for a week. All animals were maintained under standard conditions of 12-h light and dark cycle. The maintenance protocol was approved by the Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi, India.
Grouping of Mice
Mice were randomly divided into five groups (6 mice/group) and named according to sensitization/challenge/treatment protocol. Group I-normal (Nor), OVA (Ovalbumin) + alum sensitized/saline challenge/no treatment; Group II-asthmatic (Asth), OVA + alum sensitized/OVA challenge/no treatment; Group III-vehical asthmatic (Veh asth), OVA + alum sensitized/OVA challenge/treated with DMSO (dimethylsulfoxide); Group IV-curcumin-treated (Cur), OVA + alum sensitized/OVA challenge/treated with 5 mg/kg bw curcumin i.n.; and Group V-standard drug (Sd) treated, OVA + alum sensitized/OVA challenge/treated with dexamethasone (1 mg/kg bw, i.p.).
Asthma Model
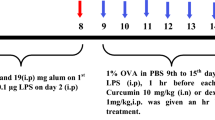
OVA-induced allergic asthma was developed in mice as described earlier with slight modification [18]. Mice were sensitized and challenged as shown in Fig. 1. Mice received 200 μl of 50 μg OVA emulsified in 4-mg aluminum hydroxide (Alum) on days 0, 7, and 14 for sensitization. From day 21, all mice were challenged except normal with 2% OVA in saline for the period of 5 weeks (twice/week). Aerosolized saline was used to challenge normal mice. After 24 h of the last OVA aerosol challenge, mice were sacrificed and different asthmatic parameters were studied in bronchoalveolar lavage fluid (BALF), lung, and blood as described earlier [17, 18].
Treatment
To evaluate the therapeutic effects of intranasal curcumin on chronic asthma, mice were treated with intranasal curcumin regularly from 21st to 55th day and on challenge day 1 h before (Pre treatment) the OVA aerosol challenge. Curcumin was dissolved in dimethysulphoxide (DMSO) and administered in each nostril in the form of nasal drops (2.5 μl /nostril) according to the dose regime (5 mg/kg) and mice were kept in supine position to ease inhalation for few minutes. Dexamethasone (1 mg/kg bw, i.p.) was used as standard drug [16, 17].
Collection of Bronchoalveolar Lavage Fluid (BALF) and Lung
After the last OVA aerosol challenge, mice were sacrificed by cervical dislocation. BALF was collected by trachea cannulation by washing off the airway lumen. Briefly, lungs were washed three times with 1 ml of ice-cold PBS and were centrifuged at 3000 rpm for 10 min at 4 °C. BALF was centrifuged and cell pellet was used to study inflammation by total and differential cell count (eosinophils and neutrophils). Differential cell count was performed by cytospin (Medspin, USA) preparations and Giemsa staining. Inflammatory cells were identified on the basis of morphological characteristics. BALF supernatant and half left lobe of lungs were fixed in 4% neutral buffer formalin rest were stored at −80 °C for further analysis.
Lung Histopathology
Lung sections (5 μm) were stained with hematoxylin and eosin (H&E) for inflammation and structural alterations. To study fibrosis (collagen deposition), lung sections were stained with Masson’s trichrome and Picrosirius red stain.
Hydroxyproline Determination in Lungs
Hydroxyproline content in lung was used to quantify collagen content in lung tissue and measured by a combined method described earlier with some modifications [19]. Briefly, lung was excised and 10% homogenate was prepared in PBS. Sample and 12 N HCL was taken in equal volume and hydrolyzed at 110 °C for 16–18 h. Samples (50 μl) were suspended in citrate-acetate buffer (5% citric acid, 1.2% glacial acetic acid, 7.24% sodium acetate, and 3.4% NaOH dissolved in distilled water pH 6.0), 150 μl of freshly prepared chloramine-T solution (1.4% chloramine-T, 10% N-propanol), and 80% citrate-acetate buffer was added and kept at room temperature for 20 min. Freshly prepared Ehrlich’s solution 150 μl (4.5 g 4-dimethylaminobenzaldehyde dissolved in 18.6 ml n-propanol, 7.8 ml of 70% perchloric acid) was added at 65 °C for 15 min. Sample was cooled on ice and absorbance was taken at 550 nm. A standard curve of hydroxyproline was generated and results were expressed as micrograms of hydroxyproline contained in total lung tissue.
Gelatin Zymography to Determine Collagenase Activity
Gelatin zymography was used to assess MMP-9 protease (collagenase) activity in BALF and lung tissue homogenate. Briefly, 10% SDS-PAGE mini-gels was prepared with gelatin (10 mg/ml), BAL fluid (20 μl) and tissue homogenate supernatant (50 μg) were run at constant voltage of 100 V under non-reducing conditions at 4 °C. Gel was washed thrice for 15 min in renaturing buffer containing 2.5% Triton X-100 to remove the sodium dodecyl sulphate (SDS). After washing, gel was incubated in incubation buffer (50 mM Tris–HCl, 50 mM Tris base, 5 mM CaCl2, 0.2 M NaCl and 0.02% NaN3 (pH 7.5) at 37 °C for 48 h. The gel was stained for 30 min with Coomassie Brilliant Blue R 250 stain and destained (5% methanol, 7% acetic acid in distilled water) till the appearance of clear white bands against blue background. Bands were analyzed by densitometry.
Protein Expressions of Eotaxin, MMP-9, and TIMP
Lung tissues were homogenized in lysis buffer containing protease inhibitor cocktail (Aprotinin (Sisco Research Laboratory-62179), Leupeptin (Amresco-J580), PMSF (Sisco Research laboratory-1648171), EDTA (Sisco Research laboratory-054448), and Na Orthovanadate (Enzo Life Science-127458). Tissue homogenate supernatant was separated and protein concentration was determined by Bradford method. Equal amount of protein (50 μg) with loading dye at ratio of 1:1 was loaded onto SDS-PAGE gel (12%) and separated at 60 V in stacking gel and 70 V in resolving gel. Separated proteins were transferred onto PVDF membranes. Membranes were blocked (4% BSA in TBST (Tris buffered saline with tween 20) for 2 h, incubated with MMP-9 (1: 1000, Thermo Scientific-PA5-3199), TIMP-1 (1:500, Thermo Scientific-MA1-773), Eotaxin (1:1000, Thermo Scientific-PA1-29219), α smooth muscle actin (5 μg/ml, e bioscience-14-9760), β actine (1: 2000, Genscript-A00702), or β actine (1: 2000, Cell Signalling Technology-4970P) antibody for overnight at 4 °C. To study eotaxin expression, 20% gel was run as its molecular weight is ∼15 KDa. After primary antibody incubation, membranes were washed thrice in TBST buffer at room temperature for 15 min followed by horseradish peroxidase (HRP)-conjugated anti-goat secondary antibody (1:10,000, Cayman-10004302) or HRP-conjugated anti-rabbit secondary antibody (1:3000, CST-7074P2) for 2 h at room temp. Membranes were again rewashed in TBST buffer and protein expressions were detected with ECL reagent (Millipore-WBKLS01000). β-actin was used as a loading control. Band intensities were determined by densitometry.
Measurement of Alanine Aminotransferase and Aspartate Aminotransferase Levels in Serum
The liver function test was performed to test the toxicity of intranasal curcumin on liver. Alanine aminotransferase (AST) and aspartate aminotransferase (ALT) levels were determined by modified Reitman and Frankel’s colorimetric DNPH method by using standard kits (Avecon).
Measurement of Creatinine Level in Blood Serum
Serum creatinine level was measured by using creatinine reagent kit (Beacon). The principle was based on the formation of orange-colored complex by the reaction of picric acid with creatinine and intensity was measured at 490 nm.
Statistical Analysis
Values are presented as the mean ± SEM. Differences between two means were evaluated using Student’s t test. Statistical significance between control and OVA inhaled animals (Asth) was estimated using the two-tailed Student’s t test. Differences between more than two groups were assessed via ANOVA with Tukey’s and post hoc test comparison. To define statistically significant differences among different groups (Nor vs Asth and Veh asth, Asth vs Cur and SD), the data were subjected to the ANOVA followed by Tukey’s and post hoc tests. Statistical significance was considered at P < 0.05 by using SPSS 16 software.
RESULTS
Curcumin Suppresses Inflammatory Cell Recruitment
Curcumin is effective in ameliorating infiltration of inflammatory cells to the lungs as total leukocyte count was reduced as compared to OVA-induced (asthmatic) group. Total inflammatory cell count and recruitment of eosinophils and neutrophils was significantly reduced in Giemsa-stained cytospin BALF preparations. Expression of eotaxin, an eosinophil chemokine, was also suppressed after intranasal curcumin treatment which could be correlated with reduced eosinophil recruitment (Fig. 2).
Curcumin Inhibits Structural Alterations in Lungs
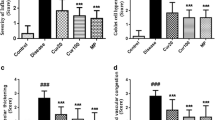
Inflammation along with airway wall and lung injury like epithelial wall shedding and ruptured alveolar space with massive accumulation of inflammatory cells around bronchioles were seen in OVA-induced lung sections after H&E staining while curcumin treatment had reduced the inflammation and structural integrity of the airways were well maintained (Fig. 3).
Histology of (top panel) showing increased infiltration of inflammatory cells in alveolar spaces, around bronchioles and loss of structural integrity in terms of epithelial shedding and destruction of airway lumen. Staining of lung sections by Masson’s trichrome (middle panel) revealed blue color collagen fiber accumulation has confirmed presence of collagen around bronchiole and blood vessel in lung tissues. The collagen fibers are blue stained and nuclei are stained black with red background. Picro Sirius red staining (bottom panel) is showing total collagen deposition. Intense orange red fibrous protein is more prominent in Asth and Veh asth group while Cur treatment has less ECM deposition.
Curcumin Ameliorates ECM Deposition
Subepithelial fibrosis is one of the well-established hallmarks of airway remodeling in chronic asthma. In OVA exposed mice, collagen deposition was promising as compared to curcumin-treated group. Masson’s trichrome and Sirius red staining is also showing fibrosis where blue-stained collagen bands and dark orange fibers around bronchioles and vessels confirm ECM deposition in asthmatic group while little collagen deposition was observed in curcumin groups. Hydroxyproline level, Masson’s trichrome and Sirius red staining results confirm regulation of fibrosis in murine model of chronic asthma (Figs. 3 and 4). Significant difference in hydroxyproline level is found in OVA-induced and curcumin-treated groups. Higher hydroxyproline content was significantly reduced after curcumin treatment.
Curcumin Inhibits Gelatinolytic Activity of MMP-9
MMP-9 is a well-known extracellular matrix degrading enzyme and has been reported for its vital role in chronic asthma disease process. OVA-exposed BALF had shown marked increased MMP-9 activity as compared to control mice. Intranasal administration of curcumin had significantly inhibited MMP-9 activity, whereas dexamethasone-treated BALF and lungs both had shown significant inhibition in MMP-9 activity (Fig. 5).
Effect of curcumin treatment on MMP-9 activity in BALF and lung tissue. MMP-9 activity has been assessed by gelatin zymography analysis. White bands indicate gelatin digestion by MMP-9 activity and band intensities were analyzed by densitometry analysis. Asth and Veh asth groups had significantly more intensity values as compared to nor. After curcumin treatment, activity was reduced. In lung tissue, no significant difference was noted. The values are ±SEM. Number sign indicates Nor vs Asth and Veh asth.
Expression Profile of Collagen Degrading and Fibrosis Factors (MMP-9, TIMP-1, Eotaxin, and Alpha-Smooth Muscle Actin)
The balance between MMPs and TIMPs is mainly responsible for the remodeling event process in tissues as they are thought to play role in trafficking of inflammatory cells and inducing airway smooth muscle cells hyperplasia. Gelatinolytic activity of MMP-9 was reduced after curcumin treatment; therefore, we looked into MMP-9 and TIMP-1 balance. TIMP-1 level remains unaltered in asthmatic and curcumin treatment groups. Eotaxin being chemokine, responsible for accumulation of eosinophils trafficking at tissue injury site, was feebly expressed after curcumin treatment as compared to asthmatic group which can be correlated with reduced number of eosinophil infiltration after curcumin treatment. As inflammation was prominent in asthmatic group as compared to curcumin-treated group, we further confirmed its expression in lungs. Airway fibrosis was confirmed by alpha-smooth muscle actin expression in lungs as curcumin was able to control the ASM mass proliferation in OVA-induced chronic asthma (Figs. 6 and 7).
Effect of curcumin treatment on protein expression levels of MMP-9 and TIMP-1 in lung tissue. No significant difference was noted in TIMP-1 expression in cur-treated group. The values are ±SEM. Number sign indicates Nor vs Asth and Veh asth. Asterisk indicates Asth and Veh asth vs Cur-treated groups (5 mg/kg), p < 0.05.
Effect of curcumin treatment on α smooth muscle actin profile in lungs. Smooth muscle protein expression was determined by western blot analysis. Band intensities were measured by densitometry. The values are ±SEM. Number sign indicates Nor vs Asth and Veh asth. Asterisk indicates Asth and Veh asth vs Cur-treated groups (5 mg/kg), p < 0.05.
Curcumin Toxicity
Although we already had studied that curcumin is safe in acute asthma. Since in chronic asthma model, it has been administered for longer duration; therefore, AST, ALT, and creatinine level was tested in blood serum to observe any toxic effect. No significant difference was noticed in AST and ALT levels while creatinine level was found significantly higher in OVA-induced and Dexa-treated groups (Fig. 8).
DISCUSSION
In the present study, murine model of chronic asthma was developed where OVA-immunized mice were given eleven OVA challenges and then fibrotic factors were measured like ECM deposition around bronchioles, hydroxyproline content, MMP-9/TIMP-1 balance, and alpha-smooth muscle actin expression. These parameters along with inflammation and eotaxin (eosinophil chemotactic factor) were significantly enhanced in the asthma. Several changes were noted in asthmatic lungs like cellular inflammation where eosinophils and neutrophil numbers were found elevated (Figs. 2 and 3). Evidences suggest that infiltrating inflammatory cells, such as eosinophils, play an active role in airway remodeling by promoting the activation of fibroblasts and endothelial cells [20–22]. These eosinophils are one of the major sources of fibrotic factors which stimulate the differentiation of airway submucosal fibroblasts to myofibroblasts which are thought to synthesize and secrete collagen into the airway submucosal space which results in airway fibrosis [11]. Airway eosinophilic inflammation is responsible for the development of airway remodeling in murine model of asthma. We also found the significant accumulation of eosinophils where IL-5 has been considered as the central mediator for eosinophilic proliferation, differentiation, and inflammation, as we already have reported elevated level of IL-5 where intranasal curcumin treatment had diminished its level in BALF [17]. Present study confirms regulation of increased level of eotaxin by intranasal curcumin treatment. Results of recent studies suggest that besides IL-5, eotaxin also contributes to asthma pathogenesis. It is a potent eosinophil chemoattractant and a critical mediator for the development and perpetuation of allergen-induced eosinophilic airway inflammation. Eotaxin selectively binds to a specific receptor (CCR3) highly expressed on eosinophils, basophils, and mast cells being important in the pathogenesis of asthma [23, 24]. Recent report suggests that eotaxin may play an important role in airway remodeling and has a pro-fibrogenic effect on lung and bronchial fibroblasts [25]. Curcumin being anti-inflammatory molecule is able to inhibit inflammation, IL-5 level, and eotaxin expression in OVA-sensitized and challenged mice which confirms reduced eosinophil recruitment to the lungs.
Subepithelial fibrosis and extracellular matrix deposition is one of the key features of remodeling [25, 26]. Various attempts have been made to control these features but none found effective. The subepithelial fibrosis occurs in the lamina reticularis layer just below the basement membrane, which leads to the thickening of the basement membrane just underneath the epithelium. Fibrosis is the result of increased deposition of extracellular matrix (ECM) proteins, including collagens I, III, and V, fibronectin, tenascin, lumican, and biglycan by fibroblasts [27, 28].
In the present study, ECM deposition was confirmed by histological assessment of lung sections (Masson’s trichrome and Sirius red staining) and hydroxyproline content in lungs. Allergen-induced mice have shown thick and intense fibers around bronchioles whereas curcumin had significantly inhibited elevated hydroxyproline level as well as collagen deposition. MMPs and their physiological inhibitors, TIMPs, which degrade and remodel the excess extracellular matrix, are believed to play an important role in the development of fibrotic tissue. Locally, in the extracellular space, MMPs are tightly regulated by TIMPs. In idiopathic pulmonary fibrosis, gelatinase B (MMP-9) expression is increased in cultured alveolar macrophages and in bronchoalveolar lavage fluid [29]. Inflammatory stimulations can lead to increased MMP-9 expression in many cell types including endothelial cells, alveolar cells, macrophages, fibroblasts, and other connective tissue cells [30]. In adults, constitutive expression of MMP-9 is restricted to neutrophils and eosinophils [31, 32]. Normal lungs contain only basal levels of MMP-2 expressed by endothelial cells and do not contain MMP-9. Under inflammatory conditions, there is an induction of MMP-9 transcription by cells such as fibroblasts, as well as increased MMP-9 expression due to the infiltration of neutrophils and eosinophils that contain pre-formed MMP-9 in their granules. Normally, the levels of the gelatinase return to baseline; however, several pulmonary pathologies are characterized by sustained, elevated expression of MMP-2 and/or MMP-9 as well as altered expression of the TIMPs. There are many reports on altered gelatinase levels in pulmonary diseases; however, detailed understanding of the involvement of gelatinases in disease pathology is still evolving [33]. Patients with stable chronic asthma also show increased levels of both MMP-2 and MMP-9 in the sputum and BAL fluid [34, 35]. Since MMP-9 and TIMP-1 balance is a key element that regulates ECM homeostasis and tissue remodeling. Earlier studies do report that MMP-9 have relevance to chronic structural airway changes in asthma, which can be generated by structural and inflammatory cells and have the ability to degrade proteoglycans and thus potentially enhance airway fibrosis and smooth muscle proliferation through their ability to release and activate latent, matrix-bound growth factors [36].
To identify the possible protective role of curcumin on MMP-9 activity and expression, we used gelatin zymography and western blot analysis in lungs. Curcumin treatment showed reduced activity and expression of MMP-9 in lungs as compared to OVA-challenged mice. These results are consistent with ECM deposition and hydroxyproline levels. This increased activity may be correlated to exaggerated airway inflammation and remodeling.
Our studies suggest that the inhibition of MMPs could be new therapeutic strategy for bronchial asthma, although further investigations are needed for clear cut understanding of the regulatory mechanisms of MMP expression and definite role of different MMPs in asthmatic airways. Fibrosis is a dynamic process that involves the balance of MMPs and their inhibitors, TIMPs. Other studies have also reported exaggerated quantities of MMP-9 in sputum and biopsies from patients with asthma [37–39]. Another report suggests that MMP-9 levels were increased in sputum after allergen challenge allergic asthmatics as compared to control subjects and correlated to the percentage of eosinophils while TIMP-1 levels did not vary significantly [40]. Increased ASM mass is the prominent feature of airway remodeling where it increases disproportionately as compared to the increase in total wall thickness. It has been documented in both fatal and non fatal asthma and correlates with both disease severity and duration. Greater ASM mass is fatal in older patients than younger patients [41–43].
ASM cells are key cells that release fibrotic growth factor and their mass spontaneously increases in severe form of asthma. We already had reported that thickening of ASM layer in lung section of asthmatic group and its expression (Fig. 7). Curcumin treatment has attenuated increase in the ASM mass over OVA-induced asthma. These findings suggest that intranasal route of curcumin administration may prove as better alternative and could be protective in interfering fibrotic changes and thereby airway remodeling by directly targeting lungs without any degradation.
Present study suggests that intranasal curcumin has significantly decreased inflammatory cell recruitment mainly eosinophils in BALF and lungs both. It is effective in maintaining ECM composition by modulating fibrotic factors involved in airway remodeling. Attenuated levels of eotaxin, MMP-9 and α-smooth muscle actin could be correlated with reduced eosinophil numbers after curcumin treatment in asthmatic mice. A detailed and integrated understanding of the cellular and molecular mechanisms involved in airway fibrosis could help to answer for effective intranasal curcumin therapy for complex disease like asthma.
References
Sumi, Y., and Q. Hamid. 2007. Airway remodeling in asthma. Allergology International 56: 341–348.
Conroy, D.M., and T.J. Williams. 2001. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respiratory Research 2: 1.
Aceves, S.S., R.O. Newbury, M.A. Dohil, J.F. Bastian, and R. Dohil. 2009. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Annals of Allergy, Asthma, and Immunology 103(5): 401–6.
Bloemen, K., S. Verstraelen, R. Van Den Heuvel, H. Witters, I. Nelissen, and G. Schoeters. 2007. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunology Letters 113: 6–18.
Darby, I.A., B. Laverdet, and A. Bonté Fand Desmoulière. 2014. Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology 7: 301.
Todd, N.W., I.G. Luzina, and S.P. Atamas. 2012. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogen Tissue Repair 5: 11.
Wynn, T.A. 2011. Integrating mechanisms of pulmonary fibrosis. The Journal of Experimental Medicine 208: 1339–1350.
Johnson, C., and Z.S. Galis. 2004. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arteriosclerosis, Thrombosis and Vascular Biology 24: 54–60.
Johnson, P.R.A., and Annual Scientific Meeting of ASCEPT. 2001. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clinical and Experimental Pharmacology and Physiology 28: 233–236.
Wenzel, S.E., S. Balzar, M. Cundall, and H.W. Chu. 2003. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9: association with asthma severity, neutrophilic inflammation, and wound repair. Journalof Allergy and Clinical Immunology 111: 1345–1352.
Tanaka, H., N. Miyazaki, K. Oashi, S. Tanaka, M. Ohmichi, and S. Abe. 2000. Sputum matrix metalloproteinase-9: tissue inhibitor of metalloproteinase-1 ratio in acute asthma. Journalof Allergy and Clinical Immunology 105: 900–905.
Holgate, S.T. 2008. Pathogenesis of asthma. Clinical Experimental Allergy 38: 872–897.
Barnes, P.J. 2006. Drugs for asthma. British Journal of Pharmacology 147: S297–S303.
Kamboj, V.P. 2000. Herbal medicine. Current Science 78: 35–38.
Anand, P., A.B. Kunnumakkara, R.A. Newman, and B.B. Aggarwal. 2007. Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics 4: 807–818.
Subhashini, Chauhan P.S., S. Kumari, et al. 2013. Intranasal curcumin and its evaluation in murine model of asthma. International Immunopharmacology 17: 733–743.
Chauhan, P.S., D. Dash, and R. Singh. 2014. Intranasal curcumin attenuates airway remodeling in murine model of chronic asthma. International Immunopharmacology 21: 63–75.
Ahmad, T., U. Mabalirajan, K. Hasija, B. Ghosh, and A. Agrawal. 2011. Mepacrine treatment attenuates allergic airway remodeling segregated from airway inflammation in mice. Internatinal Immunopharmacology 11: 74–78.
Christensen, P.J., R.E. Goodman, L. Pastoriza, B. Moore, and G.B. Toews. 1999. Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent. The American Journal of Pathology 155: 1773–1779.
Corry, D.B., K. Rishi, J. Kanellis, et al. 2002. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nature Immunology 3: 347–353.
Kay, A.B., S. Phipps, and D.S. Robinson. 2004. A role for eosinophils in airway remodelling in asthma. Trends in Immunology 25: 477–482.
Humbles, A.A., C.M. Lloyd, S.J. McMillan, D.S. Friend, G. Xanthou, and Gerard C. McKenna. 2004. A critical role for eosinophils in allergic airways remodeling. Science 305(5691): 1776–1779.
Paplińska, M., H. Grubek-Jaworska, and R. Chazan. 2007. Role of eotaxin in the pathophysiology of asthma. Polish Pneumonology and Allergology 75: 180–185.
Bousquet, J., P.K. Jeffery, W.W. Busse, M. Johnson, and A.M. Vignola. 2000. Asthma: from bronchoconstriction to airways inflammation and remodeling. American Journal of Respiratory and Critical Care Medicine 161: 1720–1745.
Postma, D.S., and W. Timens. 2006. Remodeling in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 3: 434–439.
Puxeddu, I., R. Bader, A.M. Piliponsky, R. Reich, F. Levi-Schaffer, and N. Berkman. 2006. The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. Journal of Allergy and Clinical Immunology 117: 103–110.
Roche, W., J. Williams, R. Beasley, and S. Holgate. 1989. Subepithelial fibrosis in the bronchi of asthmatics. The Lancet 33: 520–524.
Huang, J., R. Olivenstein, R. Taha, Q. Hamid, and M. Ludwig. 1999. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. American Journal of Respiratory and Critical Care Medicine 160: 725–729.
Gaggar, A., Y. Li, N. Weathington, et al. 2007. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. American Journal of Physiology-Lung Cellular and Molecular Physiology 293: L96–L104.
Nagese, H., and J.F. Woessner Jr. 1999. Matrix metalloproteinses. Journal of Biological Chemistry 274: b1.
Devarajan, P., J. Johnston, S. Ginsberg, H.E. Van Wart, and N. Berliner. 1992. Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. Journal of Biological Chemistry 267: 25228–25232.
Schwingshackl, A., M. Duszyk, N. Brown, and R. Moqbel. 1999. Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-α. Journa of Allergy and Clinical Immunology 104: 983–990.
Chakrabarti, S., and K.D. Patel. 2005. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Experimental Lung Research 31: 599–621.
Maisi, P., K. Prikk, R. Sepper, et al. 2002. Soluble membrane‐type 1 matrix metalloproteinase (MT1‐MMP) and gelatinase A (MMP‐2) in induced sputum and bronchoalveolar lavage fluid of human bronchial asthma and bronchiectasis. Apmis 110: 771–782.
Mautino, G., C. Henriquet, C. Gougat, et al. 1999. Increased expression of tissue inhibitor of metalloproteinase-1 and loss of correlation with matrix metalloproteinase-9 by macrophages in asthma. Laboratory investigation. A Journal of Technical Methods and Pathology 79: 39–47.
Han, Z., and N. Zhong. 2003. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respiratory Medicine 97: 563–567.
Hoshino, M., Y. Nakamura, J. Sim, J. Shimojo, and S. Isogai. 1998. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. Journal of Allergy and Clinical Immunology 102: 783–788.
Mautino, G., N. Oliver, P. Chanez, J. Bousquet, and F. Capony. 1997. Increased release of matrix metalloproteinase-9 in bronchoalveolar lavage fluid and by alveolar macrophages of asthmatics. American Journal of Respiratory Cellular and Molecular Biology 7: 583–591.
Kelly, E.A., W.W. Busse, and N.N. Jarjour. 2000. Increased matrix metalloproteinase-9 in the airway after allergen challenge. American Journal of Respiratory and Critical Care Medicine 162: 1157–1161.
Cataldo, D.D., J. Bettiol, A. Noël, P. Bartsch, J.M. Foidart, and R. Louis. 2002. Matrix metalloproteinase-9, but not tissue inhibitor of matrix metalloproteinase-1, increases in the sputum from allergic asthmatic patients after allergen challenge. CHEST Journal 122: 1553–1559.
Carroll, N., J. Elliot, A. Morton, and A. James. 1993. The structure of large and small airways in nonfatal and fatal asthma. The American Review of Respiratory Disease 147: 405–410.
James, A.L., P.D. Paré, and J.C. Hogg. 1989. The mechanics of airway narrowing in asthma. The American Review of Respiratory Disease 39: 242–246.
Kuwano, K., C.H. Bosken, P.D. Paré, T.R. Bai, B.R. Wiggs, and J.C. Hogg. 1993. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. The American Review of Respiratory Disease 148: 1220–1225.
ACKNOWLEDGEMENTS
Authors are thankful to Department of Science and Technology - Science and Engineering Research Board, (DST-SERB) and Council of Scientific and Industrial Research, India for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chauhan, P.S., Dash, D. & Singh, R. Intranasal Curcumin Inhibits Pulmonary Fibrosis by Modulating Matrix Metalloproteinase-9 (MMP-9) in Ovalbumin-Induced Chronic Asthma. Inflammation 40, 248–258 (2017). https://doi.org/10.1007/s10753-016-0475-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0475-3