Abstract
The peripheral nervous system is one of many organ systems that can be profoundly impacted in diabetes mellitus. Diabetic peripheral neuropathy has a significant negative effect on patients’ quality of life as it begins with loss of limbs’ sensation and may result in lower limb amputation. This investigation aimed at exploring the effect of sulforaphane on peripheral neuropathy in diabetic rats. Experimental diabetes was induced through single intraperitoneal injections of nicotinamide (50 mg/kg) and streptozotocin (52.5 mg/kg). Rats were divided into five groups. Two groups were treated with saline or sulforaphane (1 mg/kg, p.o.). Three diabetic groups were either untreated or given sulforaphane (1 mg/kg, p.o.) or pregabalin (10 mg/kg, i.p.). Two weeks after drugs’ administration, biochemical, behavioral, histopathological, and immunohistochemical investigations were carried out. Treatment with sulforaphane restored animals’ body weight, reduced blood glucose, glycated hemoglobin, and increased insulin levels. In parallel, it normalized motor coordination and the latency withdrawal time of tail flick test, increased the latency withdrawal time of cold allodynia test, and ameliorated histopathological changes. Treatment of sulforaphane, likewise, decreased sciatic nerve malondialdehyde, nitric oxide, interleukin-6, and matrix metalloproteinase-2 and -9 contents. Similarly, it reduced sciatic nerve DNA fragmentation and expression of cyclooxygenase-2 and nuclear factor kappa-B p65. Meanwhile, it increased sciatic nerve superoxide dismutase and interleukin-10 contents. These results reveal the neuroprotective effect of sulforaphane against peripheral neuropathy in diabetic rats possibly through modulating oxidative stress, inflammation, and extracellular matrix remodeling.

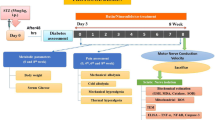
Diagram that illustrates the effects of sulforaphane in treating experimental diabetic peripheral neuropathy. In NA-STZ model of diabetes mellitus, sulforaphane, restored animals’ body weight, reduced blood glucose, glycated hemoglobin and increased insulin levels. In parallel, it normalized motor coordination and the latency withdrawal time of tail flick test, increased the latency withdrawal time of cold allodynia test and ameliorated histopathological changes. Treatment of sulforaphane, likewise, decreased sciatic nerve malondialdehyde, nitric oxide, interleukin-6, matrix metalloproteinase-2 and -9 contents. Similarly, it reduced sciatic nerve DNA fragmentation and expression of cyclooxygenase-2 and nuclear factor kappa-B p65. Meanwhile, it increased sciatic nerve superoxide dismutase and interleukin-10 contents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The peripheral nervous system is one of many systems that is immensely impacted in diabetes mellitus (DM) [1]. Nearly half of patients, who have had DM for 25 years develop diabetic peripheral neuropathy (DPN) with varying severity [2, 3]. Peripheral nerve complications first appear in distal extremities and can lead to chronic pain or loss of limbs’ sensation [4]. DPN is counted as a substantial cause of morbidity as it contributes to the development of foot ulcerations, Charcot joints, and limb amputation in diabetic patients [5]. Notably, in developing countries, up to 50% of diabetic patients are diagnosed by DPN [6].
The currently postulated mechanisms of DPN are escorted with cellular damage resulting from the activation of various cellular pathways triggered by long-term hyperglycemia, which consecutively leads to metabolic imbalances mostly oxidative stress [1, 7]. These cellular pathways include aldose reductase, hexosamine pathway, non-enzymatic glycation, and oxidative phosphorylation, which once activated result in the formation of toxic metabolites such as reactive oxygen species (ROS), advanced glycation end-products (AGE’s), and protein kinase C (PKC)-mediated cellular signaling molecules [8,9,10,11]. The over-production of such toxic metabolites results in endothelial dysfunction, microangiopathy, ischemia, and eventually peripheral nerves damage and death [2, 7, 12]. Noteworthy, increased glycolytic process and over-activation of aldose pathway lead the formation of free radicals through reducing nicotinamide adenine dinucleotide phosphate (NADPH) and overburdening the mitochondrial electron transport chain, correspondingly [9, 13]. As a consequence, the over-production of ROS within the cell triggers the activation of poly(ADP-ribose) polymerase (PARP) pathway, which results in nicotinamide adenine dinucleotide (NAD+) depletion as well as ROS, PKC, and AGE’s formation [14]. Both ROS and AGEs prompt a cascade of inflammatory reactions through the over-activation of nuclear factor-kappa B (NF-κB) pathway [15]. This pathway in turn initiates the production of interleukins from macrophages alongside the transcription of various proteins including cytokines, chemokines, adhesion molecules, nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) resulting in neuronal dysfunction and the progression of DPN [16, 17].
Noteworthy, matrix metalloproteinases (MMPs) are extensively involved in inflammation and tissue remodeling accompanied by various neuro-degenerative diseases including DPN [18, 19]. Both inflammation and oxidative stress associated with uncontrolled hyperglycemia trigger the activation of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9). This leads to extracellular matrix (ECM) degradation, constriction of arteries, ischemia of nerve tissues, and finally neural cell cessation [20, 21]. It has been investigated that activation of COX-2 stimulates the production and expression of both MMP-2 and MMP-9 from vascular smooth muscle, endothelial cells, and macrophages [22]. Furthermore, ROS prompts the oxidation of the sulfide bond of the pro-domain of MMPs, which results in the activation of pro-MMPs and the decrease of tissue inhibitors of metalloproteinases (TIMPs) [23].
Sulforaphane (SFN) is a broccoli-derived isothiocynate (ITC) that is found in cruciferous vegetables, mainly broccoli and cauliflower [24]. It has been shown that SFN has a neuroprotective effect due to its anti-oxidant and anti-inflammatory properties [25, 26]. SFN is a potent inducer of nuclear erythroid 2-related factor 2 (Nrf2) and hence provides neuroprotective effect against neurodegenerative diseases [27]. In addition to its direct effect on Nrf2, SFN exhibits anti-inflammatory effect via inhibiting NF-κB signaling pathway [28]. Several recent studies have investigated that SFN increased neurogenesis, decreased amyloid plaque deposition, and enhanced cognitive function in various animal models of Alzheimer’s disease (AD) [29, 30]. SFN, likewise, mitigated the development of Parkinson’s disease (PD) in rats through its neuroprotective, anti-apoptotic, and anti-oxidant actions [31]. Furthermore, a recent study has shown that SFN attenuated cerebral ischemic/reperfusion injury via its anti-inflammatory and neuroprotective properties [32]. Based on the described effects, this study attempted to examine the potential protective effect of SFN against streptozotocin (STZ)-induced peripheral neuropathy in diabetic rats using the pregabalin (PGB) as a standard drug for comparison. Additionally, the roles of SFN on body weight, diabetic biomarkers, behavioral alterations as well as oxidative stress, inflammation, MMPs activity, DNA fragmentation, and histological alterations were also assessed.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (250–300 g) were used through this investigation. They were obtained from the Animal House of the National Research Center, Giza, Egypt. Animals were kept at least 1 week for adaptation before being assigned to the study, in standard housing circumstances (room temperature 22 ± 2 °C, relative humidity 60 ± 10% and 12 h light/dark cycle). All animals were permitted ad libitum access to tap water and standard laboratory chow. This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Ethics Committees of Faculty of Pharmacy, Cairo University (permit number 1393) and National Research Centre (permit number 15/047).
Chemicals and Drugs
Sulforaphane and PGB were obtained from Swanson Health Products, (North Dakota, USA) and Pfizer (Cairo, Egypt), respectively. STZ and nicotinamide (NA) were purchased from Sigma-Aldrich (Missouri, USA) and Bayer (Lyon, France), respectively. All other chemicals used in this study were of highest purity and analytical grade.
Induction of Diabetes
Nicotinamide (50 mg/kg), dissolved in normal physiological saline, was injected intraperitoneally 15 min before STZ administration (52.5 mg/kg, i.p.), which dissolved in 0.1 M citrate buffer directly before use (pH 4.5) to 36 rats that were fasted overnight. For the partial protection of insulin-secreting β-cells from the damaging effect of STZ, NA is injected before STZ [33]. After STZ administration, all rats received 5% glucose solution as an alternative to tap water for 24 h to reduce death due to hypoglycemic shock. Two days after STZ administration, blood samples were withdrawn from the tail vein of each rat and blood glucose levels were measured using a portable glucometer (ACCU-Check, Roche, USA). Only rats depicting values ranging BGL ≥ 200 mg/dl were chosen and considered as diabetic [34,35,36].
Experimental Design
In the experiment, a total of 60 rats were randomly distributed into 5 groups (12 rats/group) as follows:
-
Group I contained normal rats that received saline subcutaneously daily for 15 days and served as normal.
-
Group II comprised normal rats that were given SFN (1 mg/kg, p.o.) daily for 15 days.
-
Group III included normal rats that administered single intraperitoneal doses of NA (50 mg/kg) and STZ (52.5 mg/kg) as formerly described and served as diabetic control.
-
Group IV included diabetic rats treated with SFN (1 mg/kg, p.o.) daily for 15 days. The dose of SFN was chosen based on formerly published work [37] besides a pilot experiment (data not shown).
-
Group V included diabetic rats treated with PGB (10 mg/kg, i.p.) daily for 15 days [38].
One day after the last drugs’ administration, all rats were exposed to several behavioral tests. Subsequently, all rats were weighed and anesthetized; thereafter, blood samples were collected via the retro-orbital plexus and separated into two parts. The first part was used for the determination of glycated hemoglobin (GHBA1c), while the second part was centrifuged at 3000×g for 10 min to separate serum for the estimation of insulin level. At last, all animals were euthanized by decapitation, and the sciatic nerve tissues were cautiously removed, washed with ice-cold saline, and left to dry. Sciatic nerve samples were divided into two parts and weighted; the first one was homogenized in phosphate buffer for the determination of malondialdehyde (MDA), nitric oxide (NO), superoxide dismutase (SOD), interleukin (IL)-6, IL-10, MMP-2, MMP-9, and DNA fragmentation, while the second portion was fixed in 10% neutral-buffered formalin for histopathological and immunohistochemical examination.
Determination of Body Mass Changes
Each rat was weighed at the beginning of the experiment and 24 h following the last treatment for the determination of both initial and final body weights (g). Percent change of body weight was calculated using the following equation:
Rotarod Test
Grip muscle strength of rats was evaluated using accelerating rotarod device (Ugo Basile, Italy, Model 7750) by placing rats in the opposite direction of the horizontal rotating rod (3 cm diameter and 90 cm height). The starting speed was at rpm and accelerated linearly to 40 rpm. Before starting the experiment, all rats were trained for three successive days (one session/day, 5 min each). The performance of all rats was evaluated through recording the time spent by each rat to fall from the roller, during 5-min period [39].
Tail Flick Test
Evaluation of antinociceptive threshold of rats was determined using the tail flick device (Ugo Basile, Italy, Model 37360). In this test, rats’ tails were gently subjected to the radiant heat stimulus of the device. Tail flick latency time was estimated in seconds from the initial light emission until tail withdrawal. To avoid tissue injury, a maximum cut-off of 10 s was considered during the test. For each rat, tail flick latency was determined for two times at 30 min interval and the mean reading was reported as tail flick latency [40].
Hind Paw Cold Allodynia Test
Cold-induced allodynia was assessed through immersing the hind paw of each rat in a cold-water container and keeping constant temperature (4.5 °C). Then, the hind paw withdrawal latency (HPWL) for each rat was measured. To prevent tissue injury, only one hind paw was assessed during each immersion at a time with a cut-off time 20 s. For each rat, the test was repeated two times for each hind paw at 5-min interval and the HPWL was recorded as the average of both hind paw readings. The extended contact time with cold water was interpreted as anti-allodynic effect, whereas the shorter contact was noted as more severe allodynia [41].
Tail Cold Allodynia Test
In this test, cold-induced allodynia was assessed through immersing the tail of each rat to cold-water container and maintaining constant temperature (4.5 °C). To avoid tissue injury, a cut-off time 15 s was considered while determining the tail withdrawal time (TWL) for each rat. The procedure was repeated for five times at 5 min interval for each rat. A shorter contact with cold-water was counted as a more severe allodynia, whereas the prolonged contact was noted as anti-allodynic effect [42].
Determination of Blood Glucose, Glycated Hemoglobin, and Insulin Levels
Blood glucose level was assessed 24 h after the last treatment using a portable glucometer (ACCU-Check, Roche, USA). Using tail vein puncture technique, blood was withdrawn from rats and a drop of blood was positioned on the glucometer strip loaded in the device for the assessment of rats’ BGL. Quantitative determination of rats’ GHBA1c and insulin levels was conducted by means of rat-specific immunoassay kits obtained from Cusabio (Wuhan, China) and Alpco (Boston, USA), respectively, based on the protocol provided.
Determination of Oxidative Stress Biomarkers
Sciatic nerve homogenate was used for the quantitative determination of MDA, NO, and SOD. Lipid peroxidation products were measured indirectly by the estimation of the secondary product such as MDA according to the technique of Ruiz-Larrea et al. [43] Total NOx content was measured based on the reduction of nitrate to nitrite by vanadium, and then total nitrate was determined by Griess reagent according to the method of Sastry et al. [44]. Determination of SOD content was performed using specific immunoassay kit obtained from Cusabio (Wuhan, China) according to the manufacturer’s guidelines.
Determination of Inflammatory Biomarkers and Matrix Metalloproteinases
Quantitative determination of IL-6, IL-10, MMP-2, and MMP-9 contents in sciatic nerve homogenate was carried out using rat-specific enzyme-linked immunosorbent assay (ELISA) kit purchased from Ray Biotech (Georgia, USA), Cusabio (Wuhan, China), and Cloud-Clone Corporation (Texas, USA), respectively, based on the manufacturer’s instructions.
Determination of Deoxyribonucleic Acid Fragmentation
Sciatic nerve homogenate was used of the quantitative determination of DNA fragmentation using diphenylamine reaction procedure as previously described by Gibb et al. [45]. The amount of fragmented DNA was measured spectrophotometrically at 600 nm, and the fragmented DNA percent was calculated using the following formula:
- OD(S):
-
= absorbance of sample supernatant
- OD(P):
-
= absorbance of sample pellet
Histopathologic Studies
The formalin-fixed sciatic nerve samples were routinely processed for paraffin embedding, and then, thin 4-μm sections were formed and stained with hematoxylin and eosin (H&E) for light microscope investigation. Evaluation of sciatic nerve damage was qualitatively estimated and graded based on the severity of pathologic changes. Scores were given to lesions detected as follows: (0) no observed impairment, (1) mild impairment fewer than 25% of sciatic nerve cells impacted, (2) moderate impairment ranging from 25 to 50% of sciatic nerve cells affected, and (3) severe impairment more than 50% of sciatic nerve cells impacted [46]. To eliminate bias, a blind examination of all the samples was performed.
Immunohistopathological Examination
Cyclooxygenase-2 and NF-κB p65 expression were estimated in the sciatic nerve using a modified avidin-biotin immunochemistry technique based on the method of [47]. Before immunohistopathological investigation, paraffinized sciatic nerve specimens were rehydrated in xylene and graded ethanol solutions and heated in citrate buffer (pH = 6) for 20 min. Thereafter, sciatic nerve samples were cooled and immunolabeled with primary polyclonal rabbit antibodies against NF-κB p65 (1:200 dilution; Invitrogen, Carlsbad, CA, USA) and COX-2 (1:200 dilution; Thermo Fisher scientific, Waltham, USA) and incubated overnight at 4 °C. Then, phosphate-buffered saline was used for washing sciatic nerve tissue sections before being incubated for 30 min at 37 °C with biotinylated secondary antibody (1:200 dilution, Dako, Denmark), and then with Avidin DH and biotinylated horseradish peroxidase H complex based on Elite ABC kit guidelines (Vector Laboratories Inc., Burlingame, USA). At last, sciatic nerve samples were washed one more time with phosphate-buffered saline, and the reaction was revealed by diaminobenzidine tetrahydrochloride (DAB Substrate Kit, Vector Laboratories Inc., Burlingame, USA) and the tissue samples counterstained with hematoxylin, dehydrated, and cleared in xylene then cover slipped for light microscopic investigation.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 6 (San Diego, CA, USA). Results of GHBA1c, blood glucose and insulin levels, MDA, NO, SOD, IL-6, IL-10, MMP-2, MMP-9, and DNA fragmentation contents as well as the tail flick behavior test were carried out using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Results of body weight, rotarod, hind paw, and tail cold allodynia behavior tests were statistically analyzed using two-way ANOVA followed by Tukey’s multiple comparison test. The data were presented as mean ± SD, and differences were considered when the probability level is less than 0.05.
RESULTS
Effect of Sulforaphane on Diabetes Induced Changes in Body Weight
Diabetic rats showed a significant loss in body weight by 25.54%, as compared to the basal value. Treatment with SFN normalized body weight of diabetic rats. However, treatment PGB did not show any significant enhancement in body weight as compared to the diabetic control group (Table 1).
Effect of Sulforaphane on Diabetes Induced Changes in Nociceptive Thresholds and Motor Coordination
Diabetic rats showed a significant decrease in the balancing time on the rotarod and in the latency withdrawal time of tail flick, hind paw cold allodynia, and tail cold allodynia tests to be 46.75, 48.06, 39.73, and 63.23%, respectively, as compared to the normal group. Treatment with SFN succeeded to normalize the balancing rotarod time on rotarod and the latency withdrawal time of tail flick test and significantly improved the latency withdrawal time of the hind paw cold allodynia test to be 177.53%, as compared to the diabetic control group. However, it did not show any significant enhancement in the latency withdrawal time of tail cold allodynia test as compared to the diabetic rats. Treatment with PGB succeeded to normalize the balancing rotarod time on rotarod and the latency withdrawal time of both tail flick and hind paw cold allodynia tests of diabetic rats, as compared to the diabetic control group. However, it did not show any significant improvement in the latency withdrawal time of tail cold allodynia test in comparison to the diabetic control group (Fig. 1).
Effect of sulforaphane on diabetes induced changes in nociceptive thresholds and motor coordination. a Rotarod, b tail flick, c hind paw cold allodynia test, and d tail cold allodynia tests in diabetic rats. Each bar with vertical line represents the mean ± SEM of eight rats in each group. *vs normal, #vs diabetic control, using one-way ANOVA followed by Tukey’s multiple comparisons test for latency withdrawal time in tail flick test as well as two-way ANOVA followed by followed by Tukey’s multiple comparisons test for rotarod balancing time and latency withdrawal times in hind paw cold allodynia and tail cold allodynia tests; p < 0.05. SFN sulforaphane, PGB pregabalin.
Effect of Sulforaphane on Diabetes Induced Changes in Blood Glucose, Glycated Hemoglobin, and Insulin Levels
Diabetic rats showed significant increase in blood glucose and GHBA1c levels to 523.70 and 434.92%, respectively, along with a significant decrease in insulin level to 19.38% as compared to the normal group. Treatment with SFN showed significant decrease of blood glucose and GHBA1c levels to be 40.15 and 35. 21%, respectively, and increased serum insulin level to be 289.71% as compared to the diabetic control group. Treatment with PGB significantly decreased GHBA1c level to be 65.94%; however, it did not show any significant improvement in blood glucose and insulin levels as compared to the diabetic control group (Table 2).
Effect of Sulforaphane on Diabetes Induced Changes in Oxidative and Inflammatory Biomarkers in the Sciatic Nerve
Diabetic rats showed a significant increase in MDA, NO, and IL-6 contents in the sciatic nerve to 486.66, 511.48, and 590.43%, respectively, and a decrease in SOD and IL-10 contents to 13.67 and 16.75%, respectively, as compared to normal rats. Treatment with SFN significantly reduced MDA, NO, and IL-6 contents in the sciatic nerve to be 45.06, 39.28, and 43.69%, respectively, and significantly increased SOD and IL-10 contents to be 386.88 and 315.78% as compared to the diabetic control group. Treatment with PGB significantly reduced MDA, NO, and IL-6 to 63.47, 53.94, and 63.32%, respectively, and increased SOD content to 262.29% of the diabetic control group, without improving sciatic nerve IL-10 content (Table 3).
Effect of Sulforaphane on Diabetes Induced Changes in Matrix Metalloproteinases in the Sciatic Nerve
Diabetic rats showed significant increase in MMP-2 and MMP-9 contents in the sciatic nerve to 506.62 and 563.32%, respectively, as compared to the normal group. Treatment with SFN showed significant decrease in sciatic nerve MMP-2 and MMP-9 contents to 45.36 and 52.70%, respectively, as compared to diabetic control group. Administration of PGB resulted in a significant decrease in the sciatic nerve content of MMP-2 and MMP-9 to 70.99 and 67.38%, respectively, in comparison to the diabetic control group (Fig. 2).
Effect of sulforaphane on diabetes induced changes in matrix metalloproteinases in the sciatic nerve. a MMP-2. b MMP-9. Each bar with vertical line represents the mean ± SEM of eight rats in each group. *vs normal, #vs diabetic control, @vs PGB, using one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05. SFN sulforaphane, PGB pregabalin.
Effect of Sulforaphane on Diabetes Induced Deoxyribonucleic Acid Fragmentation in the Sciatic Nerve
Diabetic rats showed significant increase in DNA fragmentation in the sciatic nerve to 400.35% in comparison to the normal group. Diabetic rats treated with SFN or PGB resulted in a significant decrease in DNA fragmentation to 72.06 and 58.82%, respectively, as compared to the diabetic control group (Fig. 3).
Effect of sulforaphane on diabetes induced deoxyribonucleic acid fragmentation in the sciatic nerve. Each bar with vertical line represents the mean ± SEM of eight rats in each group. *vs normal, #vs diabetic control, using one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05. SFN sulforaphane, PGB pregabalin.
Effect of Sulforaphane on Diabetes Induced Changes in Cyclooxygenase-2 Expression in the Sciatic Nerve
Immunohistochemical examination of sciatic nerve tissues of diabetic rats showed a significant increase in the expression of COX-2 that has been confirmed through severe intracellular and nerve sheath positive immunoreactions as well as significantly higher immuno-reactive area percentage (572.25%) as compared to the normal group. Treatment with SFN resulted in a significant suppression of COX-2 protein expression, evidenced by moderate positive immunoreaction in the nerve fiber cells and the nerve sheath along with a significantly reduced immunoreactive area percentage (55.71%) in comparison to the diabetic control group. On the other hand, treatment with PGB showed significant decrease in COX-2 protein expression, demonstrated by mild positive immunoreaction in the nerve fiber cells and the nerve sheath along with a significantly reduced immunoreactive area percentage (73.68%) in comparison to the diabetic control group (Table 4; Fig. 4).
Effect of sulforaphane on diabetes induced changes in cyclooxygenase-2 expression in the sciatic nerve. a Representative photomicrographs illustrating the immunohistochemical staining of COX-2 in sciatic nerve sections (×400). Normal group showed no immunoreaction of COX-2 in the nerve fiber cells and in the nerve sheath. SFN group showed no immunoreaction of COX-2 in the nerve fiber cells as well as the nerve sheath. Diabetic control group showed severe intracellular and nerve sheath positive immunoreactions of COX-2 (arrow). Diabetic + SFN group showed moderate positive immunoreaction of COX-2 in the cells of the nerve fibers (arrow). Diabetic + PGB group showed mild positive immunoreaction of COX-2 in the nerve fiber cells as well as the nerve sheath. b Quantification of COX-2 staining as area percentage of immunopositive cells to the total area of microscopic field across five fields. Each bar with vertical line represents the mean ± SEM of five fields. *vs normal, #vs diabetic control, @vs PGB, using one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05. SFN sulforaphane, PGB pregabalin.
Effect of Sulforaphane on Diabetes Induced Changes in Nuclear Factor Kappa-B p65 Expression in the Sciatic Nerve
Immunohistochemical examination of sciatic nerve tissues of the diabetic control group showed severe intracellular and nerve sheath positive immunoreactions as well as significantly higher immuno-reactive area percentage (623.07%) in comparison to normal rats. Treatment with SFN or PGB showed significant reduction of NF-κB p65 protein expression, proved by no positive immunoreaction in the cells of the nerve fibers along with a significantly reduced immunoreactive area percentage (76.84 and 88.17%, respectively) relative to the diabetic control group (Table 4; Fig. 5).
Effect of sulforaphane on diabetes induced changes in nuclear factor kappa-B expression in the sciatic nerve. a Representative photomicrographs illustrating the immunohistochemical staining of NF-κB in sciatic nerve sections (×400). Normal group showed no immunoreaction of NF-κB in the nerve fiber cells and in the nerve sheath. SFN group showed no immunoreaction of NF-κB in the nerve fiber cells as well as the nerve sheath. Diabetic control showed severe intracellular and nerve sheath positive immunoreactions of NF-κB indicated by brown staining (arrow). Diabetic + SFN group showed no immunoreaction of NF-κB in the cells of the nerve fibers. Diabetic + PGB showed no immunoreaction of NF-κB in the nerve fiber cells as well as the nerve sheath. b Quantification of NF-κB staining as area percentage of immunopositive cells to the total area of microscopic field across five fields. Each bar with vertical line represents the mean ± SEM of five fields. *vs normal, #vs diabetic control, @vs PGB, using one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05. SFN sulforaphane, PGB pregabalin.
Effect of Sulforaphane on Diabetes Induced Histopathological Changes in the Sciatic Nerve
Histopathological investigation of sciatic nerve sections in rats of both normal and SFN groups showed normal structure of nerve fibers cells with steady arrangement and covering sheath. However, diabetic rats showed severe extravasations of red blood cells between the nerve trunk and nerve sheath, moderate increase in collagen proliferation and severe myelin ballooning and vascular degenerations with moderate irregular arrangement of nerve fiber cells. Treatment with SFN or PGB maintained the normal histopathological structure of the sciatic nerve (Table 5; Fig. 6).
Effect of sulforaphane on diabetes induced histopathological changes in the sciatic nerve. Representative photomicrographs of sciatic nerve from normal group showed normal histological structure of nerve fibers cells with regular arrangement and covering sheath; SFN group showed normal histological structure; diabetic control displayed severe extravasations of red blood cells (H) in-between the nerve trunk and nerve sheath, moderate increase in collagen proliferation and severe myelin ballooning and vascular degenerations with moderate irregular arrangement of nerve fiber cells were observed (arrow); Diab + SFN group showed normal histological structure; Diab + PGB showed normal histological structure (H&E ×40).
DISCUSSION
The findings of the current study revealed that SFN has a neuro-protective effect against peripheral neuropathy induced by NA-STZ model similar to that of the standard drug PGB. Peripheral neuro-protection of SFN is based on the observed enhancement of diabetic rats’ motor functions and nociceptive threshold, alleviation of histopathological impairment of sciatic nerve, coupled with reducing of oxidative stress, inflammatory load, MMPs, and DNA fragmentation.
In the current study, NA and STZ injections showed significant increase in blood glucose and GHBA1c levels coupled with a marked decrease in serum insulin level and rats’ average body weight, which is in agreement with former studies [34, 48,47,50]. It has been investigated that NA-STZ promotes oxidative stress leading to partial destruction of insulin secreting β-cells, thus diminishing their insulin secreting function and consequently increasing BGL [51]. Furthermore, Balamurugan and Ignacimuthu found that insulin deficiency co-occurs with extravagant breakdown of tissue proteins and wasting muscle mass leading to the observed loss in rats’ average body weight [52]. Herein, SFN treatment attenuated NA-STZ-induced loss in diabetic rats’ body weight which is in consistent with a former study of Sun et al., who found that SFN treatment improved body weight in mdx mice via triggering Nrf-2 pathway and thereby mitigating oxidative damage [53]. Additionally, SFN mitigated NA-STZ-induced diabetes in rats demonstrated by reducing blood glucose and insulin levels and increasing serum insulin level. In support, Song et al. and de Souza et al. demonstrated that the anti-hyperglycemic effect of SFN is mainly due to its ability to reduce the overproduction of ROS and inhibit NF-κB pathway in the pancreatic islets cells, hence preserving insulin secretion [28, 37].
It has been shown that long-term hyperglycemia can induce worsening in rats’ motor functions as well as nociceptive threshold [54]. Herein, NA-STZ-induced diabetes resulted in significant impairment of muscle coordination on the rotating rod coupled with sensory dysfunction in diabetic rats demonstrated by reduction in latency withdrawal time in the tail flick, hind paw cold allodynia, and tail cold allodynia tests. In support, Solanki and Bhavsar found that STZ injection resulted in impairment of motor coordination in diabetic rats [55]. Notably, uncontrolled hyperglycemia resulted in obvious nerve degeneration possibly due to partial destruction of pancreatic β-cells, overproduction of blood glucose, and reduced consumption of glucose by tissues [56]. Additionally, Pandhare et al. and Al-Rejaie et al. found that STZ injection resulted in the development of thermal and cold hyperalgesia in diabetic rats as well as worsening in their motor performance as a consequence to the release of pro-inflammatory meditators from the resident macrophages, Schwann cells, and the area near the nerve lesion [54, 57]. In the current study, treatment with SFN showed significant improvement in motor coordination of diabetic rats as well as nociceptive threshold. In agreement, Morroni et al. found that SFN improved motor coordination in 6-hydroxydopamine-lesioned mouse model of PD through enhancing glutathione levels and its dependent enzymes (glutathione-S-transferase and glutathione reductase) as well as modulating neuronal survival pathways such as extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) in the brain of mice [58]. In addition, Chen et al. found that SFN mitigated motor impairment in early brain injury after experimental subarachnoid hemorrhage in rats [59]. Herein, treatment with PGB showed marked enhancement in the motor coordination as well as nociceptive threshold of NA-STZ-induced diabetic rats. In support to our findings, Bender et al. alluded that PGB has anti-hyperalgesic and anti-allodynic effects possibly via selectively blocking calcium alpha-2 delta-2 (α2–δ) channels and therefore reduce the calcium ion level and oxidative stress in the sciatic nerve [60].
Noteworthy, oxidative stress and inflammation seem to take a center-stage in the development of DPN [8, 61]. In the current study, NA and STZ injections induced a significant increase in sciatic nerve contents of MDA and NO as well as a marked decrease in SOD content. In support to our results, Kamble and Bodhankar demonstrated that uncontrolled hyperglycemia accompanied by NA and STZ injections results in oxidative stress and ROS overproduction that in turn prompt the development of DPN [36]. Herein, SFN treatment boosted endogenous anti-oxidant defense through attenuating the effect of NA-STZ-induced diabetes on the aforementioned oxidative biomarkers. In support, Karin et al. and Guerrero-Beltrán found that the protective effect of SFN against neurodegenerative diseases arises from induction of many cyto-protective proteins through Nrf2-antioxidant response element pathway [62, 63]. Noteworthy, when cell is in equilibrium state, Nrf2 is segregated in the cytosol through two molecules of Kelch-like ECH-associated protein 1 (Keap 1) [64]. Nevertheless, whenever there is a massive generation of ROS, oxidation of cysteine thiol groups of Keap1 takes place that leads to conformational changes resulting in the detachment of Nrf2 from Keap1, allowing nuclear translocation of Nrf2. Nrf2 interaction with antioxidant response element (ARE), the specific DNA-binding domain, results in augmenting the expression of antioxidant enzymes including heme oxygenase-1 (HO-1), glutamylcysteine synthetase, gamma-glutamyl cysteine ligase (γ-GCS), and thioredoxin reductase as well as phase 2 detoxifying enzymes such as NAD(P)H:quinone oxidoreductase (NQO1), glutathione S-transferases (GST), and UDP-glucuronyltransferases [27]. SFN acts on Nrf2-Keap1 cytosolic complex through its isothiocyanates part and dislocates this complex through altering the cysteine thiol residues of Keap1 that in turn leads to allow Nrf2 nuclear translocation and induction of ARE gene transcription [65].
Notably, oxidative stress triggers a cascade of inflammatory responses, through the activation of NF-κB pathway that in turn results in the devastation of lipid membrane and tissue damage, and lastly leads to the progression of DPN [16, 17]. Herein, NA-STZ-induced diabetes resulted in significant increase in IL-6 as well as NF-κB p65 and COX-2 expression coupled with a marked reduction in sciatic nerve IL-10 content. These findings are in accordance with Kamble and Bodhankar, who found that long-term hyperglycemia triggered various inflammatory mediators through the activation of NF-κB in STZ-induced diabetic rats [36]. It is worth mentioning that NF-κB plays a fundamental role in acute and chronic inflammatory disorders. In normal condition, NF-κB dimers are retained in the cytoplasm in an inactive form by binding to a specific NF-κB inhibitory protein and IκB protein that prevent NF-κB-DNA binding. Different stimuli such as pro-inflammatory cytokines can activate the IκB kinase (IKK) complex that phosphorylates NF-κB-IκB complex resulting in their dislocation. Thus, the liberated NF-κB can interact with DNA and triggers the expression of diverse pro-inflammatory mediators and inducible enzymes [66]. Besides its anti-oxidant effects, SFN has been documented as a regulator of inflammatory responses through inhibiting the activation NF-κB pathway in sciatic nerve and in turn contributes to neuroprotection in diabetic neuropathy [62]. Herein, PGB treatment ameliorated the NA-STZ-induced changes in oxidative and inflammatory biomarkers. These findings are in agreement with former studies of Thiagarajan et al., who demonstrated that PGB is an antagonist of voltage-dependent calcium channels that binds to α2–δ subunit and hence decreases the calcium ion level and oxidative stress in the sciatic nerve [67]. Furthermore, Park et al. found that the neuroprotective action of PGB is mediated via its anti-inflammatory effect as it suppresses the NF-κB-regulated gene products and the expression of COX-2 and thus prevents the synthesis of substance P-induced cytokine in DPN [68].
Notably, long-term hyperglycemia leads to oxidative stress and overproduction of ROS that in turn results in over-activation of oxidative poly(ADPribose) polymerase (PARP), which trigger DNA fragmentation and also contribute markedly to the development of DPN [69]. In this context, NA-STZ-induced diabetic rats exhibited sciatic nerve DNA fragmentation. On the other hand, treatment with SFN significantly reduced DNA fragmentation in sciatic nerve of diabetic rats similar to that observed in PGB-treated rats. In accordance with our findings, Angeloni et al. showed that SFN activates Nrf2 that serves as a defense mechanism against oxidative stress through producing antioxidants, cytoprotective proteins, and phase II detoxifying enzymes and hence increases cell viability and decreases DNA fragmentation [70]. Furthermore, Tarozzi et al. found that SFN mediated DNA fragmentation into oligosomes, which is counted as an irreversible late stage of apoptosis via averting mitochondrial depolarization resulting from the activation of caspase 9 and 3 in dopaminergic-like human neuroblastoma cell line [25].
Herein, histopathological investigation revealed that NA-STZ-induced diabetic rats resulted in severe extravasation of red blood cells between the nerve trunk and nerve sheath, moderate increase in collagen proliferation, severe myelin ballooning, and vascular degeneration with moderate irregular arrangement of nerve fiber cells. In support, former studies showed that uncontrolled DM provoked sciatic nerve damage [18, 71]. Treatment with SFN and PGB retained the normal histopathological structure of sciatic nerve tissue in diabetic rats. In support, Tarozzi et al. alluded that SFN mitigated neurodegenerative diseases via its dual anti-oxidant and anti-inflammatory neuroprotective effects [25].
Ultimately, MMP-2 and MMP-9 are widely associated with many neuro-degenerative diseases including DPN as they destruct ECM components in the basement membrane via acting on various substrates such as fibronectin, elastin, desaturated interstitial collagen, and IV collagen [72]. Notably, ECM devastation results in constriction as well as alteration in the structure of arteries that in turn leads to impairment in neural cells, causing ischemia of nerve tissue and eventually nerve death [18]. Herein, NA and STZ injections resulted in significant increase in sciatic nerve MMP-2 and MMP-9 contents. In support to these findings, Creager et al. and Parkar and Addepalli found that uncontrolled hyperglycemia induced by STZ activates PKC that prompts ROS overproduction coupled with a cascade of inflammatory responses via activating NF-κB [16, 18]. ROS and inflammation upregulate MMPs especially MMP-2 and MMP-9 via oxidizing of the sulfide bond of the pro-domain of MMPs, which results in the triggering of pro-MMPs and the reduction of TIMPs [21]. In this study, SFN treatment showed marked suppression of the expression of MMP-2 and MMP-9 in sciatic nerve similar to those of PGB-treated rats. In accordance with these findings, studies of Benedict et al. found that SFN suppressed the expression MMPs via its anti-inflammatory effect in traumatic spinal cord injury in rats [26]. Furthermore, Sun et al. alluded that SFN mitigated the expression of MMP-9 via inhibiting TNF-α-induced NF-κB activation in spinal cord injury in mice [53].
CONCLUSION
Taken together, the present investigation demonstrates that SFN exerted anti-peripheral neuropathic effect against the NA-STZ-induced diabetic rats. Its beneficial effect might be attributed to its ability to modulate hyperglycemia, oxidative stress, inflammation, and its ability to remodel ECM (Fig. 7). Given to the efficacy of SFN, it would be recommended to perform more pre-clinical studies to elucidate further mechanisms that might be involved in the anti-peripheral neuropathic effect of SFN. In addition, clinical studies are necessary to investigate the applicability of SFN as therapeutic agent for the treatment of peripheral neuropathy in patients suffering from diabetes.
Abbreviations
- AD:
-
Alzheimer’s disease
- AGEs:
-
Advanced glycation end products
- ANOVA:
-
Analysis of variance
- COX-2:
-
Cyclooxygenase-2
- DM:
-
Diabetes mellitus
- DPN:
-
Diabetic peripheral neuropathy
- ECM:
-
Extracellular matrix
- ERK1/2:
-
Extracellular signal-regulated protein kinases 1 and 2
- GHBA1c:
-
Glycated hemoglobin
- H&E:
-
Hematoxylin and eosin
- HPWL:
-
Hind paw withdrawal latency
- IL-10:
-
Interleukin-10
- IL-6:
-
Interleukin-6
- iNOS:
-
Nitric oxide synthase
- Keap1:
-
Kelch-like ECH associated protein 1
- MDA:
-
Malondialdehyde
- MMP-2:
-
Matrix metalloproteinase-2
- MMP-9:
-
Matrix metalloproteinase-9
- NA:
-
Nicotinamide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- PD:
-
Parkinson’s disease
- PGB:
-
Pregabalin
- PKC:
-
Protein kinase C
- ROS:
-
Reactive oxygen species
- SFN:
-
Sulforaphane
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- TWL:
-
Tail withdrawal latency
References
Grote, Caleb W., and Douglas E. Wright. 2016. A role for insulin in diabetic neuropathy. Frontiers in Neuroscience 10: 1–10. https://doi.org/10.3389/fnins.2016.00581 Frontiers Media SA.
Solmaz, Volkan, Bilge Piri Çınar, Gürkan Yiğittürk, Hatice Köse Özlece, Hüseyin Avni Eroglu, Aslan Tekatas, Oytun Erbaş, and Dilek Taşkıran. 2017. Neuroprotective effects of octreotide on diabetic neuropathy in rats. Biomedicine & Pharmacotherapy 89: 468–472. https://doi.org/10.1016/j.biopha.2017.02.027.
Maria, Galuppo, Giacoppo Sabrina, Bramanti Placido, and Emanuela Mazzon. 2014. Use of natural compounds in the management of diabetic peripheral neuropathy. Molecules 19: 2877–2895. https://doi.org/10.3390/molecules19032877.
Vileikyte, Loretta, Richard R. Rubin, and Howard Leventhal. 2004. Psychological aspects of diabetic neuropathic foot complications: An overview. Diabetes/Metabolism Research and Reviews 20: S13–S18. https://doi.org/10.1002/dmrr.437.
Katulanda, Prasad, Priyanga Ranasinghe, Ranil Jayawardena, R. Godwin, M.H. Rezvi Sheriff Constantine, and David R. Matthews. 2012. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetology & Metabolic Syndrome 4: 2–8. https://doi.org/10.1186/1758-5996-4-21 BioMed Central.
Sobhani, Sahar, Hamid Asayesh, Farshad Sharifi, Shirin Djalalinia, Hamid Reza Baradaran, Seyed Masoud Arzaghi, Morteza Mansourian, Aziz Rezapoor, Hossein Ansari, Mohammad Parvaresh Masoud, and Mostafa Qorbani. 2014. Prevalence of diabetic peripheral neuropathy in Iran: A systematic review and meta-analysis. Journal of Diabetes & Metabolic Disorders 13: 2–7. https://doi.org/10.1186/s40200-014-0097-y.
Bruschi, Lídia Karla Martinho, Dayvson Araújo da Rocha, Eusínio Lavigne Gesteira Filho, Nathália de Moura Pancoti Barboza, P.A.B. Frisanco, Raquel Milanesi Callegaro, Larissa Bianca Paiva Cunha de Sá, and Alberto Krayyem Arbex. 2017. Diabetes mellitus and diabetic peripheral neuropathy. Open Journal of Endocrine and Metabolic Diseases 7: 12–21. https://doi.org/10.4236/ojemd.2017.71002.
Chen, Long, Bing Li, Biqin Chen, Yiye Shao, Qiong Luo, Xiaohong Shi, and Yinghui Chen. 2016. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Scientific Reports 6: 1–11. https://doi.org/10.1038/srep31656.
Oh, Yoon. 2016. Bioactive compounds and their Neuroprotective effects in diabetic complications. Nutrients 8: 1–20. https://doi.org/10.3390/nu8080472.
Vincent, Andrea M., Brian C. Callaghan, Andrea L. Smith, and Eva L. Feldman. 2011. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nature Reviews Neurology 7: 573–583. https://doi.org/10.1038/nrneurol.2011.137.
Oyenihi, Ayodeji Babatunde, Ademola Olabode Ayeleso, Emmanuel Mukwevho, and Bubuya Masola. 2015. Antioxidant strategies in the management of diabetic neuropathy. BioMed Research International. Hindawi: 1–15. https://doi.org/10.1155/2015/515042.
Zangiabadi, Nasser, Vahid Sheibani, Majid Asadi-Shekaari, Mohammad Shabani, Mandana Jafari, Ali Reza Asadi, Haleh Tajadini, and Morteza Jarahi. 2011. Effects of melatonin in prevention of neuropathy in STZ-induced diabetic rats. American Journal of Pharmacology and Toxicology 6: 59–67. https://doi.org/10.3844/ajptsp.2011.59.67.
Yagihashi, Soroku, Hiroki Mizukami, and Kazuhiro Sugimoto. 2011. Mechanism of diabetic neuropathy: Where are we now and where to go? Journal of Diabetes Investigation 2: 18–32. https://doi.org/10.1111/j.2040-1124.2010.00070.x Wiley-Blackwell.
Edwards, James L., Andrea M. Vincent, Hsinlin T. Cheng, and Eva L. Feldman. 2008. Diabetic neuropathy: Mechanisms to management. Pharmacology & Therapeutics 120: 1–34. https://doi.org/10.1016/j.pharmthera.2008.05.005 NIH Public Access.
Ramasamy, Ravichandran, Shi Fang Yan, Kevan Herold, Raphael Clynes, and Ann Marie Schmidt. 2008. Receptor for advanced glycation end products: Fundamental roles in the inflammatory response: Winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Annals of the New York Academy of Sciences 1126: 7–13. https://doi.org/10.1196/annals.1433.056 NIH Public Access.
Creager, M.A., Thomas F. Lüscher, Francesco Cosentino, and Joshua A. Beckman. 2003. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108: 1527–1532. https://doi.org/10.1161/01.CIR.0000091257.27563.32.
Eid, Ahmed H., Noha F. Abdelkader, Ola M. Abd El-Raouf, Hala M. Fawzy, and Ezz-El-Din S. El-Denshary. 2016. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Archives of Pharmacal Research 39: 1693–1702. https://doi.org/10.1007/s12272-016-0833-6.
Parkar, N., and V. Addepalli. 2014. Effect of nobiletin on diabetic neuropathy in experimental rats. Austin Journal of Pharmacology and Therapeutics. 2: 2–5.
Ji, Ru-Rong, Zhen-Zhong Xu, Xiaoying Wang, and Eng H. Lo. 2009. Matrix metalloprotease regulation of neuropathic pain. Trends in Pharmacological Sciences 30: 336–340. https://doi.org/10.1016/j.tips.2009.04.002 NIH Public Access.
Kawasaki, Yasuhiko, Zhen-Zhong Xu, Xiaoying Wang, Jong Yeon Park, Zhi-Ye Zhuang, Ping-Heng Tan, Yong-Jing Gao, et al. 2008. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nature Medicine 14: 331–336. https://doi.org/10.1038/nm1723 Nature Publishing Group.
Opris, Razvan, Corina Tatomir, Diana Olteanu, Remus Moldovan, Bianca Moldovan, Luminita David, Andras Nagy, Nicoleta Decea, Mihai Ludovic Kiss, and Gabriela Adriana Filip. 2017. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids and Surfaces B: Biointerfaces 150: 192–200. https://doi.org/10.1016/j.colsurfb.2016.11.033.
Nunez, O., A. Fernández-Martínez, P.L. Majano, A. Apolinario, M. Gómez-Gonzalo, I. Benedicto, M. López-Cabrera, et al. 2004. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: Role of viral core and NS5A proteins. Gut 53: 1665–1672. https://doi.org/10.1136/gut.2003.038364.
Kowluru, Renu A., and Mamta Kanwar. 2009. Oxidative stress and the development of diabetic retinopathy: Contributory role of matrix metalloproteinase-2. Free Radical Biology and Medicine 46: 1677–1685. https://doi.org/10.1016/j.freeradbiomed.2009.03.024.
Kaufman-Szymczyk, Agnieszka, Grzegorz Majewski, Katarzyna Lubecka-Pietruszewska, and Krystyna Fabianowska-Majewska. 2015. The role of sulforaphane in epigenetic mechanisms, including interdependence between histone modification and DNA methylation. International Journal of Molecular Sciences 16: 29732–29743. https://doi.org/10.3390/ijms161226195.
Tarozzi, Andrea, Cristina Angeloni, Marco Malaguti, Fabiana Morroni, Silvana Hrelia, and Patrizia Hrelia. 2013. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Medicine and Cellular Longevity 2013: 415078. https://doi.org/10.1155/2013/415078 Hindawi.
Benedict, Andrea L., Andrea Mountney, Andres Hurtado, Kelley E. Bryan, Ronald L. Schnaar, Albena T. Dinkova-Kostova, and Paul Talalay. 2012. Neuroprotective effects of sulforaphane after contusive spinal cord injury. Journal of Neurotrauma 29: 2576–2586. https://doi.org/10.1089/neu.2012.2474.
Sita, Giulia, Patrizia Hrelia, Andrea Tarozzi, and Fabiana Morroni. 2016. Isothiocyanates are promising compounds against oxidative stress, neuroinflammation and cell death that may benefit neurodegeneration in Parkinson’s disease. International Journal of Molecular Sciences 17: 63–71. https://doi.org/10.3390/ijms17091454.
Song, Mi-Young, Eun-Kyung Kim, Woo-Sung Moon, Jin-Woo Park, Hyung-Jin Kim, Hong-Seob So, Raekil Park, Kang-Beom Kwon, and Byung-Hyun Park. 2009. Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicology and Applied Pharmacology 235: 57–67. https://doi.org/10.1016/j.taap.2008.11.007.
Zhang, Rui, Jingzhu Zhang, Lingduo Fang, Xi Li, Yue Zhao, Wanying Shi, and Li An. 2014. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. International Journal of Molecular Sciences 15: 14396–14410. https://doi.org/10.3390/ijms150814396.
Sun, Y., T. Yang, L. Mao, and F. Zhang. 2017. Sulforaphane protects against brain diseases: roles of cytoprotective. Enzyme 4: 1–7.
Zhou, Qian, Bin Chen, Xindong Wang, Lixin Wu, Yang Yang, Xiaolan Cheng, Zhengli Hu, et al. 2016. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2 and autophagy pathways. Scientific Reports 6: 32206. https://doi.org/10.1038/srep32206.
Yu, Chang, He Qi, Jing Zheng, Ling Yu Li, Yang Hao Hou, and Fang Zhou Song. 2017. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. International Immunopharmacology 45: 74–78. https://doi.org/10.1016/j.intimp.2017.01.034.
Ghasemi, Asghar, S. Khalifi, and S. Jedi. 2014. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiologica Hungarica 101: 408–420. https://doi.org/10.1556/APhysiol.101.2014.4.2.
Mikaili, P., A.A. Hemmati, M.J. Khodayar, M. Ghafurian, and I. Rashidi. 2011. Evaluation of the effects of nicotinamide on the blemycin-induced pulmonary fibroses in rat. International Journal of Animal and Veterinary 3: 330–336 Maxwell Scientific Organization.
El-Marasy, Salma A., Heba M.I. Abdallah, Siham M. El-Shenawy, Aiman S. El-Khatib, Osama A. El-Shabrawy, and Sanaa A. Kenawy. 2014. Anti-depressant effect of hesperidin in diabetic rats. Canadian Journal of Physiology and Pharmacology 92: 945–952. https://doi.org/10.1139/cjpp-2014-0281 NRC Research Press.
Kamble, Hemant Vinayak, and Subhash Laxmanrao Bodhankar. 2014. Concomitant administration of trigonelline and sitagliptin attenuates nicotinamide-streptozotocin induced diabetic neuropathy in wistar rats. Journal of Chemical and Pharmaceutical Research 6: 616–624.
Souza, de, Carolina Guerini, José Augusto Sattler, Adriano Martimbianco de Assis, Anderson Rech, Marcos Luiz Santos Perry, and Diogo Onofre Souza. 2012. Metabolic effects of sulforaphane oral treatment in streptozotocin-diabetic rats. Journal of Medicinal Food 15: 795–801. https://doi.org/10.1089/jmf.2012.0016.
Rocha-González, Héctor Isaac, Magali Ramírez-Aguilar, Vinicio Granados-Soto, Juan Gerardo Reyes-García, Jorge Elías Torres-López, Juan Carlos Huerta-Cruz, and Andrés Navarrete. 2014. Antineuropathic effect of 7-hydroxy-3,4-dihydrocadalin in streptozotocin-induced diabetic rodents. BMC Complementary and Alternative Medicine 14: 2–12. https://doi.org/10.1186/1472-6882-14-129.
Lundblad, M., E. Vaudano, and M.A. Cenci. 2003. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. Journal of Neurochemistry 84: 1398–1410. https://doi.org/10.1046/j.1471-4159.2003.01632.x.
Liepinsh, Edgars, Reinis Vilskersts, Liga Zvejniece, Baiba Svalbe, Elina Skapare, Janis Kuka, Helena Cirule, Solveiga Grinberga, Ivars Kalvinsh, and Maija Dambrova. 2009. Protective effects of mildronate in an experimental model of type 2 diabetes in Goto-Kakizaki rats. British Journal of Pharmacology 157: 1549–1556. https://doi.org/10.1111/j.1476-5381.2009.00319.x.
Ameyaw, E.O., J.N. Boampong, K.E. Kukuia, P. Amoateng, E. Obese, C. Osei-Sarpong, and E. Woode. 2014. Effect of xylopic acid on paclitaxel-induced neuropathic pain in rats. Journal of Medical and Biomedical Sciences 2: 6–12. https://doi.org/10.4314/jmbs.v2i4.2 School of Medicine and Health Sciences, University for Development Studies.
Lee, Ji-Hye, Dong Xing Li, Heera Yoon, Donghyun Go, Fu Shi Quan, Byung-Il Min, and Sun Kwang Kim. 2014. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complementary and Alternative Medicine 14: 2–7. https://doi.org/10.1186/1472-6882-14-471.
Ruiz-Larrea, M.B., A.M. Leal, M. Liza, M. Lacort, and H. de Groot. 1994. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 59: 383–388. https://doi.org/10.1016/0039-128X(94)90006-X.
Sastry, K.V.H., R.P. Moudgal, J. Mohan, J.S. Tyagi, and G.S. Rao. 2002. Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Analytical Biochemistry 306: 79–82. https://doi.org/10.1006/abio.2002.5676.
Gibb, R.K., D.D. Taylor, T. Wan, D.M. O’Connor, D.L. Doering, and C. Gerçel-Taylor. 1997. Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecologic Oncology 65: 13–22. https://doi.org/10.1006/gyno.1997.4637.
Tasci, Ilker, Mehmet Refik Mas, Sevil Atalay Vural, Salih Deveci, Bilgin Comert, Gunay Alcigir, Nuket Mas, C. Akay, M. Bozdayi, C. Yurdaydin, H. Bozkaya, O. Uzunalimoglu, A.T. Isik, and H.M. Said. 2007. Pegylated interferon-alpha plus taurine in treatment of rat liver fibrosis. World Journal of Gastroenterology 13: 3237–3244. https://doi.org/10.3748/wjg.v13.i23.3237.
Shi, Guang, Dong Li, Jinling Fu, Yan Sun, Yarong Li, Rongfeng Qu, Xin Jin, and Dongfu Li. 2015. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. American Journal of Translational Research 7: 1612–1620.
Ahangarpour, Akram, Hamid Heidari, Ali Akbar Oroojan, Farhang Mirzavandi, Khalil Nasr Esfehani, and Zeinab Dehghan Mohammadi. 2017. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna Journal of Phytomedicine 7: 169–179 Mashhad University of Medical Sciences.
Ghamarian, Abdolreza, Mohammad Abdollahi, Xiaogang Su, Azita Amiri, Ali Ahadi, and Azin Nowrouzi. 2012. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU Journal of Pharmaceutical Sciences 20: 2–9. https://doi.org/10.1186/2008-2231-20-56.
Saravanan, Ramalingam, and Leelavinothan Pari. 2008. Effect of succinic acid monoethyl ester on hemoglobin glycation and tail tendon collagen properties in type 2 diabetic rats. Fundamental & Clinical Pharmacology 22: 291–298. https://doi.org/10.1111/j.1472-8206.2008.00581.x.
Yilmaz, Okkes, Yasemin Ersan, Ayse Dilek Ozsahin, Ali Ihsan Ozturk, and Yusuf Ozkan. 2013. Consequences of the combined α-tocopherol, ascorbic acid and α-lipoic acid on the glutathione, cholesterol and fatty acid composition in muscle and liver of diabetic rats. Iranian Journal of Basic Medical Sciences 16: 165–172 Mashhad University of Medical Sciences.
Balamurugan, Rangachari, and Savarimuthu Ignacimuthu. 2011. Antidiabetic and hypolipidemic effect of methanol extract of Lippia nodiflora L. in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine 1: S30–S36. https://doi.org/10.1016/S2221-1691(11)60117-2.
Sun, Chengcao, Cuili Yang, Ruilin Xue, Shujun Li, Ting Zhang, Lei Pan, Xuejiao Ma, Liang Wang, and Dejia Li. 2015. Sulforaphane alleviates muscular dystrophy in mdx mice by activation of Nrf2. Journal of Applied Physiology 118: 224–237. https://doi.org/10.1152/japplphysiol.00744.2014.
Pandhare, Ramdas B., B. Sangameswaran, Popat B. Mohite, and Shantaram G. Khanage. 2012. Attenuating effect of seeds of Adenanthera pavonina aqueous extract in neuropathic pain in streptozotocin-induced diabetic rats: an evidence of neuroprotective effects. Revista Brasileira de Farmacognosia 22: 428–435. https://doi.org/10.1590/S0102-695X2012005000008 Sociedade Brasileira de Farmacognosia.
Solanki, Nilay D., and Shailesh K. Bhavsar. 2015. An evaluation of the protective role of Ficus racemosa Linn. in streptozotocin-induced diabetic neuropathy with neurodegeneration. Indian Journal of Pharmacology 47: 610–615. https://doi.org/10.4103/0253-7613.169579.
Sharma, Ashish Kumar, Akash Sharma, Rita Kumari, Kunal Kishore, Divya Sharma, Bharthu Parthsarthi Srinivasan, Ashok Sharma, S.K. Singh, S. Gaur, V.S. Jatav, P. Sharma, V. Srivastava, S. Joshi, M. Joshi, P.K. Dhakad, D.S. Kanawat, A. Mishra, A. Sharma, D. Singh, R.P. Singh, H.S. Chawda, R. Singh, S.K. Raikwar, M.K. Kurmi, P. Khatri, A. Agarwal, and A. Munajjam. 2012. Sitagliptin, sitagliptin and metformin, or sitagliptin and amitriptyline attenuate streptozotocin-nicotinamide induced diabetic neuropathy in rats. Journal of Biomedical Research 26: 200–210. https://doi.org/10.7555/JBR.26.20110054.
Al-Rejaie, Salim S., Hatem M. Abuohashish, Mohammed M. Ahmed, Aws S. Arrejaie, Abdulaziz M. Aleisa, and Shakir D. AlSharari. 2015. Telmisartan inhibits hyperalgesia and inflammatory progression in a diabetic neuropathic pain model of Wistar rats. Neurosciences (Riyadh, Saudi Arabia) 20: 115–123. https://doi.org/10.17712/nsj.2015.2.20140511.
Morroni, Fabiana, Andrea Tarozzi, Giulia Sita, Cecilia Bolondi, Juan Manuel Zolezzi Moraga, Giorgio Cantelli-Forti, and Patrizia Hrelia. 2013. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 36: 63–71. https://doi.org/10.1016/j.neuro.2013.03.004.
Chen, Long, Bing Li, Biqin Chen, Yiye Shao, Qiong Luo, Xiaohong Shi, and Yinghui Chen. 2016. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Scientific Reports 6: 31656. https://doi.org/10.1038/srep31656 Nature Publishing Group.
Bender, Gregor, Jeffry A. Florian, Stephen Bramwell, Mark J. Field, Keith K.C. Tan, Scott Marshall, Joost DeJongh, Robert.R. Bies, and Meindert Danhof. 2010. Pharmacokinetic–pharmacodynamic analysis of the static allodynia response to pregabalin and sildenafil in a rat model of neuropathic pain. Journal of Pharmacology and Experimental Therapeutics 334: 599–608.
Sandireddy, Reddemma, Veera Ganesh Yerra, Aparna Areti, Prashanth Komirishetty, and Ashutosh Kumar. 2014. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. International Journal of Endocrinology 2014: 674987. https://doi.org/10.1155/2014/674987 Hindawi.
Karin, Michael, Yumi Yamamoto, and Q. May Wang. 2004. The IKK NF-κB system: A treasure trove for drug development. Nature Reviews Drug Discovery 3: 17–26. https://doi.org/10.1038/nrd1279.
Guerrero-Beltrán, Carlos Enrique, Mariel Calderón-Oliver, José Pedraza-Chaverri, and Yolanda Irasema Chirino. 2012. Protective effect of sulforaphane against oxidative stress: Recent advances. Experimental and Toxicologic Pathology 64: 503–508. https://doi.org/10.1016/j.etp.2010.11.005.
Itoh, Ken, Junsei Mimura, and Masayuki Yamamoto. 2010. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxidants & Redox Signaling 13: 1665–1678. https://doi.org/10.1089/ars.2010.3222.
Xue, M., Q. Qian, A. Adaikalakoteswari, N. Rabbani, R. Babaei-Jadidi, and P.J. Thornalley. 2008. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 57: 2809–2817. https://doi.org/10.2337/db06-1003.
González-Ramos, Reinaldo, Jacques Donnez, Sylvie Defrère, Isabelle Leclercq, Jean Squifflet, Jean Christophe Lousse, and Anne Van Langendonckt. 2007. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Molecular Human Reproduction 13: 503–509. https://doi.org/10.1093/molehr/gam033.
Thiagarajan, Venkata R.K., Palanichamy Shanmugam, Uma M. Krishnan, and Arunachalam Muthuraman. 2014. Ameliorative potential of Vernonia cinerea on chronic constriction injury of sciatic nerve induced neuropathic pain in rats. Anais da Academia Brasileira de Ciencias 86: 1436–1449.
Park, Seyeon, Eun Sook Ahn, Dong Woo Han, Jong Hwa Lee, Kyung Tae Min, Hyunkyung Kim, and Yong-Woo Hong. 2008. Pregabalin and gabapentin inhibit substance P-induced NF-κB activation in neuroblastoma and glioma cells. Journal of Cellular Biochemistry 105: 414–423. https://doi.org/10.1002/jcb.21837.
Kumar, Ashutosh, Ravinder K. Kaundal, Seethalakshmi Iyer, and Shyam S. Sharma. 2007. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sciences 80: 1236–1244. https://doi.org/10.1016/j.lfs.2006.12.036.
Angeloni, Cristina, Emanuela Leoncini, Marco Malaguti, Sabrina Angelini, Patrizia Hrelia, and Silvana Hrelia. 2009. Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. Journal of Agricultural and Food Chemistry 57: 5615–5622. https://doi.org/10.1021/jf900549c.
Zangiabadi, Nasser, Hossein Mohtashami, Mahboobeh Hojatipour, Mandana Jafari, Majid Asadi-Shekaari, and Mohammad Shabani. 2014. The effect of Angipars on diabetic neuropathy in STZ-induced diabetic male rats: A study on behavioral, electrophysiological, sciatic histological and ultrastructural indices. The Scientific World Journal 2014: 1–8. https://doi.org/10.1155/2014/721547.
Martin, Alexandra, Michael R. Komada, and David C. Sane. 2003. Abnormal angiogenesis in diabetes mellitus. Medicinal Research Reviews 23: 117–145. https://doi.org/10.1002/med.10024.
Acknowledgments
The authors are thankful to Dr. Adel Bakeir (Histology Department, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt) for carrying out the histopathological and immunohistochemical examinations of this study.
Funding
This study was funded by the National Research Centre, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Ethics Committees of Faculty of Pharmacy, Cairo University (permit number 1393) and National Research Centre (permit number 15/047).
Conflict of Interest
The authors affirm that there were no conflicts of interest associated with this research work.
Additional information
Highlights
• Sulforaphane is neuroprotective against diabetic peripheral neuropathy
• Sulforaphane has anti-hyperglycemic, anti-oxidant, anti-inflammatory activities
• Sulforaphane suppressed matrix metalloproteinases involved in the neuropathic pain
Rights and permissions
About this article
Cite this article
Moustafa, P.E., Abdelkader, N.F., El Awdan, S.A. et al. Extracellular Matrix Remodeling and Modulation of Inflammation and Oxidative Stress by Sulforaphane in Experimental Diabetic Peripheral Neuropathy. Inflammation 41, 1460–1476 (2018). https://doi.org/10.1007/s10753-018-0792-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0792-9